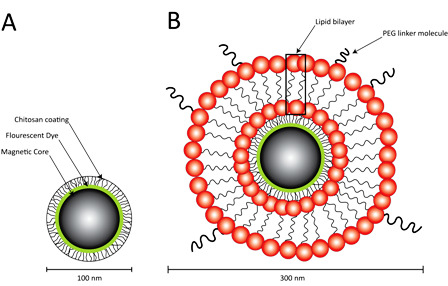
Development of a Novel Lipophilic, Magnetic Nanoparticle for in Vivo Drug Delivery
Abstract
:Abbreviations
| chi-MNPs | chitosan-coated magnetic nanoparticle |
| DDAB | dimethyldioctadecylammonium bromide |
| DSPE-PEG2000-MAL | 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[maleimide (polyethylene glycol)-2000] |
| DLS | dynamic light scattering |
| MNP | magnetic nanoparticle |
| pDNA | plasmid pHcRed1-C1 |
| RBE4 | rat brain endothelial 4 |
| RES | reticuloendothelial system |
| Soy PC | l-α-phosphatidylcholine |
1. Introduction
2. Materials and Methods
2.1. Preparation of Lipid-Encapsulated Magnetic Nanoparticles
2.2. Size and the ζ-Potential Particle Characterization
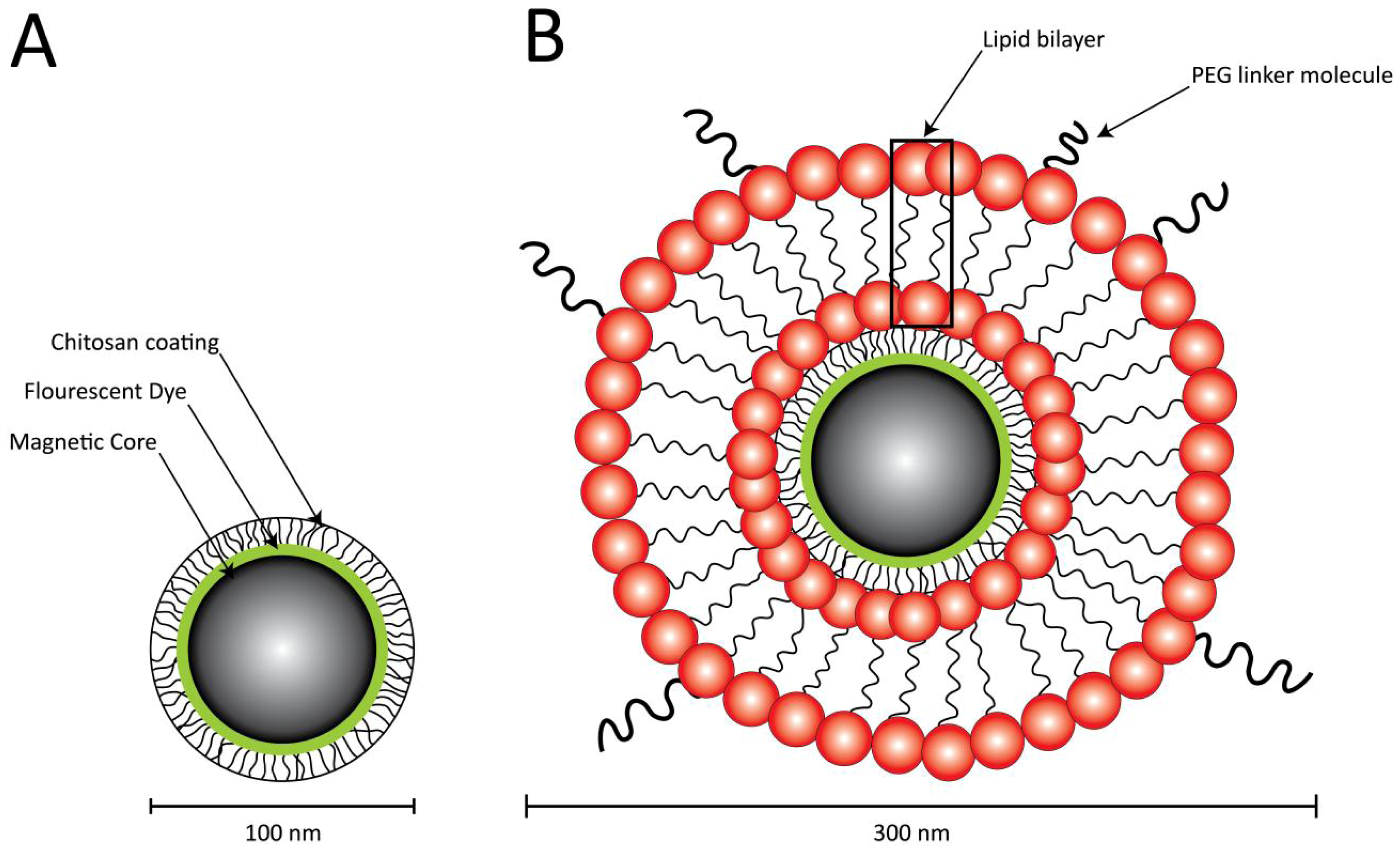
2.3. Assessment of DNA Binding Capacity
2.4. In Vitro Transfection Studies
2.4.1. Cell Culture
2.4.2. Preparation of Plasmid
2.4.3. Magnetofection
2.4.4. In Vivo Distribution
3. Results
3.1. Particle Characterization
3.2. Assessment of DNA Binding Capacity

3.3. In Vitro Transfection Studies
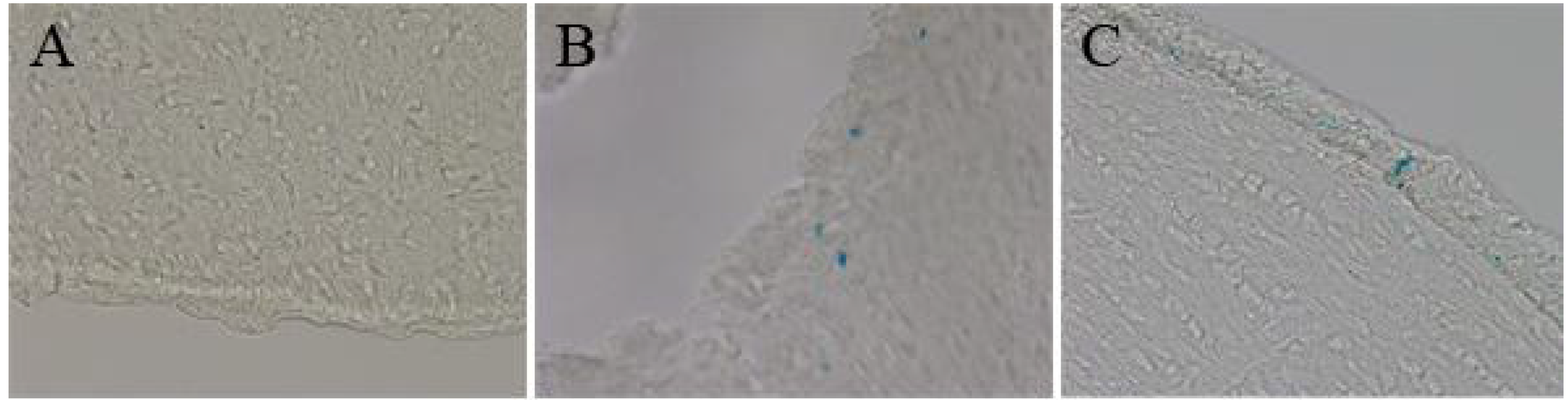
3.4. Distribution Experiments
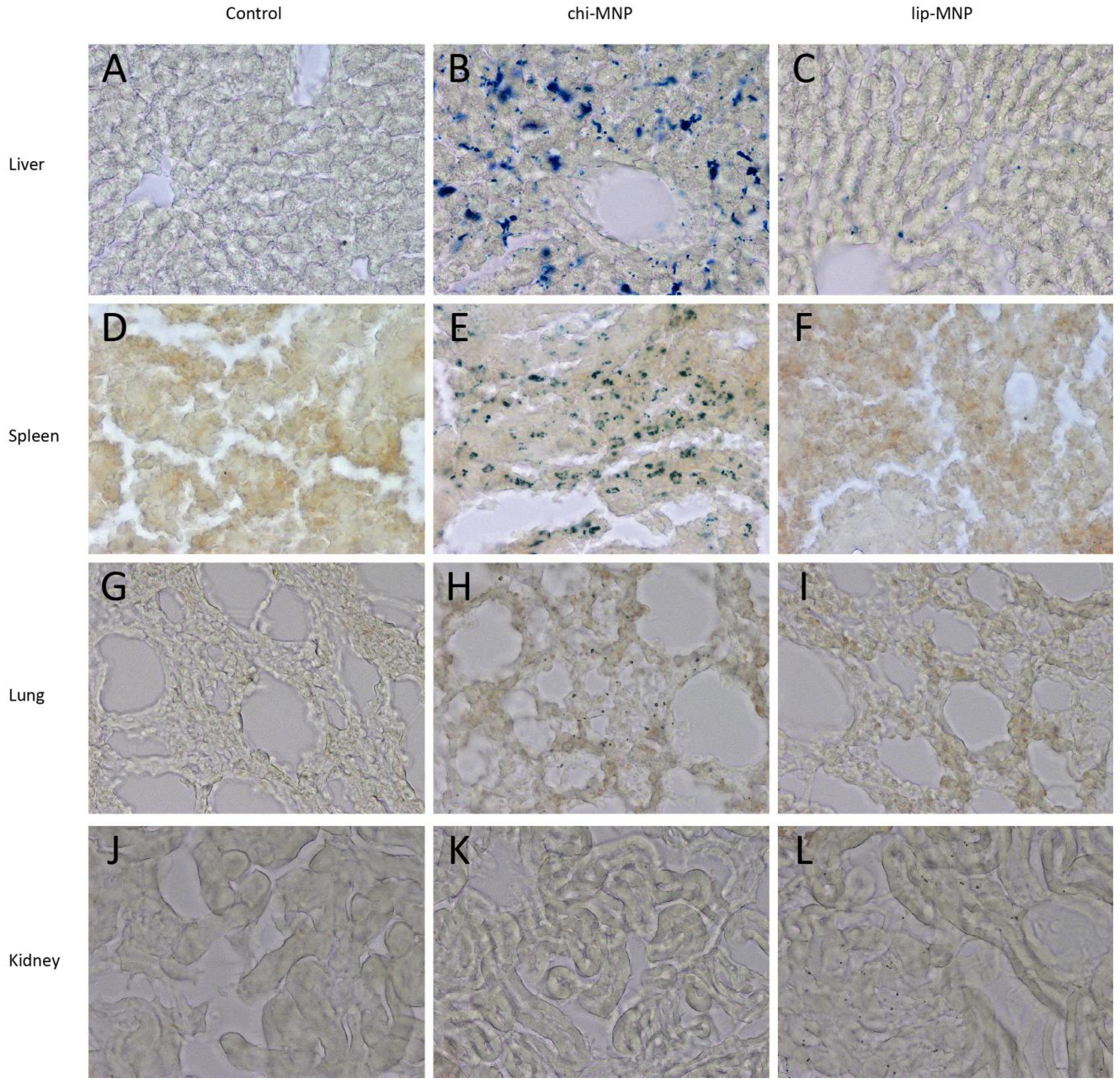
| Organ | chi-MNP | Lip-MNP |
|---|---|---|
| Liver | +++ | + |
| Spleen | +++ | 0 |
| Lungs | + | 0 |
| Kidneys | 0 | + |
| Brain | + | + |
4. Discussion
4.1. In Vitro Magnetofection
4.2. In Vivo Distribution of Magnetic Nanoparticles
4.3. Targeted Therapy Using Magnetic Nanoparticles
5. Conclusions
Acknowledgements
References
- Alexiou, C.; Jurgons, R.; Seliger, C.; Iro, H. Medical applications of magnetic nanoparticles. J. Nanosci. Nanotechnol. 2006, 6, 2762–2768. [Google Scholar] [CrossRef]
- Ang, D.; Nguyen, Q.V.; Kayal, S.; Preiser, P.R.; Rawat, R.S.; Ramanujan, R.V. Insights into the mechanism of magnetic particle assisted gene delivery. Acta Biomat. 2011, 7, 1319–1326. [Google Scholar] [CrossRef]
- Chertok, B.; Moffat, B.A.; David, A.E.; Yu, F.; Bergemann, C.; Ross, B.D.; Yang, V.C. Iron oxide nanoparticles as a drug delivery vehicle for MRI monitored magnetic targeting of brain tumors. Biomaterials 2008, 29, 487–496. [Google Scholar] [CrossRef]
- Chertok, B.; David, A.E.; Moffat, B.A.; Yang, V.C. Substantiating in vivo magnetic brain tumor targeting of cationic iron oxide nanocarriers via adsorptive surface masking. Biomaterials 2009, 30, 6780–6787. [Google Scholar] [CrossRef]
- Thomsen, L.B.; Lichota, J.; Eskehave, T.N.; Linemann, T.; Mortensen, J.H.; du Jardin, K.G.; Moos, T. Brain delivery systems via mechanism independent of receptor-mediated endocytosis and adsorptive-mediated endocytosis. Curr. Pharm. Biotech. 2012, 13, 2349–2354. [Google Scholar] [CrossRef]
- Carrion, C.; Domingo, J.C.; de Madariaga, M.A. Preparation of long-circulating immunoliposomes using PEG-cholesterol conjugates: Effect of the spacer arm between PEG and cholesterol on liposomal characteristics. Chem. Phys. Lipids 2001, 113, 97–110. [Google Scholar] [CrossRef]
- Chonn, A.; Semple, S.C.; Cullis, P.R. Association of blood proteins with large unilamellar liposomes in vivo. Relation to circulation lifetimes. J. Biol. Chem. 1992, 267, 18759–18765. [Google Scholar]
- Thomsen, L.B.; Lichota, J.; Kim, K.S.; Moos, T. Gene delivery by pullulan derivatives in brain capillary endothelial cells for protein secretion. J. Ctrl. Rel. 2011, 151, 45–50. [Google Scholar] [CrossRef]
- Kievit, F.M.; Veiseh, O.; Bhattarai, N.; Fang, C.; Gunn, J.W.; Lee, D.; Ellenbogen, R.G.; Olson, J.M.; Zhang, M. PEI-PEG-Chitosan Copolymer Coated Iron Oxide Nanoparticles for Safe Gene Delivery: Synthesis, complexation, and transfection. Adv. Funct. Mater. 2009, 19, 2244–2251. [Google Scholar] [CrossRef]
- Pan, X.; Guan, J.; Yoo, J.W.; Epstein, A.J.; Lee, L.J.; Lee, R.J. Cationic lipid-coated magnetic nanoparticles associated with transferrin for gene delivery. Int. J. Pharm. 2008, 358, 263–270. [Google Scholar] [CrossRef]
- Moos, T. Developmental profile of non-heme iron distribution in the rat brain during ontogenesis. Dev. Brain Res. 1995, 87, 203–213. [Google Scholar] [CrossRef]
- Hartig, S.M.; Greene, R.R.; Dasgupta, J.; Carlesso, G.; Dikov, M.M.; Prokop, A.; Davidson, J.M. Multifunctional nanoparticulate polyelectrolyte complexes. Pharm. Res. 2007, 24, 2353–2369. [Google Scholar] [CrossRef]
- Senyei, A.; Widder, K.; Czerlinski, G. Magnetic Guidance of drug-carrying microspheres. J. Appl. Phys. 1978, 49, 3578–3584. [Google Scholar] [CrossRef]
- Prow, T.; Smith, J.N.; Grebe, R.; Salazar, J.H.; Wang, N.; Kotov, N.; Lutty, G.; Leary, J. Construction, gene delivery, and expression of DNA tethered nanoparticles. Mol. Vis. 2006, 12, 606–615. [Google Scholar]
- Pedroso De Lima, M.C.; Simões, S.; Pires, P.; Faneca, H.; Düzgünes, N. Cationic lipid-DNA complexes in gene delivery: From biophysics to biological applications. Adv. Drug Deliv. Rev. 2001, 47, 277–294. [Google Scholar] [CrossRef]
- Mykhaylyk, O.; Antequera, Y.S.; Vlaskou, D.; Plank, C. Generation of magnetic nonviral gene transfer agents and magnetofection in vitro. Nat. Protoc. 2007, 2, 2391–2411. [Google Scholar] [CrossRef]
- Li, S.D.; Huang, L. Nanoparticles evading the reticuloendothelial system: Role of the supported bilayer. Biochim. Biophys. Acta 2009, 1788, 2259–2266. [Google Scholar] [CrossRef]
- Alam, M.I.; Beg, S.; Samad, A.; Baboota, S.; Kohli, K.; Ali, J.; Ahuja, A.; Akbar, M. Strategy for effective brain drug delivery. Eur. J. Pharm. Sci. 2010, 40, 385–403. [Google Scholar]
- Gosk, S.; Vermehren, C.; Storm, G.; Moos, T. Targeting anti-transferrin receptor antibody (OX26) and OX26-conjugated liposomes to brain capillary endothelial cells using in situ perfusion. J. Cereb. Blood Flow Metab. 2004, 24, 1193–1204. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Linemann, T.; Thomsen, L.B.; Jardin, K.G.d.; Laursen, J.C.; Jensen, J.B.; Lichota, J.; Moos, T. Development of a Novel Lipophilic, Magnetic Nanoparticle for in Vivo Drug Delivery. Pharmaceutics 2013, 5, 246-260. https://doi.org/10.3390/pharmaceutics5020246
Linemann T, Thomsen LB, Jardin KGd, Laursen JC, Jensen JB, Lichota J, Moos T. Development of a Novel Lipophilic, Magnetic Nanoparticle for in Vivo Drug Delivery. Pharmaceutics. 2013; 5(2):246-260. https://doi.org/10.3390/pharmaceutics5020246
Chicago/Turabian StyleLinemann, Thomas, Louiza B. Thomsen, Kristian G. du Jardin, Jens C. Laursen, Jesper B. Jensen, Jacek Lichota, and Torben Moos. 2013. "Development of a Novel Lipophilic, Magnetic Nanoparticle for in Vivo Drug Delivery" Pharmaceutics 5, no. 2: 246-260. https://doi.org/10.3390/pharmaceutics5020246