Delivery of Intraocular Triamcinolone Acetonide in the Treatment of Macular Edema
Abstract
:1. Introduction
2. Barriers Affecting Drug Delivery and Metabolism
2.1. Static Barriers
2.2. Dynamic Barriers
2.3. Metabolic Barriers
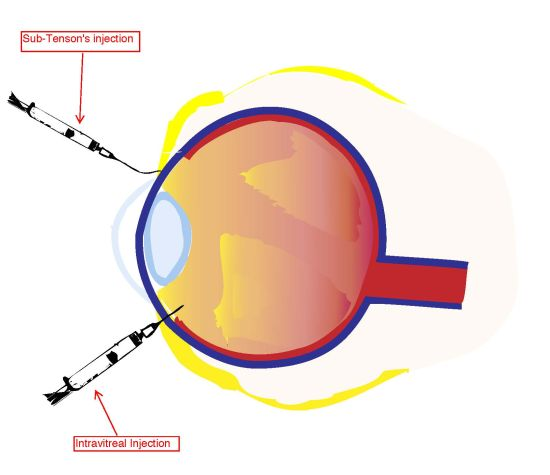
3. Intravitreal Injection of Corticosteroids to the Posterior Pole
3.1. Triamcinolone Acetonide
3.2. Efficacy of IV-TA
3.3. Drawbacks of IVTA
4. Sub-Tenon’s Infusion of Corticosteroids to the Posterior Pole
4.1. Efficacy of STI-TA
4.2. Drawbacks of STI-TA

| IVI | STI | ||
|---|---|---|---|
| Pro | Cons | Pro | Cons |
| Effectively reduces macular thickness and improves BCVA | Faster development of cataracts | Small improvement in BCVA and macular thickness | Technically more difficult to perform Correctly (i.e., risk of reflux) |
| Increased drug bioavailability | Increased risk of endophthalmitis | Less risk of cataract development | Many barriers that ultimately reduce drug bioavailablity |
| Increased risk of elevated IOP and secondary glaucoma | Less risk of secondary intraocular hypertension (if no reflux during procedure) | ||
5. Conclusions
References
- Baruch, D. Kuppermann. Underlying Basis and Goals of Macular Edema Therapy; Galen Publishing, LLC: Boston, MA, USA, 2007; pp. 182–186. [Google Scholar]
- Peyman, G.A.; Lad, E.M.; Moshfeghi, D.M. Intravitreal injection of therapeutic agents. Ret. J. Ret. Vit. Dis. 2009, 29, 875–912. [Google Scholar]
- Tao, Y.; Jonas, J.B. Intravitreal triamcinolone. Ophthalmologica 2011, 225, 1–20. [Google Scholar] [CrossRef]
- Ahmed, I.; Gokhale, R.D.; Shah, M.V.; Patton, T.F. Physicochemical determinants of drug diffusion across the conjunctiva, sclera, and cornea. J. Pharm. Sci. 1987, 76, 583–586. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Noonan, J.S. Permeability of cornea, sclera and conjunctiva: A literature analysis for drug delivery to the eye. J. Pharm. Sci. 1998, 87, 1479–1488. [Google Scholar] [CrossRef]
- Kim, S.H.; Lutz, R.J.; Wang, N.S.; Robinson, M.R. Transport barriers in transscleral drug delivery for retinal diseases. Ophthalmic Res. 2007, 39, 244–254. [Google Scholar] [CrossRef]
- Ambati, J.; Adamis, A.P. Transscleral drug delivery to the retina and choroid. Prog. Retin. Eye Res. 2002, 21, 145–151. [Google Scholar] [CrossRef]
- Ambati, J.; Canakis, C.S.; Miller, J.W.; Gragoudas, E.S.; Edwards, A.; Weissgold, D.J.; Kim, I.; Delori, F.C.; Adamis, A.P. Diffusion of high molecular weight compounds through sclera. Invest. Ophthalmol. Vis. Sci. 2000, 41, 1181–1185. [Google Scholar]
- Boubriak, O.A.; Urban, J.P.; Bron, A.J. Differential effects of aging on tranport porperties of anterior and posterior human sclera. Eye Res. 2003, 76, 701–713. [Google Scholar] [CrossRef]
- Edwards, A.; Prausnitz, M.R. Fiber matrix model of scleral and corneal stroma for drug delivery to the eye. AIChE J. 1998, 44, 214–225. [Google Scholar] [CrossRef]
- Cruysberg, L.P.; Nuijts, R.M.; Geroski, D.H.; Koole, L.H.; Hendriske, F.; Edelhauser, H.F. In vitro human scleral permeability of fluorescein, dexamethasone-fluorescein, methotrexate-fluorescein and rhodamine 6G and the use of a coated oil as a new drug delivery system. J. Ocul. Pharmacol. Ther. 2002, 18, 559–569. [Google Scholar] [CrossRef]
- Cheruvu, N.P.; Kompella, U.B. Bovine and por-cine transscleral solute transport: Influence of lipophilicity and the choroid-Bruch’s layer. Invest. Ophthalmol. Vis. Sci. 2006, 47, 4513–4522. [Google Scholar]
- Maurice, D.M.; Polgar, J. Diffusion across the sclera. Exp. Eye Res. 1977, 25, 577–582. [Google Scholar] [CrossRef]
- Dunlevy, J.R.; Rada, J.A. Interaction of lumican with aggrecan in the aging human sclera. Invest. Ophthalmol. Vis. Sci. 2004, 3849–3846. [Google Scholar] [CrossRef]
- Moore, D.J.; Hussain, A.A.; Marshall, J. Age-related variation in the hydraulic conductivity of Bruch's Membrane. Invest. Ophthalmol. Vis. Sci. 1995, 36, 1290–1297. [Google Scholar]
- Hussain, A.A.; Rowe, L.; Marshall, J. Age-related alterations in the diffusional transport of amino acids across the human Bruch’s-choroid complex. J. Opt. Soc. Am. A. Opt. Image Sci. Vis. 2002, 19, 166–172. [Google Scholar] [CrossRef]
- Pitkänen, L.; Ranta, V.P.; Moilanen, H.; Urtti, A. Permeability of retinal pigment epithelium: Effects of permeant molecular weight and lipophilicity. Invest. Ophthalmol. Vis. Sci. 2005, 46, 641–646. [Google Scholar] [CrossRef]
- Robinson, M.R.; Lee, S.S.; Kim, H.; Kim, S.; Lutz, R.J.; Galban, C.; Bungay, P.M.; Yuan, P.; Wang, N.S.; Kim, J.; et al. A rabbit model for assessing the ocular barriers to the transscleral delivery of triamcinolone acetonide. Exp. Eye Res. 2006, 82, 479–487. [Google Scholar] [CrossRef]
- Lee, S.J.; He, W.; Robinson, S.B.; Robinson, M.R.; Csaky, K.G.; Kim, H. Evaluation of clearance mechanisms with transscleral drug delivery. Invest. Ophthalmol. Vis. Sci. 2010, 51, 5205–5212. [Google Scholar]
- Inomata, H.; Bill, A.; Smelser, G.K. Unconventional routes of aqueous humor outflow in cynomolgus monkey (Macaca irus). Am. J. Ophthalmol. 1972, 73, 893–907. [Google Scholar]
- Kumar, G. Drug Metabolizing Enzymes Systems in the Eye. In Ocular Therapeutics and Drug Delivery: A Multidisciplanary Approach; Reddy, I.K., Ed.; Technomic Publishing Company: Lancaster, CA, USA, 1996; pp. 149–167. [Google Scholar]
- Peyman, G.A.; Herbst, R. Bacterial endophthalmitis: Treatment with intraocular injection of gentamicin and dexamethasone. Arch. Ophthalmol. 1974, 91, 416–418. [Google Scholar] [CrossRef]
- Ciulla, T.A.; Walker, J.D.; Fong, D.S.; Criswell, M.H. Corticosteroids in posterior segment disease: An update on new delivery systems and new indications. Curr. Opin. Ophthalmol. 2004, 15, 211–220. [Google Scholar] [CrossRef]
- Intravitreal Injection Survery. Anti-infection procedures are still evolving. Retin. Physciana 2006, 3 (3).
- Bhavsar, A.R.; Ip, M.S.; Glassman, A.R. The risk of endophthalmitis following intravitreal triamcinolone injection in the DRCRnet and SCORE clinical trials. Am. J. Ophthalmol. 2007, 144, 454–456. [Google Scholar] [CrossRef]
- Jonas, J.B.; Degenring, R.F.; Kamppeter, B.A.; Kreissig, I.; Akkoyun, I. Duration of the effect of intravitreal triamcinolone acetonide as treatment for diffuse diabetic macular edema. Am. J. Ophthalmol. 2004, 138, 158–160. [Google Scholar]
- Scholes, G.N.; O'Brien, W.J.; Abrams, G.W.; Kubicek, M.F. Clearance of triamcinolone from vitreous. Arch. Ophthalmol. 1985, 103, 1567–1569. [Google Scholar] [CrossRef]
- Gillies, M.C.; Sutter, F.K.; Simpson, J.M.; Larsson, J.; Ali, H.; Zhu, M. Intravitreal triamcinolone for refractory diabetic macular edema: Two-year results of a double-masked, placebo-controlled, randomized clinical trial. Ophthalmology 2006, 113, 1533–1538. [Google Scholar]
- Sutter, F.K.; Simpson, J.M.; Gillies, M.C. Intravitreal triamcinolone for diabetic macular edema that persists after laser treatment: Three-month efficacy and safety results of a prospective, randomized, double-masked, placebo-controlled clinical trial. Ophthalmology 2004, 111, 2044–2049. [Google Scholar]
- Gillies, M.C.; McAllister, I.L.; Zhu, M.; Wong, W.; Louis, D.; Arnold, J.J.; Wong, T.Y. Intravitreal triamcinolone prior to laser treatment of diabetic macular edema: 24-month results of a randomized controlled trial. Ophthalmology 2011, 118, 866–872. [Google Scholar]
- Larsson, J.; Zhu, M.; Sutter, F.; Gillies, M.C. Relation between reduction of foveal thickness and visual acuity in diabetic macular edema treated with intravitreal triamcinolone. Am. J. Ophthalmol. 2005, 139, 802–806. [Google Scholar] [CrossRef]
- Browning, D.J.; Glassman, A.R.; Aiello, L.P.; Beck, R.W.; Brown, D.M.; Fong, D.S.; Bressler, N. M.; Danis, R.P.; Kinyoun, J.L.; et al. Relationship between optical coherence tomography-measured central retinal thickness and visual acuity in diabetic macular edema. Ophthalmology 2007, 114, 525–536. [Google Scholar] [CrossRef]
- Maia, M.; Farah, M.E.; Belfort, R.N.; Penha, F.M.; Lima Filho Acacio, A.S.; Aggio, F.B.; Belfort, R., Jr. Effects of intravitreal triamcinolone acetonide injection with and without preservative. Br. J. Ophthalmol. 2007, 91, 1122–1124. [Google Scholar] [CrossRef]
- Nelson, M.L.; Tennant, M.T.; Sivalingam, A.; Regillo, C.D.; Belmont, J.B.; Martidis, A. Infectious and presumed noninfectious endophthalmitis after intravitreal triamcinolone acetonide injection. Retina 2003, 23, 686–691. [Google Scholar]
- Friedman, D.M.; Moore, M.E. The efficacy of intraarticular steroids in osteoarthritis: a double-blind study. J. Rheumatol. 1980, 7, 850–856. [Google Scholar]
- Charalampidou, S.; Nolan, J.; Ormonde, G.O.; Beatty, S. Visual perceptions induced by intravitreous injections of therapeutic agents. Eye (Lond.) 2011, 25, 494–501. [Google Scholar] [CrossRef]
- Jonas, J.B.; Kreissig, I.; Degenring, R. Intraocular pressure after intravitreal injection of triamcinolone acetonide. Br. J. Ophthalmol. 2003, 87, 24–27. [Google Scholar] [CrossRef]
- Gillies, M.C.; Islam, F.M.; Larsson, J.; Pasadhika, S.; Gaston, C.; Zhu, M.; Wong, T.Y. Triamcinolone-induced cataract in eyes with diabetic macular oedema: 3-year prospective data from a randomized clinical trial. Clin. Exp. Ophthalmol. 2010, 38, 605–612. [Google Scholar] [CrossRef]
- Gillies, M.C.; Kuzniarz, M.; Craig, J.; Ball, M.; Luo, W.; Simpson, J.M. Intravitreal triamcinolone-induced elevated intraocular pressure is associated with the development of posterior subcapsular cataract. Ophthalmology 2005, 112, 139–143. [Google Scholar]
- Becker, B.; Ballin, N. Glaucoma and corticosteroid provocative testing. Arch. Ophthalmol. 1965, 74, 621–624. [Google Scholar] [CrossRef]
- Becker, B.; Bresnick, G.; Chevrette, L.; Kolker, A.E.; Oaks, M.C.; Cibis, A. Intraocular pressure and its response to topical corticosteroids in diabetes. Arch. Ophthalmol. 1966, 76, 477–483. [Google Scholar] [CrossRef]
- Inatani, M.; Iwao, K.; Kawaji, T.; Hirano, Y.; Ogura, Y.; Hirooka, K.; Shiraga, F.; Nakanishi, Y.; Yamamoto, H.; Negi, A.; et al. Intraocular pressure elevation after injection of triamcinolone acetonide: A multicenter retrospective case-control study. Am. J. Ophthalmol. 2008, 145, 676–681. [Google Scholar]
- Okada, A.A.; Wakabayashi, T.; Morimura, Y.; Kawahara, S.; Kojima, E.; Asano, Y.; Hida, T. Trans-Tenon's retrobulbar triamcinolone infusion for the treatment of uveitis. Br. J. Ophthalmol. 2003, 87, 968–971. [Google Scholar]
- Koga, T.; Mawatari, Y.; Inumaru, J.; Fukushima, M.; Tanihara, H. Trans-Tenon's retrobulbar triamcinolone acetonide infusion for reractory diabetic macular edema after vitrectomy. Graefe's Arch. Clin. Exp. Ophthalmol. 2005, 243, 1247–1252. [Google Scholar]
- Bonini-Filho, M.A.; Jorge, R.; Barbosa, J.C.; Calucci, D.; Cardillo, J.A.; Costa, R.A. Intravitreal injection versus sub-Tenon's infusion of triamcinolone acetonide for refractory diabetic macular edema: A randomized clinical trial. Invest. Ophthalmol. Vis. Sci. 2005, 46, 3845–3849. [Google Scholar]
- Cardillo, J.A.; Melo, L.A., Jr.; Costa, R.A.; Skaf, M.; Belfort, R., Jr.; Souza-Filho, A.A.; Farah, M.E.; Kuppermann, B.D. Comparison of intravitreal versus posterior sub-Tenon's capsule injection of triamcinolone acetonide for diffuse diabetic macular edema. Ophthalmology 2005, 112, 1557–1563. [Google Scholar] [CrossRef]
- Massin, P.; Audren, F.; Haouchine, B.; Erginay, A.; Bergmann, J.F.; Benosman, R.; Caulin, C.; Gaudric, A. Intravitreal triamcinolone acetonide for diabetic diffuse macular edema: Preliminary results of a prospective controlled trial. Ophthalmology 2004, 111, 218–224, discussion 224-215. [Google Scholar]
- Mueller, A.J.; Jian, G.; Banker, A.S.; Rahhal, F.M.; Capparelli, E.; Freeman, W.R. The effect of deep posterior subtenon injection of corticosteroids on intraocular pressure. Am. J. Ophthalmol. 1998, 125, 158–163. [Google Scholar]
- Smithen, L.M.; Ober, M.D.; Maranan, L.; Spaide, R.F. Intravitreal triamcinolone acetonide and intraocular pressure. Am. J. Ophthalmol. 2004, 138, 740–743. [Google Scholar] [CrossRef]
- Shimura, M.; Yasuda, K.; Nakazawa, T.; Shiono, T.; Sakamoto, T.; Nishida, K. Drug reflux during posterior subtenon infusion of triamcinolone acetonide in diffuse diabetic macular edema not only brings insufficient reduction but also causes elevation of intraocular pressure. GraefesArch. Clin. Exp. Ophthalmol. 2009, 247, 907–912. [Google Scholar] [CrossRef]
- Boddu, S.H.; Jwala, J.; Vaishya, R.; Earla, R.; Karla, P.K.; Pal, D.; Mitra, A.K. Novel nanoparticulate gel formulations of steroids for the treatment of macular edema. J. Ocul. Pharmacol. Ther. 2010, 26, 37–48. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pickrell, A.; Harris, A.; Ngo, S.; Amireskandari, A.; Stewart, E.; Siesky, B. Delivery of Intraocular Triamcinolone Acetonide in the Treatment of Macular Edema. Pharmaceutics 2012, 4, 230-242. https://doi.org/10.3390/pharmaceutics4010230
Pickrell A, Harris A, Ngo S, Amireskandari A, Stewart E, Siesky B. Delivery of Intraocular Triamcinolone Acetonide in the Treatment of Macular Edema. Pharmaceutics. 2012; 4(1):230-242. https://doi.org/10.3390/pharmaceutics4010230
Chicago/Turabian StylePickrell, Aaron, Alon Harris, Sandra Ngo, Annahita Amireskandari, Erin Stewart, and Brent Siesky. 2012. "Delivery of Intraocular Triamcinolone Acetonide in the Treatment of Macular Edema" Pharmaceutics 4, no. 1: 230-242. https://doi.org/10.3390/pharmaceutics4010230