1. Introduction
Microbicides are being developed as a pre-exposure prophylaxis of human immunodeficiency virus (HIV) and other sexually transmitted infections (STIs) [
1,
2,
3]. A number of microbicide candidates are currently in clinical trials but a clinical impact is yet to be realized, as several promising candidates have shown mixed results. For example, 1% tenofovir (TFV) gel is being investigated in several clinical trials, of which some have shown efficacy [
1], while others not [
4]. Participant adherence to the study protocol appears to be a major factor governing the efficacy of microbicide candidates in clinical trials [
5]. This is reflected in the CAPRISA 004 trial results, where women who used a TFV gel in more than 80% of their sex acts showed a 54% reduction in HIV infections, whereas women who used the gel in less than half of their sex acts had only a 28% reduction [
1]. Subsequently, the VOICE trial studying the efficacy of a TFV gel had the gel study arm discontinued due to lack of efficacy [
4]. In a detailed evaluation of patient compliance, only 36% of women assessed had actually used the gel within the week, despite 90% reporting to do so [
6]. These data suggest user adherence is a critical factor governing microbicide success, and formulations, while biologically efficacious, are effective only if used as directed. Accordingly, a number of methods are being investigated to track and/or improve adherence to microbicide use during clinical trials including daily reporting using interactive voice response systems [
7,
8] and imaging of used applicators [
9].
Varied studies have been conducted to understand product attributes that influence acceptability and thus adherence [
10,
11]. Several studies have explored how physical attributes of products and rheological properties can influence women’s perceived efficacy [
12,
13], which, in turn affects acceptability. These product attributes include appearance [
14,
15], smell [
14,
15], taste, and textural properties that may affect sexual pleasure (how the product feels during intercourse) [
11,
12] and leakage (the propensity of the product to seep out of the body) [
10,
12,
14,
16], as well as vaginal coating [
12]. Our group has previously conducted focus groups to assess how physical properties such as size, shape and firmness of suppositories developed in the lab influence women’s perception of efficacy and willingness to try [
15]. During our study, in spite of being instructed that different sizes would contain the same amount of medication, bigger sizes were perceived to be more effective, especially against HIV [
15]. One of the primary drivers for selection of a particular size, shape or firmness was the perceived ease of insertion, which also influenced their willingness to try the product [
15]. Li
et al. [
17] showed a high correlation between the anticipated
ease-of-insertion and
willingness-to-try ratings, suggesting physical characteristics of the drug carrier are an equally important consideration in the design of effective microbicides.
To address different needs and preferences of women, microbicides are being developed in different physical forms: Tablets [
18], quick dissolving films [
19,
20], gels [
21,
22] and removable rings [
23,
24]. These choices are rheologically diverse and include products that dissolve within the body, eventually being discharged, as well as products that need to be removed and replaced periodically. Notably, many so-called “gels” in the literature are not gels in a technical sense, as they are highly viscous non-Newtonian liquids, not solids. Recently, we have developed microbicide prototypes that fall between the two extremes of tablets and viscous liquid “gels” to improve upon negative attributes that have been reported with these existing technologies. For example, leakage has been cited as one of the drawbacks of liquid “gels” [
25], while the dissolution of tablets creates a hypertonic solution. Ideally, our prototypes would disintegrate inside the body and be eliminated with vaginal mucus secretions. Leveraging soft-gel technology, our delivery system consists of semisoft vaginal suppositories prepared from carrageenan, which has several advantages over gelatin, the traditional soft-gel matrix. Use of carrageenan helps circumvent negative aspects of gelatin, such as the risk of zoonotic infections, the lack of acceptability by vegetarians and the insufficient heat stability for storage in tropical climates. Carrageenan also has a distinct advantage because of its reported anti-viral activity against viruses other than HIV [
26,
27,
28]. We have previously conducted acceptability studies with gel suppository prototypes and identified physical attributes (size, shape and firmness) and user preferences (frequency of application and duration of protection) most favorable to women [
15,
17,
29]. In this iterative design process to identify physical characteristics that influence perceived
ease-of-insertion and
willingness-to-try, we have developed products across a range of firmness, size and shape. The firmness of the prototype gels used to prepare the suppositories was characterized by the storage modulus (G’) of the gels at 25 °C and ranged from 250 to 25,000 Pa. The size of the products varied from 1 to 5 mL and shapes included both standard (long oval, tampon) and non-standard (spherical) shapes for suppositories. Using this approach, we have identified preferred shapes, size and firmness for the microbicide prototypes using mixed methods (e.g., qualitative focus groups and large scale quantitative
ex vivo consumer acceptability trials [
1,
15,
17,
29]).
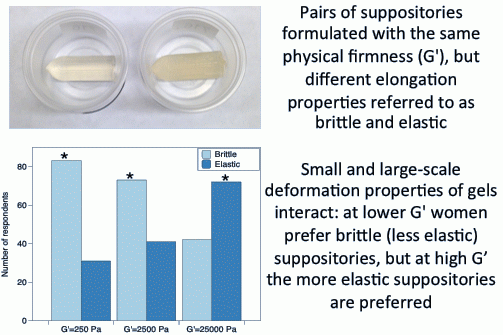
In our prior work, suppositories, especially those at the lower firmness (G’), would often break while being manipulated in the hand, causing women to consider them difficult to insert. To overcome this, we reformulated the suppositories so they could be deformed to a greater extent before fracturing. The current study was designed to investigate whether suppositories with the same physical firmness (defined in terms of storage modulus, G’) but with different elongation properties influence women’s imagined ease-of-insertion and thus firmness preferences.
2. Experimental Section
2.1. Materials
Commercial samples of κ-carrageenan (Gelcarin NF 911, batch number: 10,707,011) and ι-carrageenan (Gelcarin NF 379, batch number: 10,514,011 were kindly provided by FMC Biopolymers (Philadelphia, PA, USA). Tenofovir (TFV) was kindly provided by Gilead Sciences (Foster City, CA, USA). All other reagents were purchased from VWR International (Bridgeport, NJ, USA) and used as received.
2.2. Participant Recruitment
Women (Test 1:
n = 114 and Test 2:
n = 120) were recruited as described elsewhere [
17,
29] to evaluate prototypes
ex vivo (in their hands) at the Sensory Evaluation Center at Penn State. Inclusion criteria included: (a) female; (b) between 18 and 55 years of age; (c) reported having had vaginal sex with a man in the last 12 months; (d) were willing to manipulate prototypes with their hands and evaluate them using a computer-guided assessment in an isolated test booth. All procedures were approved by the Pennsylvania State University Institutional Review Board (protocol #36943, approved 25 April 2013). Participants provided informed consent, and were reimbursed for their time.
2.3. Sample Design and Preparation
Based on previous acceptability studies, 4 firmness levels ranging in G’ from 250 to 25,000 Pa (25 °C) were selected [
15]. To prepare gels of constant firmness with different elongation properties, the types and amounts of ι- and κ-carrageenan were varied, as was the amount of potassium chloride (
Table 1). Gels were prepared by mixing the dry ingredients (carrageenan and KCl) in deionized water and holding the mixture at 85 °C for at least 1 h. The gel was stirred intermittently till it formed a homogenous dispersion. Prior to preparing the suppositories, the gel was characterized in a rheometer to confirm the storage modulus closely (within 10%) matched the desired value (
Table 1) at 25 °C. Small deformation rheological measurements were performed at a frequency of 1 Hz and strain of 1% on a strain-controlled oscillatory rheometer (ARES, TA Instrument, New Castle, DE, USA) using a cone and plate geometry (probe diameter = 25 mm, cone angle = 5.73°). The gel was loaded between the plates when hot (80 °C) and the edges sealed with a light coating of mineral oil to prevent moisture evaporation. Data were recorded first on cooling to 15 °C, followed by heating to 60 °C at a rate of 5 °C per minute. Bullet shaped suppositories (
Figure 1) were prepared by filling plastic syringes with the hot gel and injecting it into acrylonitrile butadiene styrene molds, followed by cooling in a refrigerator (4 °C) for 15 min and holding at room temperature for at least 2 h in sealed glass vessels to allow the gels to set [
17,
29]. Samples were presented in 0.75 oz Solo transparent plastic cups (Solo Cup Company, Urbana, IL, USA), kept at 25 °C with the lids sealed tightly until evaluated.
Table 1.
Composition of brittle and elastic gels used to prepare suppositories.
Table 1.
Composition of brittle and elastic gels used to prepare suppositories.
| Nominal Storage Modulus (G’) (Pa) | Brittle Formula | Elastic Formula |
|---|
| κ (% w/v) | ι (% w/v) | KCl (M) | κ (% w/v) | ι (% w/v) | KCl (M) |
|---|
| 250 | 0.5 | 0 | 0.025 | 0 | 2 | 0.05 |
| 2,500 | 1.25 | 0 | 0.04 | 0.5 | 3 | 0.05 |
| 12,500 | 2 | 0 | 0.05 | 1.25 | 3 | 0.05 |
| 12,500 with TFV | 2.5 | 0 | 0.05 | 2 | 4 | 0.05 |
| 25,000 | 2.5 | 0 | 0.05 | 2 | 3 | 0.05 |
Figure 1.
Bullet-shaped suppositories of size 3 mL.
Figure 1.
Bullet-shaped suppositories of size 3 mL.
κ-Carrageenan gels are relatively brittle vis-à-vis those containing ι-carrageenan. For convenience, we refer to the samples as either “brittle” or “elastic” in the remainder of the manuscript, but these names were never used with participants, who only referred to products using random 3-digit blinding codes.
2.4. Sample Evaluation
Participants were asked to watch a 90 s video in which a medical professional demonstrates how a participant should evaluate a prototype in her hand [
17,
29]. Participants were instructed to: (1) Take the sample and put it into her non-dominant hand; (2) Gently stroke the sample with the index finger of her dominant hand; (3) Put the sample between her fingers and pinch gently (hand not specified verbally; shown as dominant hand in video); (4) Finally hold the sample between her fingers and imagine she was trying to insert the sample into her vagina (hand not specified). After watching the video, participants were provided with a consent form to read. Women who wished to participate were assigned individual ID codes and sent to the testing area.
2.4.1. Sample Evaluation for Test 1
Participants were presented with three pairs of samples: Each pair consisting of the brittle and elastic suppositories of the same storage modulus (G’ = 250, 2500 or 25,000 Pa). For each pair of brittle and elastic suppositories, participants were asked to select their preferred sample in a forced-choice task; they were also provided with an open-ended comment box to provide reasons for their selection if they choose to.
2.4.2. Sample Evaluation for Test 2
Based on the results of Test 1 (see below), the preferred samples were selected and compared to an additional intermediate firmness level with storage modulus G’ = 12,500 Pa, so a total of four suppositories were evaluated. Participants rated their imagined ease-of-insertion and willingness-to-try on separate 100-point visual analog scales for each of the suppositories. After the individual ratings were obtained, women were asked to rank the four suppositories in order from most to least preferred.
In both tests, participants provided self-report of their age, race, ethnicity, education, marital status, number of vaginal deliveries and vaginal product usage after product evaluation was complete. The majority were married, college-educated white women. Data on prior experience with other vaginal products, collected using a check all that apply question, is summarized in
Table 2.
Table 2.
Prior vaginal product usage from a check that all apply question (column totals may exceed the number of participants).
Table 2.
Prior vaginal product usage from a check that all apply question (column totals may exceed the number of participants).
| Vaginal Products | Test 1 (n = 114) | Test 2 (n = 120) |
|---|
| Vaginal contraceptive products such as Nuvaring | 5 | 4 |
| Spermicidal gels and films | 3 | 5 |
| Yeast infection medicines such as Vagisil and Monistat | 26 | 27 |
| Douche | 5 | 7 |
| Menstruation products such as tampons | 71 | 85 |
| Lubrication products such as KY gels, liquibeads and Vitamin-E suppositories | 39 | 40 |
| Decline to answer | 13 | 13 |
2.5. Mechanical Characterization of Elongation Properties of the Gels
To characterize the large-deformation rheological properties of brittle and elastic gels, the gels were molded into oval rings (outer length of oval = 5.90 cm, center-to-center length = 2.48 cm, radius of outer arc = 1.71 cm, width of gel leg = 1.03 cm, depth of gel = 1.0 cm) and stretched in tension at 5 mm/s on two 1.4 cm aluminum dowel pins (
Figure S1A) attached to a TAXT2i Texture Analyzer (Stable Micro Systems, Halsemere, Surrey, UK) [
30]. Eight to ten measurements of tensile force and deformation at fracture were made for each gel type and firmness. Tensile force and deformation was also compared with gels prepared with and without TFV to study the effect of TFV addition on the elongation properties. Gels with TFV were prepared with additional amounts of carrageenan as compared to gels without TFV to obtain the same G’ value (12,500 Pa) (
Table 1). Dowel pins and the contacting surfaces of the ring were lightly coated with mineral oil to reduce friction. The force and deformation at fracture were measured to calculate the stress at fracture (σ) and strain at fracture (ε) using the equations:
and
where: σ is the true stress at fracture (g/m
2);
F is the force at fracture (g);
A0 the initial cross sectional area (m
2); Δ
L the change in leg length (m);
Ca the average circumference (m); and ϵ the Hencky or true strain at fracture.
As fracture behavior might be altered by exposure to vaginal fluids, we quantified the compression force required to fracture spherical suppositories (size 3 mL) that had been soaked in 5 mL vaginal simulant fluid (VSF) at 37 °C with constant shaking. Compression force was measured at 0, 2, 6 and 24 h using parallel plates and the TAXT2i Texture Analyzer (
Figure S1B). Compression force was also compared between gels prepared with and without TFV to study the fracture behavior of the suppositories in the presence of TFV. The vaginal simulant fluid was prepared as described by Owen and Katz [
31].
2.6. Characterization of Drug Release
The rate of release of the antiretroviral drug tenofovir (TFV) was determined for spherical suppositories made using the brittle and elastic gel formulations. Gels with G’ = 12,500 Pa were selected for the drug release studies, as this firmness was the most favored in Test 2 (results below). Based on the dose of TFV employed in clinical trials with 4 mL of 1% TFV gel [
1], each suppository was prepared as previously described [
17], with the addition of 40 mg of TFV. The acidic pH of TFV in water (pH = 3–4) disrupted carrageenan gel formation, as the pK
a of carrageenan is 4.9. Therefore, the required concentration of TFV was dissolved in deionized water and the pH of the TFV solution was adjusted to 7.2–7.4 by addition of sodium hydroxide. Gels were then prepared as described above and characterized in a rheometer (see above) to confirm the storage modulus closely (within 10%) matched the desired value of G’ = 12,500 at 25 °C. Spherical suppositories were prepared by filling plastic syringes with the hot gel and injecting into molds, followed by cooling and storing as described above. Dissolution studies were performed (a) using USP apparatus 2 (basket apparatus) with 100 mL glass vessels or (b) in a shaking incubator with 50 mL screw-cap test tubes. Studies were run at 150 rpm, 37 °C with 80 mL VSF for the basket apparatus and 5 mL VSF for the shaking incubator. Dissolution studies with 5 mL VSF were designed to better mimic the vaginal environment, as the average volume of vaginal fluid obtained from healthy donors has been shown to vary in the range of 0.5–8 mL/day [
32,
33,
34].
2.7. Statistical Analyses
Data were analyzed using JMP v9.0.2 (Cary, NC, USA). For Test 1, preference for brittle and elastic suppository was compared using a binomial test with a p value less than 0.05 considered significant. The effect of gel type and storage modulus on the imagined ease-of-insertion and willingness-to-try was tested via ANOVA, with participant as a random effect and suppository type as a fixed effect. Tukey’s Honest Significant Difference (HSD) was used for post hoc comparisons with p < 0.05 considered significantly different. Similarly, the effect of suppository type and storage modulus on gel compression and elongation was tested via ANOVA. The least square means (L.S.M.) were then compared across different suppository types or across different G’ values separately using Tukey’s HSD test with p < 0.05 considered significantly different. For the TFV release data in water VSF mean and standard error were calculated (n = 7) for each time point. For comparing the initial rates of diffusion (0–2 h) across different VSF volumes and suppository types, the slopes for individual samples were computed from the release data. The least square means (L.S.M.) of slopes were calculated and compared using Tukey’s HSD test with p < 0.05 considered significantly different.
4. Discussion
Microbicides are an active area of research in the field of HIV prevention; they have the particular advantage of being a woman-controlled method of prevention [
2]. While shown to be biologically efficacious, a number of the microbicides have failed to demonstrate efficacy in clinical trials, largely due to poor compliance by study participants [
4,
6]. Compliance may depend on women’s perception of efficacy as well as product acceptability hence, research efforts are being directed to determine product features affecting women’s perception and liking of the microbicide candidates [
12,
13,
15,
17,
29]. We have developed carrageenan-based soft-gel suppositories with varying physical characteristics to determine favorable product characteristics that drive acceptability and willingness to try. In our previous development efforts, the different design parameters that we varied in our prototypes are firmness (5 levels), size (3 levels) and shape (6 distinct shapes) [
15]. In this iterative design process, we developed two types of carrageenan gels with the same storage modulus but different elongation properties to determine if the extensibility plays a role in women’s preference and willingness to try.
Previous optimization efforts indicated that ease of insertion is a critical factor governing women’s willingness to try this product [
15,
17], and that the rheological properties, both small and large deformation, may play a role in the imagined ease of handling and insertion.
The current study investigated the role of large deformation properties on preference, willingness to try, and imagined ease of insertion. Our initial hypothesis was that women would prefer the elastic samples to brittle samples in the lower firmness range, as they would not break as easily during handling and insertion as the brittle ones. However, women preferred the brittle samples at G’ = 250 and 2500 Pa and preferred the elastic samples at G’ = 25,000 Pa. Contrary to our initial hypothesis, the elastic samples were perceived to be softer, flimsy, easily breakable and difficult to insert at lower firmness. Based on the results of the physical compression test, the elastic suppositories with G’ = 2500 Pa could withstand greater deformation prior to fracturing however the force required to fracture them prior to VSF contact was significantly lower than brittle samples. Thus the brittle samples were perceived to be sturdier, which may explain why women preferred brittle samples to elastic ones at the lower firmness. Focus group discussions conducted previously established that imagined comfort upon insertion plays a role in women’s preferences, along with imagined ease of insertion [
15]. Brittle samples with G’ = 25,000 Pa were preferred in previous work. However when offered the choice here, women preferred the elastic sample at G’ = 25,000 Pa as it retained the desirable quality of being easy to insert and was also perceived soft enough to be comfortable once inserted. Additionally data from focus groups also indicated that color plays a role in women’s preferences, especially since the color of the suppository corresponds to the appearance of the vaginal discharge [
15]. The brittle suppositories were slightly clearer in appearance than the elastic ones and this may have influenced women’s preference for brittle suppositories. However with ι-carrageenan (required for elastic gels), higher firmness requires higher concentration and the color/opacity differences although small may be unavoidable. The storage modulus value (G’) has been used for quantifying firmness of the prototypes developed in the laboratory and previous development efforts did not include elongation properties as a design parameter. Present results provide support for combining the storage modulus with large deformation testing to fully characterize the gels during preclinical development.
In our second test, when an intermediate firmness level was evaluated alongside the preferred samples from Test 1, the most preferred firmness shifted from G’ = 25,000 Pa [
17,
29] to a brittle suppository with G’ = 12,500 Pa. Notably, preferences may be influenced by the other options presented within the choice set [
37]. The gels of varying firmness prepared previously [
17,
29] had varied ratios of κ- and ι-carrageenan; thus, firmness was partially confounded with elongation properties. Based on comments from focus group participants [
15], here we prepared gels with the same storage modulus yet different elongation properties to assess their effect on preference to refine our understanding of women’s preferences. The ranking data indicated that brittle suppositories with G’ = 12,500 Pa were most preferred; still all the samples except elastic at G’ = 12,500 Pa were highly rated for
ease-of-insertion and
willingness-to-try, suggesting brittle samples in the range G’ = 2500–12,500 Pa and the elastic samples with G’ = 25,000 Pa were well accepted.
Our design process simultaneously optimizes different physical characteristics believed to drive acceptability, and performance parameters such as drug release and residence time in the vagina. Critically, producing microbicides in a wide range of firmness that are all highly acceptable may allow for better optimization of the drug release profile. The release of TFV from the carrageenan matrix depends on various factors such as drug loading, density of the carrageenan gel, partition coefficient of the drug between water and carrageenan, and molecular size of the drug. Among these factors, the density of the gel depends on the total carrageenan concentration [
38], which greatly varies between brittle and elastic gels at the same firmness level. The elastic gels at G’ = 12,500 Pa have twice the total carrageenan concentration as compared to the brittle gels, thus slowing down the diffusion from the elastic gel suppositories. The initial rate of diffusion is higher in 80 mL VSF as compared to 5 mL VSF for both the brittle and elastic suppositories. The mass transport of TFV from the carrageenan matrix depends on the concentration of TFV in the saturated zone surrounding the suppository. The lower volume of VSF (5 mL) results in faster saturation in the surrounding medium as compared to 80 mL, thus slowing down TFV release. Due to insufficient sink condition with 5 mL VSF, TFV release plateaus at 24 h, and only replenishing with fresh VSF stimulates additional release. Since TFV prophylaxis works by diffusing into the vaginal walls, this will help maintain a concentration gradient across the suppository, vaginal lumen and vaginal tissue [
3]. The vaginal discharge in women is composed of several components such as vulvar secretions, cervical mucus, and endometrial fluid, and its amount varies based on several factors such as menstrual cycle, hormone levels, and sexual arousal [
39]. Due to physiologic processes, there is regular flushing of the fluids in the vagina, which may help maintain a concentration gradient that allows TFV diffusion into the vaginal lumen.
While characterizing the elastic properties of the gel, the strain required to fracture rings was lower for brittle gels as compared to elastic gels. However, in the case of brittle gels, the strain increased with increasing firmness whereas for elastic gels it progressively decreased with increasing firmness. This can be explained in part by the varying ratio of ι- to κ-carrageenan used to prepare elastic gels of increasing firmness. Elastic gels of G’ = 2500 Pa were prepared at an ι:κ ratio of 6:1 and ι is reported to form elastic gels [
40], hence it can withstand the highest fracture strain. Addition of increasing amounts of κ-carrageenan to prepare elastic gels with increasing firmness led to more brittle gels (though not as brittle as κ-carrageenan alone).
Upon contact with vaginal fluid, our suppositories are intended to break down and be eliminated with vaginal mucous secretions. To test the disintegration or matrix softening of carrageenan in the vaginal environment, suppositories were soaked in VSF for 24 h at body temperature with continuous stirring. The current prototypes do not completely disintegrate in contact with VSF. However, there is a significant decrease in the amount of force required to fracture ovules upon soaking in VSF for 2 h. Since this force does not continually decrease with time, efforts are on-going to reformulate gels to aid disintegration in the vagina.