Development of a Taste-Masked Orodispersible Film Containing Dimenhydrinate
Abstract
:1. Introduction
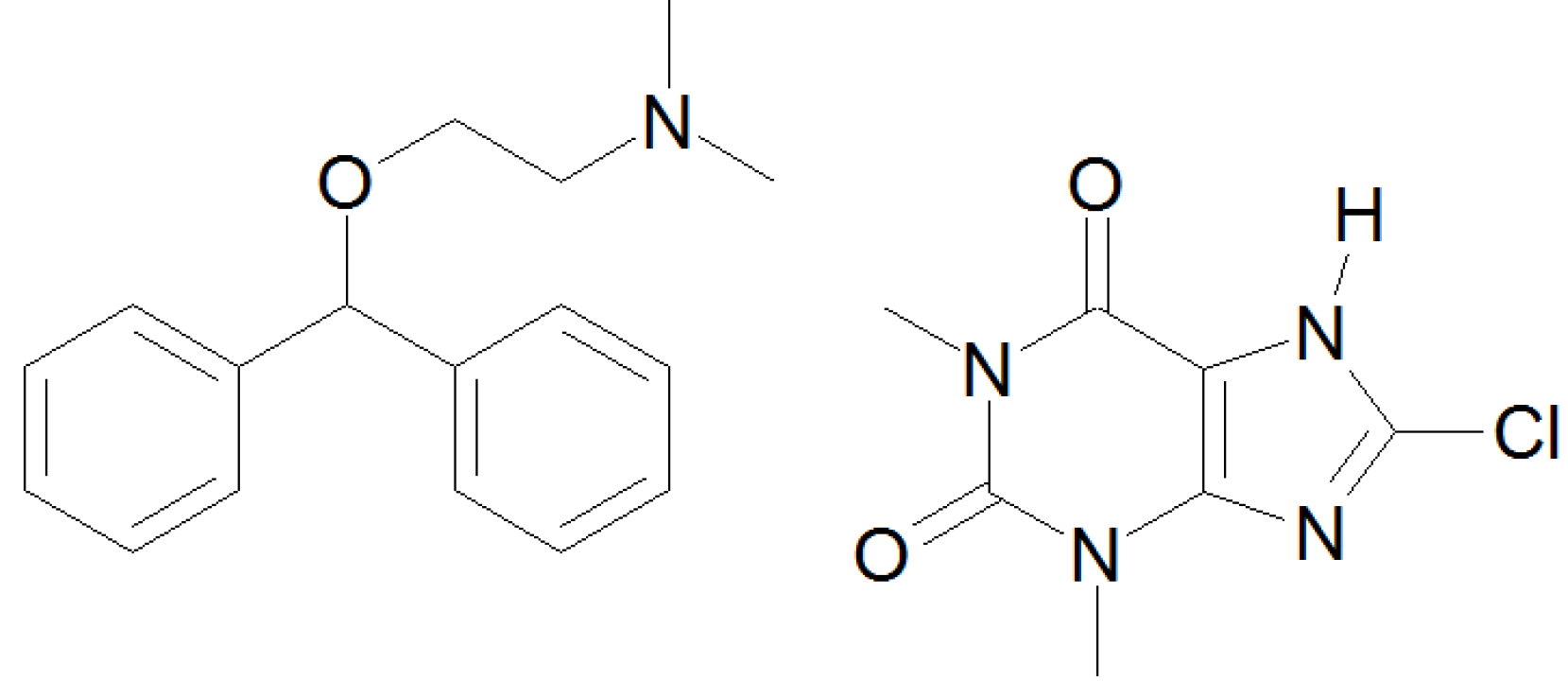
2. Materials and Methods
2.1. Materials
| Substance | Function | Distributor | Brand name |
|---|---|---|---|
| dimenhydrinate | drug | Pharma Roth (D) | |
| modified pea starch polymer | film forming agent | Roquette (F) | Lycoat RS 720 |
| glycerol (anhydrous) | plasticizer | Caesar & Loretz (D) | |
| water | solvent | ||
| ethanol abs. | co-solvent | VWR (D) | |
| E 124 (red) | coloring agent | Caesar & Loretz (D) | |
| hydroxypropyl-β-cyclodextrin | solubilizer taste | Roquette (F) | Kleptose® HPB oral grade |
| masking agent | |||
| maltodextrin (pea starch based) | solubilizer taste | Roquette (F) | Kleptose® linecaps |
| masking agent | |||
| sulfobutylether-β-cyclodextrin (+sodium salts) | solubilizer taste | Cydex (US) | Captisol® |
| masking agent | |||
| saccharin sodium | sweetener | Caesar & Loretz (D) |
2.2. Sample Preparation

| Batch code: | D | P | DCA | PCA | DCD | PCD | DCDS | PCDS | DMD | PMD | DS | PS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimenhydrinate | x | - | x | - | x | - | x | - | x | - | x | - |
| HP-β-CD | - | - | - | - | x | x | x | x | - | - | - | - |
| SBE-β-CD | - | - | x | x | - | - | - | - | - | - | - | - |
| Maltodextrin | - | - | - | - | - | - | - | - | x | x | - | - |
| Saccharin sodium | - | - | - | - | - | - | x | x | - | - | x | x |
2.3. Film Thickness and Weight
2.4. Drug Content
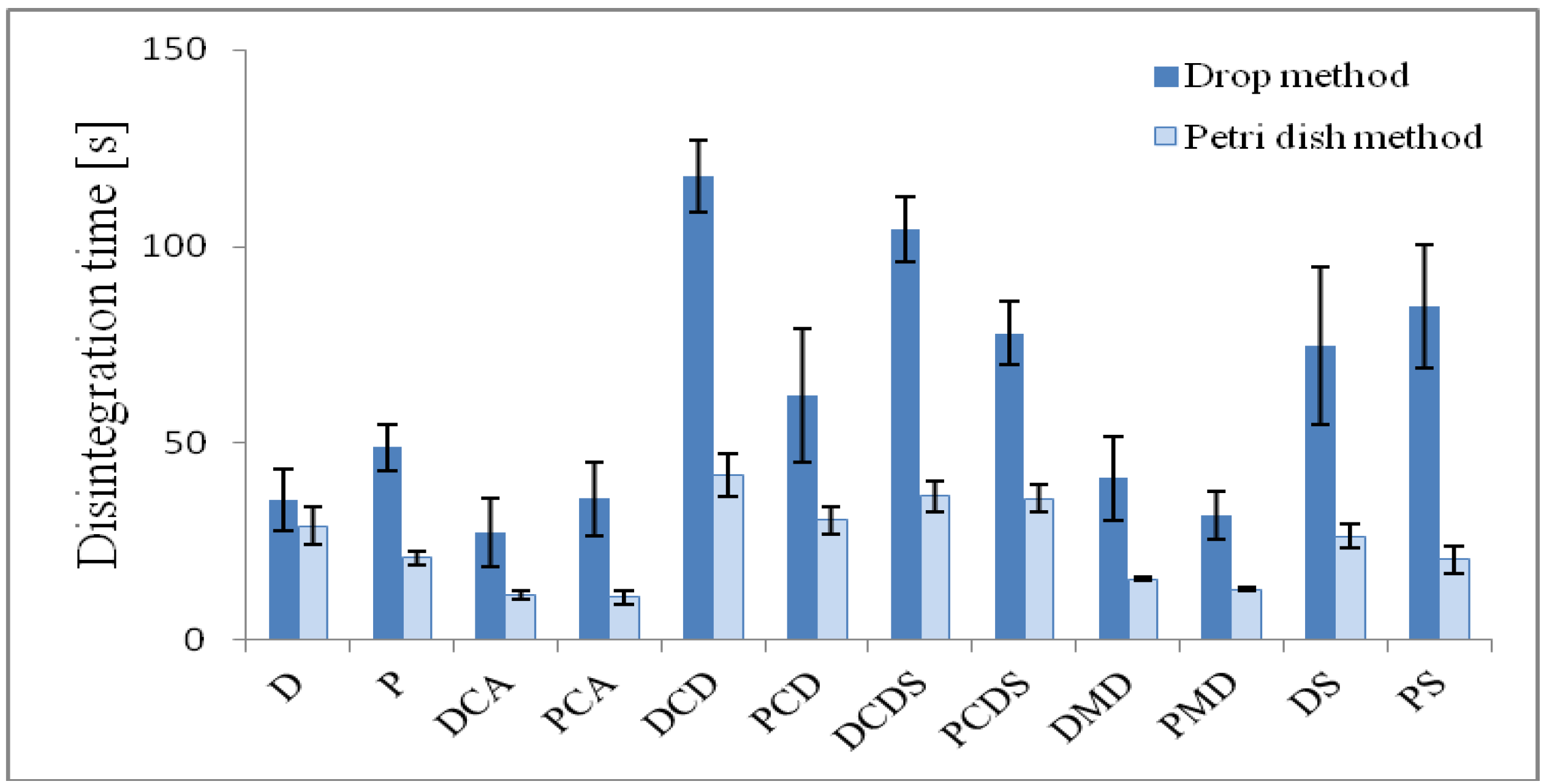
2.5. Determination of Disintegration time
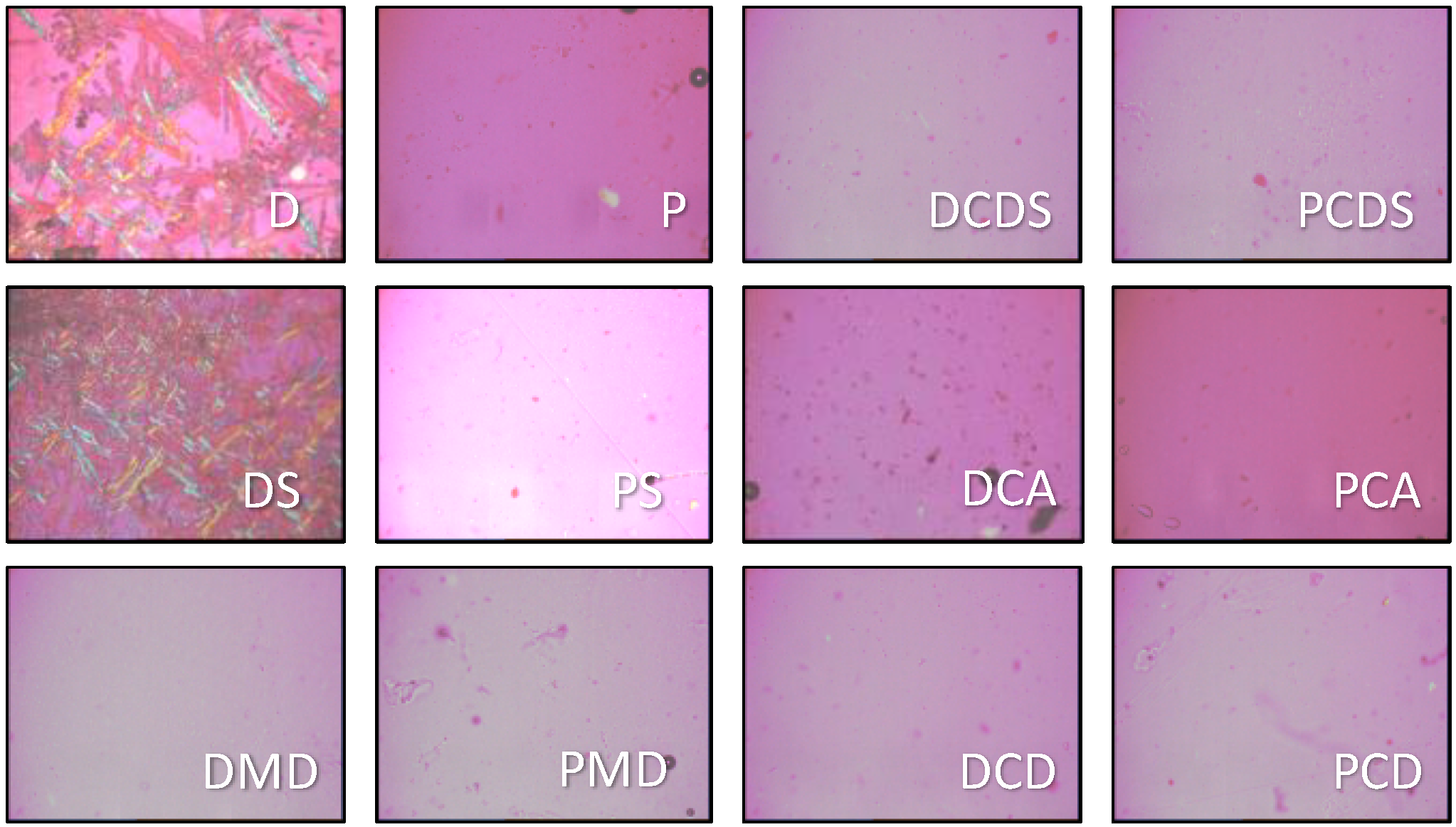
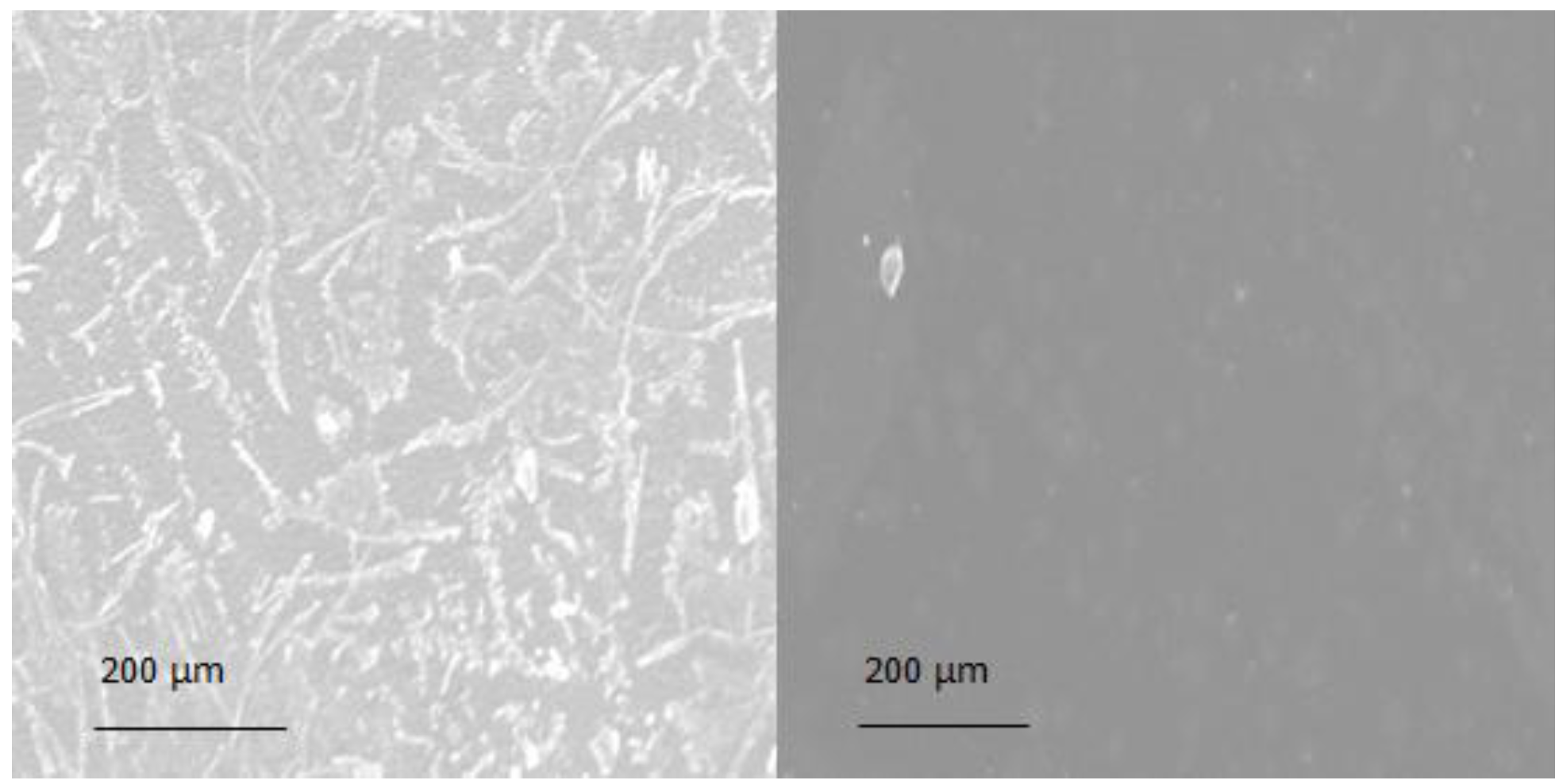
2.6. Morphology
| operational voltage | 40 kV |
|---|---|
| operational amperage | 40 mA |
| angular step size | 0.0167113° 2 θ |
| range | 10°-50° 2 θ |
| scanning rate | 0.417782°/s |
2.7. Electronic Taste Sensing
| Insent | α Astree | ||
|---|---|---|---|
| SB2AAE | umami | ZZ | Cross selective |
| SB2CA0 | sourness | AB | |
| SB2CT0 | saltiness | BA | |
| SB2AE1 | astringency | BB | |
| SB2AC0 | bitterness | CA | |
| SB2AN0 | bitterness | DA | |
| SB2C00 | bitterness (anionic) | JE | |
3. Results and Discussion
3.1. Film Properties
| Thickness (µm) | Weight (µm) | Drug content | Disintegration time | ||||
|---|---|---|---|---|---|---|---|
| (mg) | AV | Q (%) | Drop (s) | Petri dish (s) | |||
| D | 143.4 ± 7.2 | 59.4 ± 2.3 | 4.7 ± 0.1 | 0.5 | 94.8 | 35.5 ± 7.8 | 29.1 ± 4.8 |
| P | 136.6 ± 7.0 | 55.6 ± 1.7 | - | - | - | 49.0 ± 5.9 | 21.0 ± 1.7 |
| DCA | 114.7 ± 3.2 | 50.5 ± 1.8 | 5.2 ± 0.1 | 0.3 | 104.5 | 27.4 ± 8.6 | 11.3 ± 1.1 |
| PCA | 116.0 ± 6.7 | 51.2 ± 3.3 | - | - | - | 36.0 ± 9.4 | 10.9 ± 1.7 |
| DCD | 158.0 ± 3.4 | 64.8 ± 1.1 | 4.7 ± 0.1 | 2.7 | 94.0 | 117.9 ± 9.3 | 41.7 ± 5.5 |
| PCD | 142.4 ± 25.4 | 66.8 ± 2.7 | - | - | - | 62.0 ± 17.0 | 30.6 ± 3.4 |
| DCDS | 171.2 ± 7.5 | 71.9 ± 3.0 | 5.6 ± 0.1 | 0.9 | 112.4 | 104.4 ± 8.2 | 36.6 ± 4.1 |
| PCDS | 152.7 ± 6.7 | 67.1 ± 5.2 | - | - | - | 78.1 ± 8.2 | 35.9 ± 3.4 |
| DMD | 125.0 ± 2.3 | 55.3 ± 2.6 | 5.0 ± 0.1 | 0.1 | 100.1 | 41.1 ± 10.6 | 15.5 ± 0.5 |
| PMD | 119.4 ± 9.4 | 52.2 ± 3.3 | - | - | - | 31.7 ± 6.0 | 12.9 ± 0.5 |
| DS | 156.6 ± 11.4 | 64.9 ± 3.9 | 5.5 ± 0.4 | 1.2 | 109.2 | 74.7 ± 20.1 | 26.4 ± 3.0 |
| PS | 127.5 ± 7.6 | 53.4 ± 2.7 | - | - | - | 85.0 ± 15.7 | 20.5 ± 3.6 |
3.2. Morphology

3.3. Taste Assessment by Electronic Taste Sensing Systems
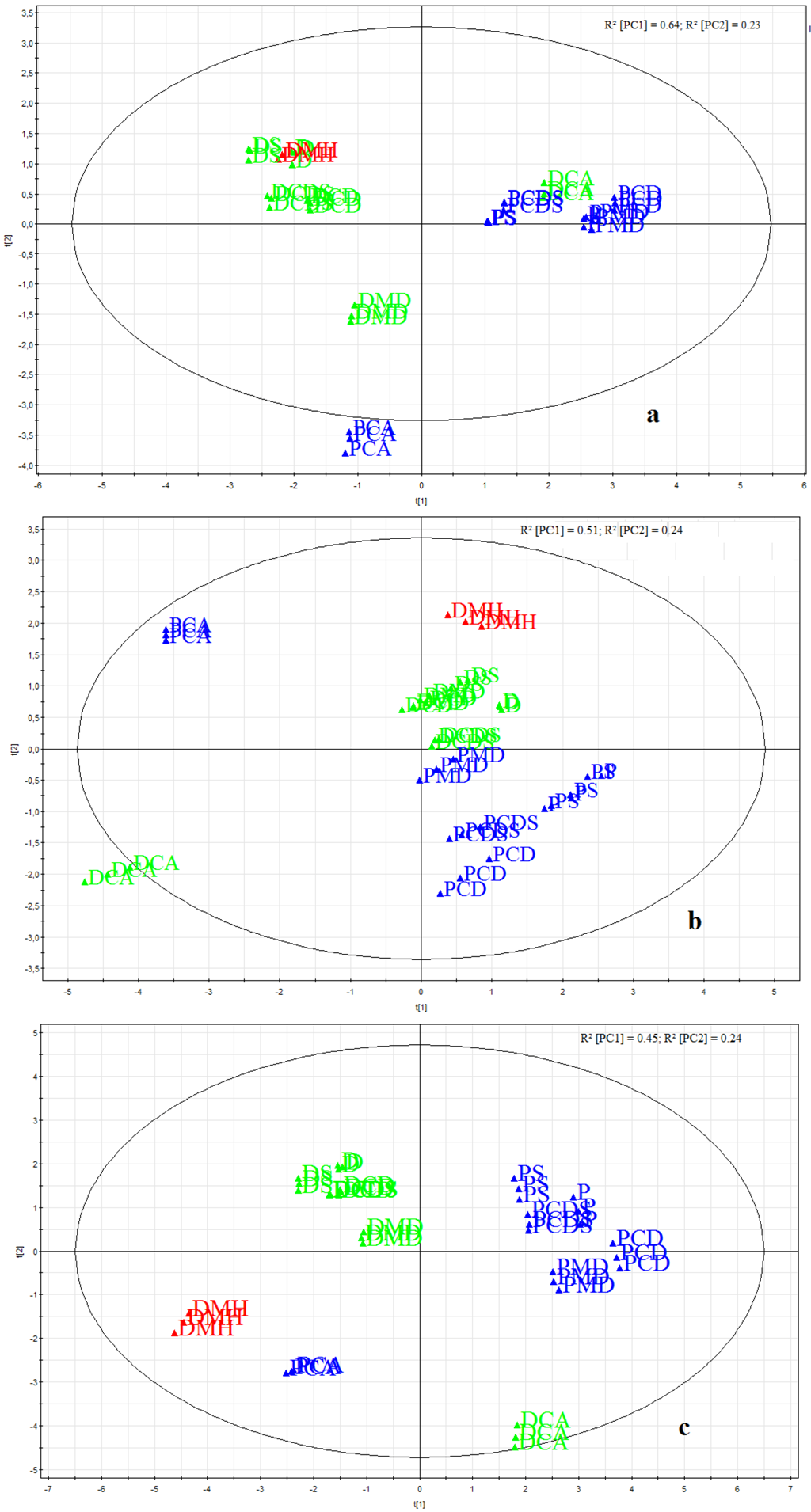

4. Conclusions
Acknowledgments
Conflict of Interest
References
- Hoffmann, E.M.; Breitenbach, A.; Breitkreutz, J. Advances in orodispersible films for drug delivery. Expet. Opin. Drug. Deliv. 2011, 8, 299–316. [Google Scholar] [CrossRef]
- European Pharmacopoeia Commission, Oromucosal Preparations. In European Pharmacopoeia 7.4; European Directorate for the Quality of Medicines (EDQM): Strasbourg, France, 2012; pp. 4257–4259.
- Watcha, M.F.; White, P.F. Postoperative nausea and vomiting: Its etiology, treatment, and prevention. Anesthesiology 1992, 77, 162–184. [Google Scholar] [CrossRef]
- Astellas Pharma GmbH, Vomex® A Sirup. Astellas Pharma: Berlin, Germany, 2011.
- World Health Orgnization (WHO) Report of the Informal Expert Meeting on Dosage Forms of Medicines for Children. 2008. Available online: http://www.who.int/selection_medicines/committees/expert/17/application/paediatric/Dosage_form_reportDEC2008.pdf (accessed on 16 December 2008).
- Loftsson, T.; Brewster, M.E. Pharmaceutical applications of cyclodextrins: Basic science and product development. J. Pharm. Pharmacol. 2010, 62, 1607–1621. [Google Scholar]
- Ayenew, Z.; Puri, V.; Kumar, L.; Bansal, A.K. Trends in pharmaceutical taste masking technologies: A patent review. Recent Pat. Drug Deliv. Formul. 2009, 3, 26–39. [Google Scholar] [CrossRef]
- European Pharmacopoeia Commission, Tablets. In European Pharmacopoeia 7.0; European Directorate for the Quality of Medicines (EDQM): Strasbourg, France, 2008; pp. 736–738.
- Siqueira, W.L.; Nicolau, J. Stimulated whole saliva components in children with Down syndrome. Spec. Care Dentist. 2002, 22, 226–230. [Google Scholar] [CrossRef]
- European Pharmacopoeia Commission, Uniformity of Dosage Forms (2.9.40.). In European Pharmacopoeia 7.4; European Directorate for the Quality of Medicines (EDQM): Strasbourg, France, 2012; pp. 4101–4103.
- Garsuch, V.; Breitkreutz, J. Novel analytical methods for the characterization of oral wafers. Eur. J. Pharm. Biopharm. 2009, 73, 195–201. [Google Scholar] [CrossRef]
- Garsuch, V.; Breitkreutz, J. Comparative investigations on different polymers for the preparation of fast-dissolving oral films. J. Pharm. Pharmacol 2010, 62, 539–545. [Google Scholar]
- Woertz, K.; Tissen, C.; Kleinebudde, P.; Breitkreutz, J. A comparative study on two electronic tongues for pharmaceutical formulation development. J. Pharmaceut. Biomed. Anal. 2011, 55, 272–281. [Google Scholar] [CrossRef]
- Pein, M.; Eckert, C.; Preis, M.; Breitkreutz, J. Taste sensing system αAstree as analytical tool—Performance Qualification using caffeine citrate as model substance. In Proceedings of the 8th Pharmaceutics & Biopharmaceutics World Meeting, Istanbul, Turkey, 2012.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Preis, M.; Pein, M.; Breitkreutz, J. Development of a Taste-Masked Orodispersible Film Containing Dimenhydrinate. Pharmaceutics 2012, 4, 551-562. https://doi.org/10.3390/pharmaceutics4040551
Preis M, Pein M, Breitkreutz J. Development of a Taste-Masked Orodispersible Film Containing Dimenhydrinate. Pharmaceutics. 2012; 4(4):551-562. https://doi.org/10.3390/pharmaceutics4040551
Chicago/Turabian StylePreis, Maren, Miriam Pein, and Jörg Breitkreutz. 2012. "Development of a Taste-Masked Orodispersible Film Containing Dimenhydrinate" Pharmaceutics 4, no. 4: 551-562. https://doi.org/10.3390/pharmaceutics4040551




