Compaction Behavior of Isomalt after Roll Compaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Roll Compaction
2.2.2. Analysis of Powders and Granules
2.2.3. Tableting and Characterization of Tablets
2.2.4. Nomenclature
Granules (G) or tablets (T)-isomalt (A) or isomalt-DCP (B)-specific compaction force (kN/cm)_tableting force (kN) (if tableted)
3. Results and discussion
3.1. Characterization of Granules
| Specific compaction force [kN/cm] | Isomalt granules | Isomalt-DCP granules | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 6 | 10 | 14 | 0 | 6 | 10 | 14 | |
| Hausner factor | 1.33 | 1.21 | 1.16 | 1.15 | 1.11 | 1.44 | 1.24 | 1.15 | 1.15 |
| ffc value | 3.6 | 6.8 | 12.1 | 16.6 | 16.5 | 3.2 | 4.8 | 10.1 | 8.4 |
| x10 [µm] | 1.7 | 25.0 | 141.5 | 184.0 | 186.5 | 1.0 | 36.6 | 37.5 | 41.0 |
| x50 [µm] | 17.7 | 546 | 642 | 607 | 617 | 7.2 | 556 | 590 | 554 |
| x90 [µm] | 44.6 | 923 | 934 | 921 | 938 | 31.1 | 920 | 927 | 928 |
3.2. Characterization of Tablets
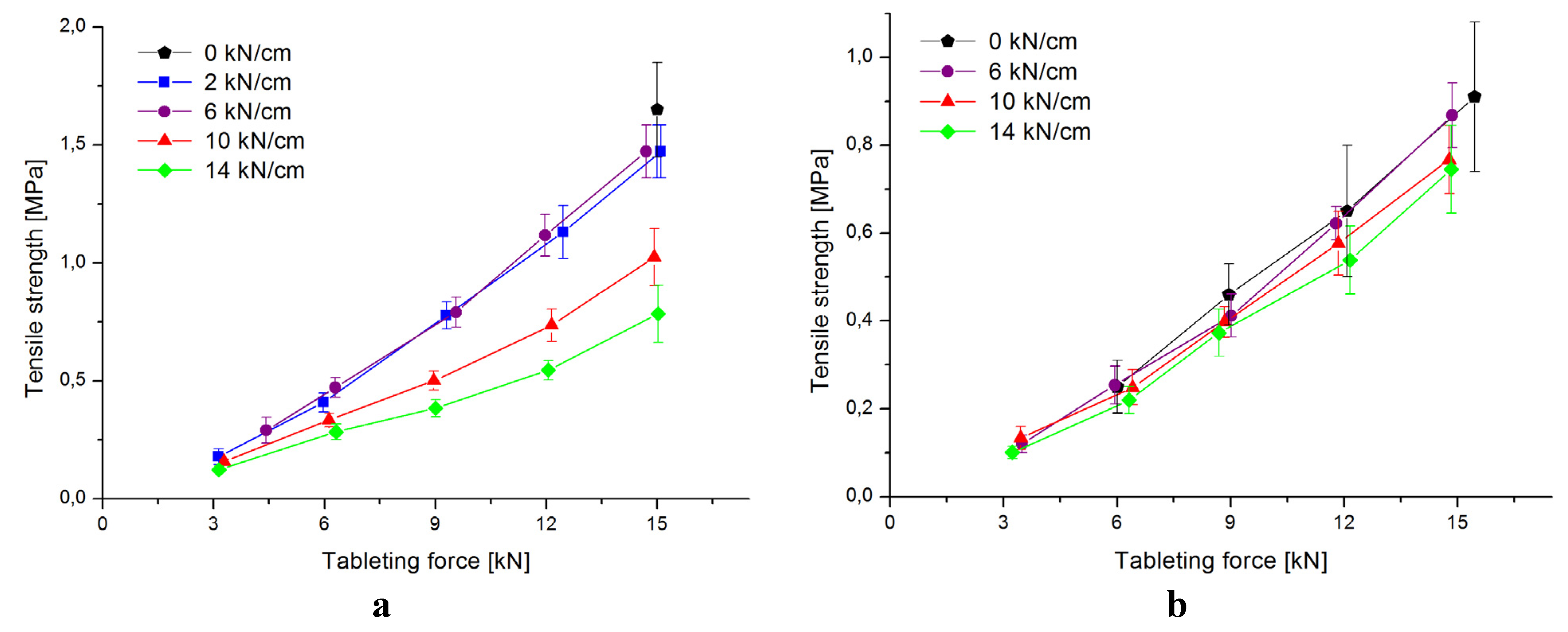
| 2.0 kN/cm | 6.0 kN/cm | 10.0 kN/cm | 14.0 kN/cm | |
|---|---|---|---|---|
| 3 kN | not passed | not passed | not passed | not passed |
| 6 kN | not passed | not passed | not passed | not passed |
| 9 kN | not passed | 0.91% | not passed | not passed |
| 12 kN | 0.62% | 0.75% | not passed | not passed |
| 15 kN | 0.74% | 0.60% | not passed | not passed |
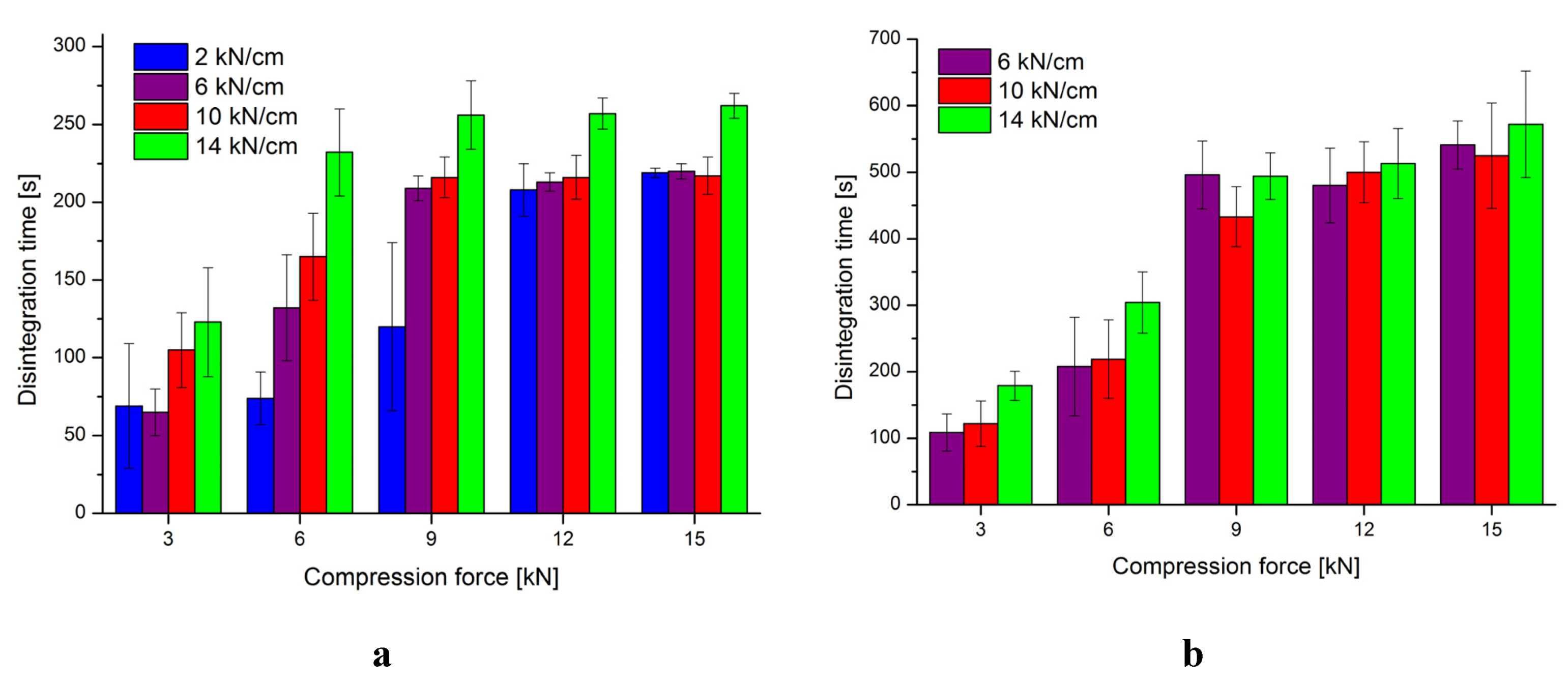
4. Conclusion
Acknowledgments
Conflict of Interest
References
- Petzoldt, R. Palatinite in type II diabetics: Effect on blood-glucose, serum-insulin, C-peptide and free fatty acids. Deutsch. Med. Wochenschr. 1982, 107, 1910–1913. [Google Scholar] [CrossRef]
- Gehring, F. The sugar substitute Palatinit® with special regard to microbiological and caries-prophylactic aspects. Zeitschrift für Ernährungswissenschaft 1981, 20, 96–106. [Google Scholar] [CrossRef]
- Bolhuis, G.K. Excipients for direct compaction—An update. Pharm. Dev. Technol. 2006, 11, 111–124. [Google Scholar] [CrossRef]
- Ndindayino, F.; Henrist, D.; Kiekens, F.; Vervaet, C.; Remon, J.P. Characterization and evaluation of isomalt performance in direct compression. Int. J. Pharm. 1999, 189, 113–124. [Google Scholar] [CrossRef]
- Ndindayino, F.; Vervaet, C.; van den Mooter, G.; Remon, J.P. Direct compression and moulding properties of co-extruded isomalt/drug mixtures. Int. J. Pharm. 2002, 235, 159–168. [Google Scholar]
- Chawla, M. Evaluation of galen IQ polymer in Tramadol Hydrochloride orally disintegrating tablet. Int. J. Drug Deliv. 2011, 3, 439–455. [Google Scholar]
- Saska, Z. Effect of isomalt as novel binding agent on compressibility of poorly compactable paracetamol evaluated by factorial design. Powder Technol. 2010, 201, 123–129. [Google Scholar]
- Kleinebudde, P. Roll compaction/dry granulation: Pharmaceutical applications. Eur. J. Pharm. Biopharm. 2004, 58, 317–326. [Google Scholar]
- Kochhar, S.K.; Rubinstein, M.H.; Barnes, D. The effects of slugging and recompression on pharmaceutical excipients. Int. J. Pharm. 1995, 115, 35–43. [Google Scholar]
- Malkowska, S.; Khan, K.A. Effect of re-conpression on the properties of tablets prepared by dry granulation. Drug Dev. Ind. Pharm. 1983, 9, 331–347. [Google Scholar]
- Fell, J.T.; Newton, J.M. Determination of tablet strength by diametral-compression test. J. Pharm. Sci. 1970, 59, 688. [Google Scholar]
- Schulze, D. Flow properties of powders and bulk solids (fundamentals). Available online: http://www.dietmar-schulze.de/grdle1.pdf (accessed on 24 September 2012).
- Ritschel, W.A.; Bauer-Brandl, A. Die Tablette: Handbuch der Entwicklung, Herstellung und Qualitätssicherung (In German); Editio Cantor Verlag: Aulendorf, Germany, 2002; p. 518. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Quodbach, J.; Mosig, J.; Kleinebudde, P. Compaction Behavior of Isomalt after Roll Compaction. Pharmaceutics 2012, 4, 494-500. https://doi.org/10.3390/pharmaceutics4040494
Quodbach J, Mosig J, Kleinebudde P. Compaction Behavior of Isomalt after Roll Compaction. Pharmaceutics. 2012; 4(4):494-500. https://doi.org/10.3390/pharmaceutics4040494
Chicago/Turabian StyleQuodbach, Julian, Johanna Mosig, and Peter Kleinebudde. 2012. "Compaction Behavior of Isomalt after Roll Compaction" Pharmaceutics 4, no. 4: 494-500. https://doi.org/10.3390/pharmaceutics4040494




