Optimizing Prednisolone Loading into Distiller’s Dried Grain Kafirin Microparticles, and In vitro Release for Oral Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Kafirin Source and Extraction, and Formulating Kafirin Microparticles
2.2. Response Surface Methodology
2.3. Optimization Data Analysis and Validation of Optimization Model
2.4. Scanning Electron Microscopy
2.5. Particle Size Distribution
2.6. Stability Testing
2.7. In vitro Drug Release
2.7.1 Simulated Gastric and Small Intestinal Conditions
2.7.2. Simulated Colonic Conditions
Fecal Inoculum
Substrates and Medium
2.8. Statistical Analysis
3. Results
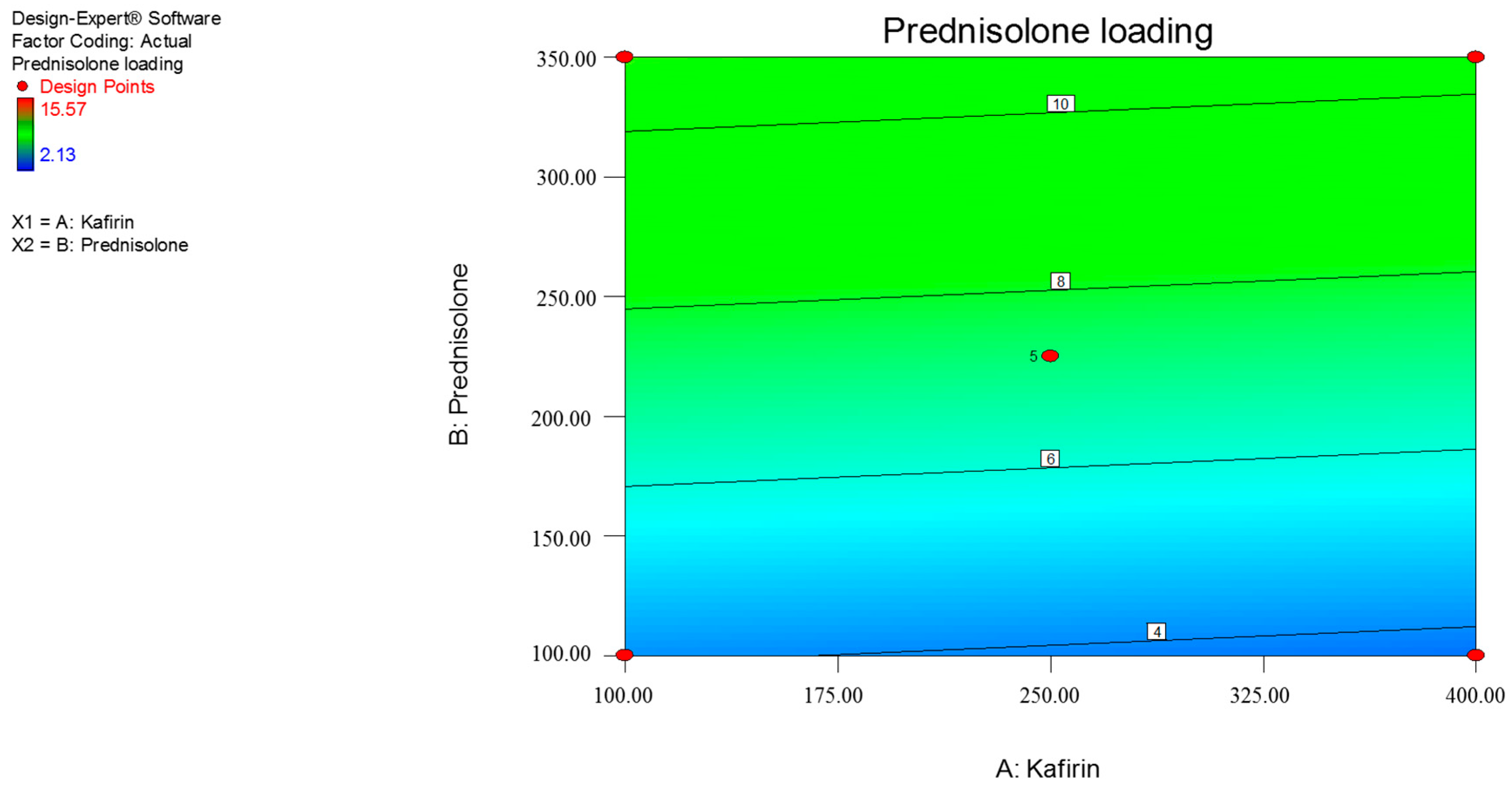
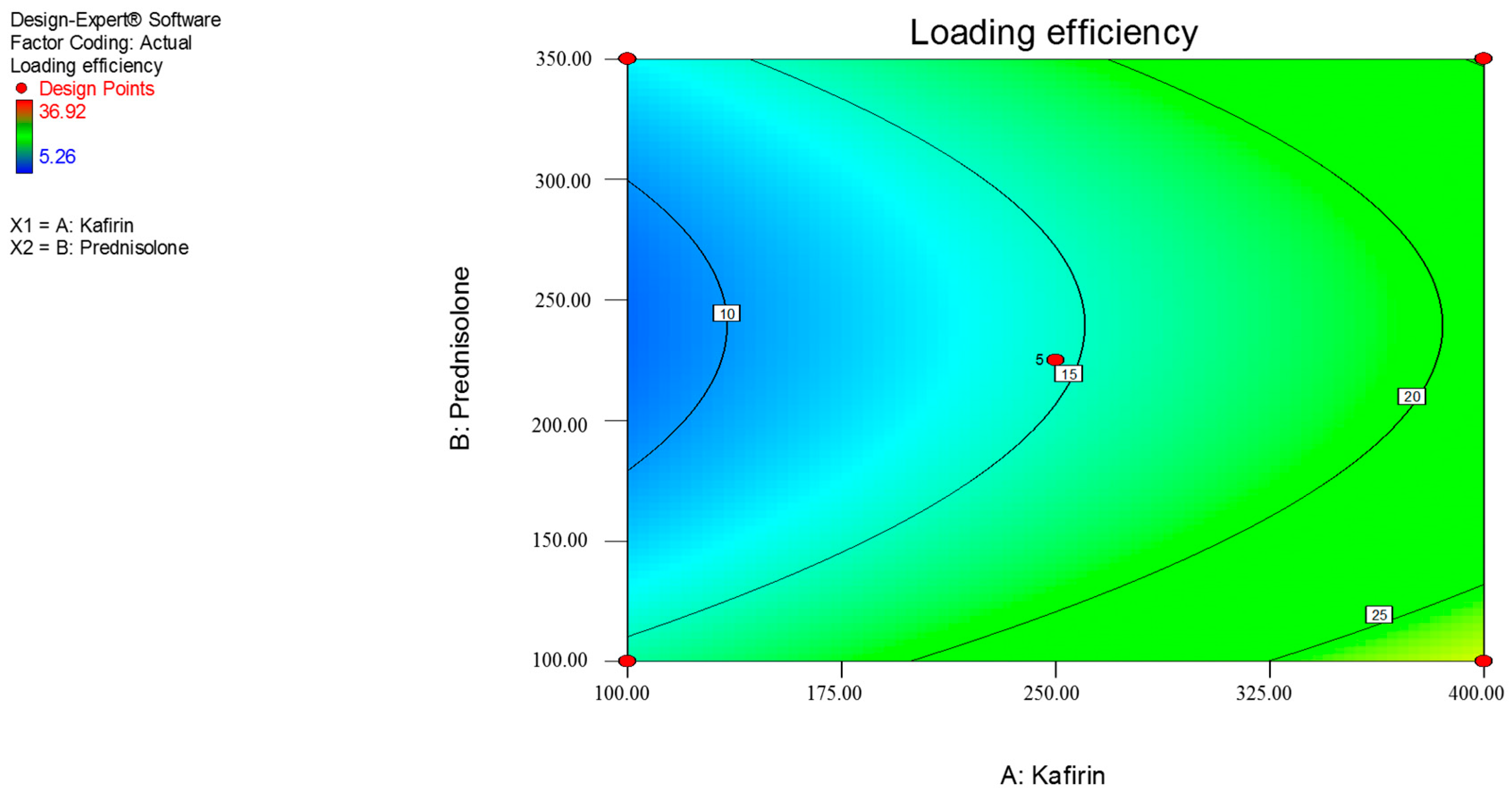
3.1. Response Surface Methdology
3.2. Optimization Data Analysis and Validation of Optimization Model and Thermodynamic Stability
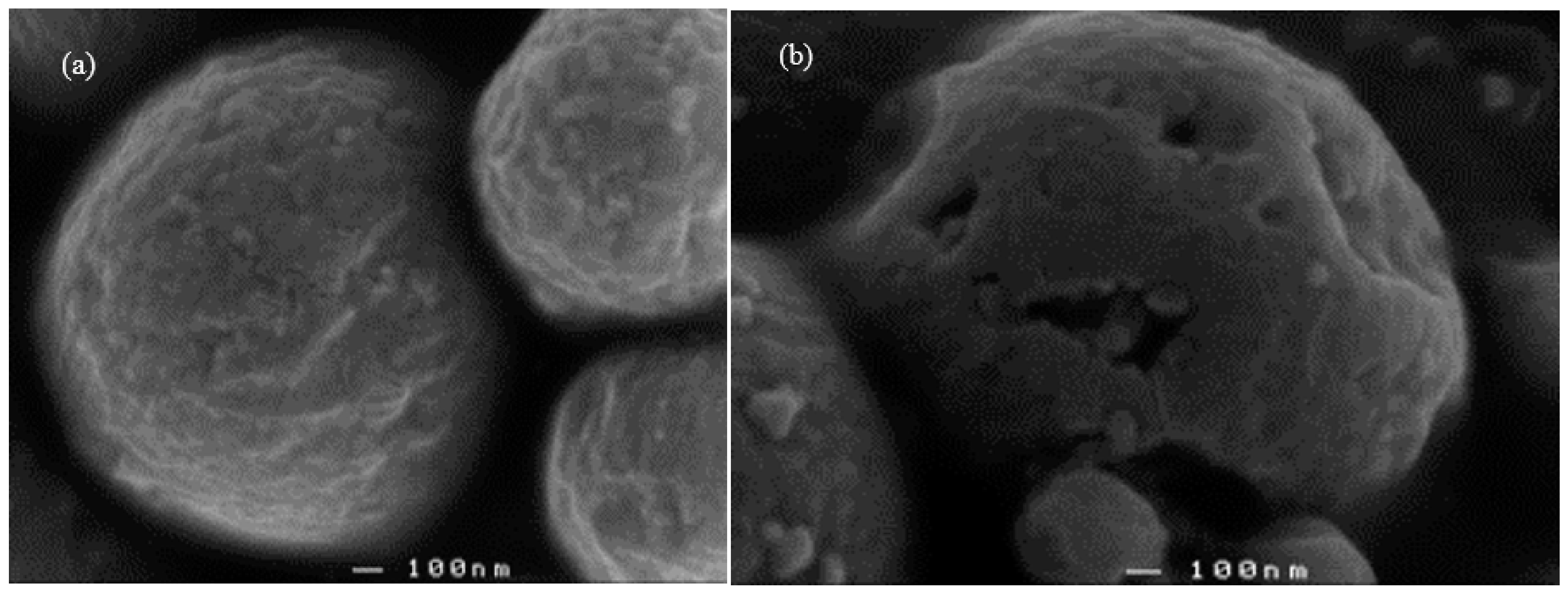
3.3. Scanning Electron Micrographs
3.4. Particle Size Distribution
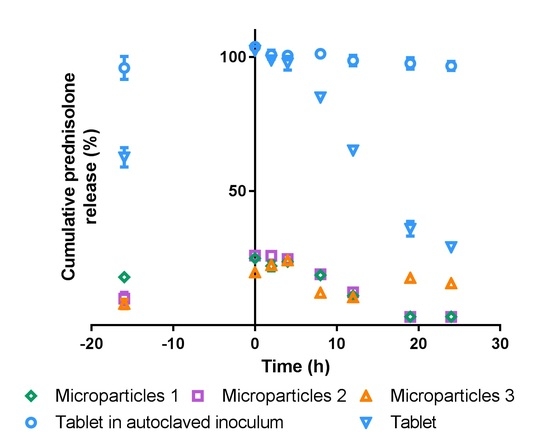
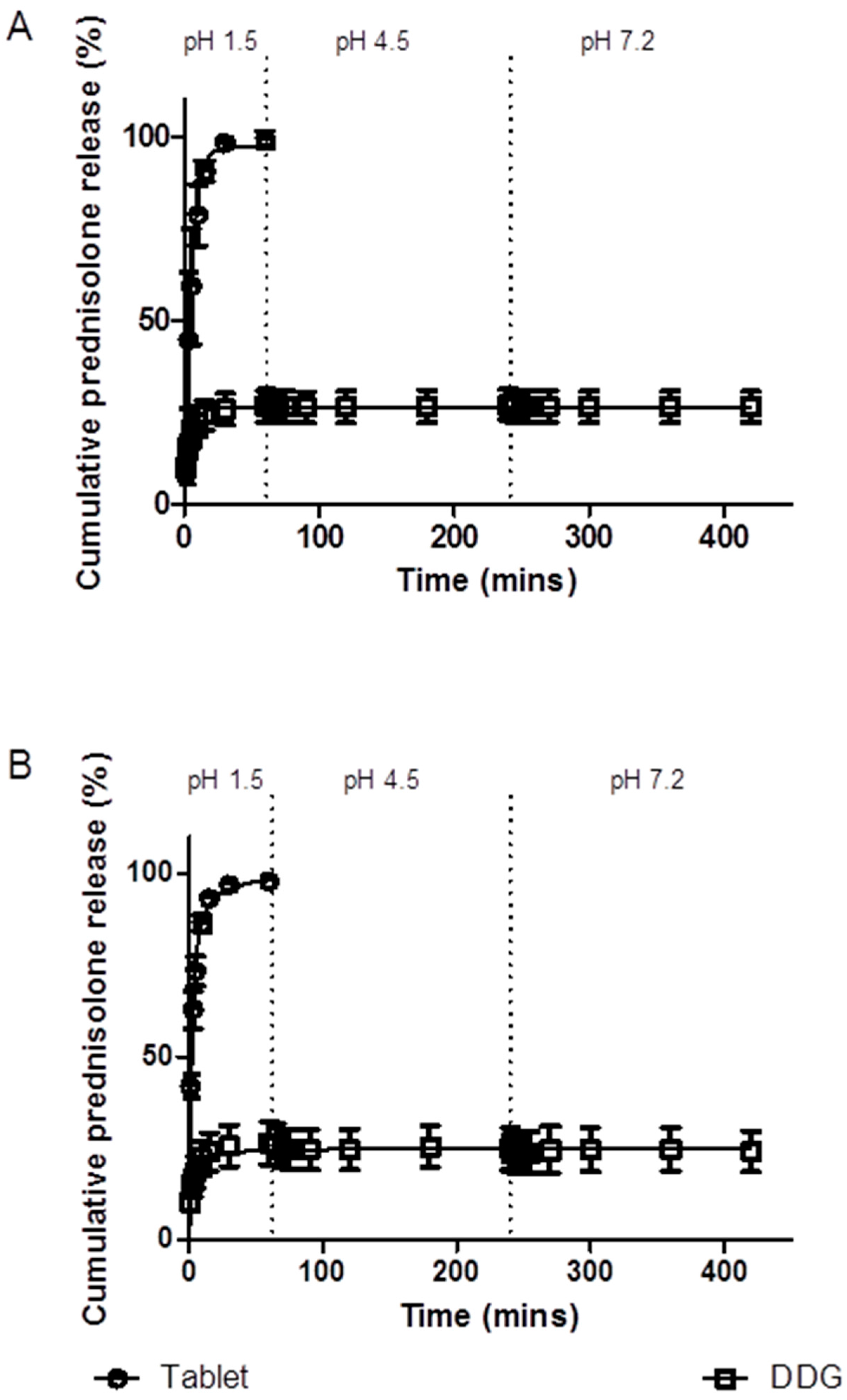
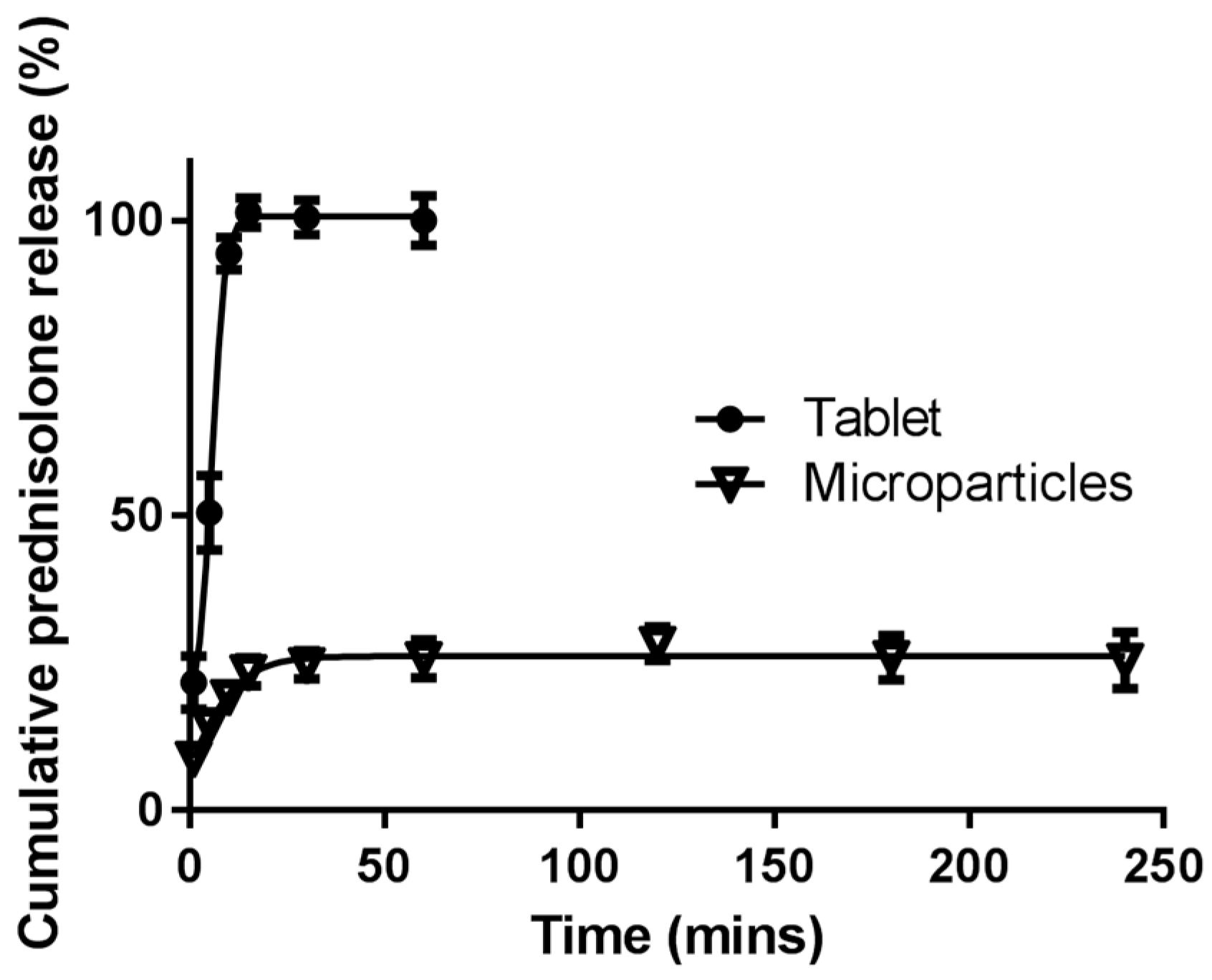
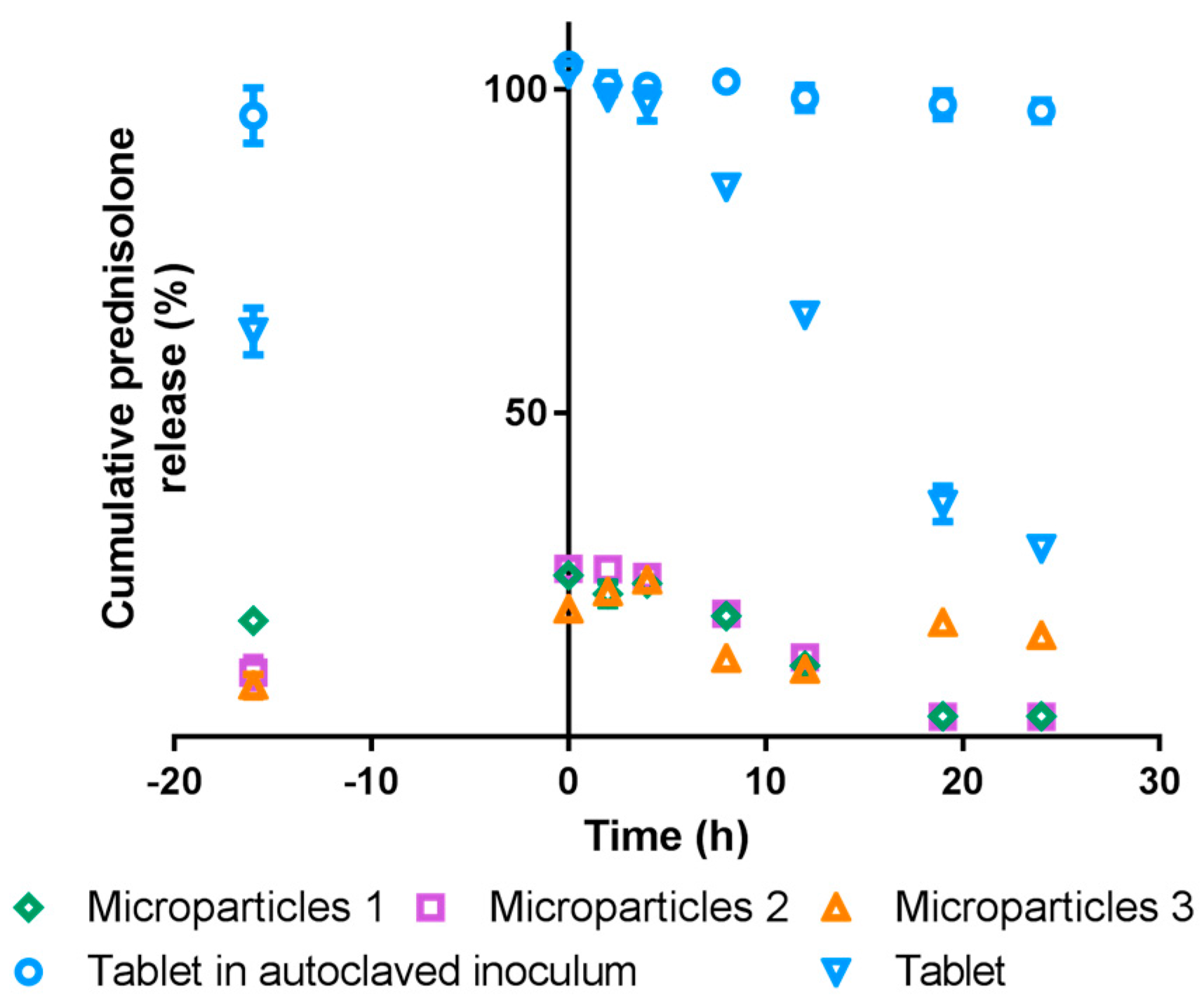
3.5. In vitro Drug Release
3.5.1. Simulated Stomach and Small Intestinal Conditions
3.5.2. Simulated Colonic Conditions
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Taylor, J.; Taylor, J.R.N.; Belton, P.S.; Minnaar, A. Kafirin microparticle encapsulation of catechin and sorghum condensed tannins. J. Agric. Food Chem. 2009, 57, 7523–7528. [Google Scholar] [CrossRef] [PubMed]
- Duodu, K.G.; Taylor, J.R.N.; Belton, P.S.; Hamaker, B.R. Factors affecting sorghum protein digestibility. J. Cereal Sci. 2003, 38, 117–131. [Google Scholar] [CrossRef]
- Belton, P.S.; Delgadillo, I.; Halford, N.G.; Shewry, P.R. Kafirin structure and functionality. J. Cereal Sci. 2006, 44, 272–286. [Google Scholar] [CrossRef]
- Lau, E.T.L.; Johnson, S.K.; Stanley, R.A.; Mikkelsen, D.; Fang, Z.; Halley, P.J.; Steadman, K.J. Preparation and in vitro release of drug-loaded microparticles for oral delivery using wholegrain sorghum kafirin protein. Int. J. Polym. Sci. 2015, 2015, 343647. [Google Scholar] [CrossRef]
- Hamaker, B.R.; Kirleis, A.; Butler, L.G.; Axtell, J.; Mertz, E. Improving the in vitro protein digestibility of sorghum with reducing agents. Proc. Natl. Acad. Sci. USA 1987, 84, 626–628. [Google Scholar] [CrossRef] [PubMed]
- Oria, M.P.; Hamaker, B.R.; Shull, J.M. Resistance of sorghum α-, β-, and γ-kafirins to pepsin digestion. J. Agric. Food Chem. 1995, 43, 2148–2153. [Google Scholar] [CrossRef]
- Weaver, C.; Hamaker, B.R.; Axtell, J. Discovery of grain sorghum germ plasm with high uncooked and cooked in vitro protein digestibilites. Cereal Chem. 1998, 75, 665–670. [Google Scholar] [CrossRef]
- Li, J.; Yang, L.; Ferguson, S.M.; Hudson, T.J.; Watanabe, S.; Katsuma, M.; Fix, J.A. in vitro evaluation of dissolution behaviour for a colon-specific drug delivery system (CodesTM) in multi-pH media using united states pharmacopeia apparatus II and III. AAPS Pharm. Sci. Tech. 2002, 3, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Watanabe, S.; Li, J.; Chu, J.S.; Katsuma, M.; Yokohama, S.; Fix, J.A. Effect of colonic lactulose availability on the timing of drug release onset in vivo from a unique colon-specific drug delivery system (codesTM). Pharm. Res. 2003, 20, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Watts, P.J.; Illum, L. Colonic drug delivery. Drug Dev. Ind. Pharm. 1997, 23, 893–913. [Google Scholar] [CrossRef]
- Sinha, V.R.; Kumria, R. Microbially triggered drug delivery to the colon. Eur. J. Pharm. Sci. 2003, 18, 3–18. [Google Scholar] [CrossRef]
- Sinko, P.J. Martin’s Physical Pharmacy and Pharmaceutical Sciences, 5th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006. [Google Scholar]
- Jain, S.K.; Jain, A. Target-specific drug release to the colon. Expert Opin. Drug Deliv. 2008, 5, 483–498. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Kim, J.S.; You, Y.H.; Park, H.J.; Lee, S.; Park, J.S.; Woo, J.S.; Hwang, S.J. Development and optimization of novel oral controlled delivery system for tamsulosin hydrochloride using response surface methodology. Int. J. Pharm. 2007, 341, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Akhgari, A.; Garekani, H.A.; Sadeghi, F.; Azimaie, M. Statistical optimization of indomethacin pellets coated with pH-dependent methacrylic polymers for possible colonic drug delivery. Int. J. Pharm. 2005, 305, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Quispe-Condori, S.; Saldaña, M.D.A.; Temelli, F. Microencapsulation of flax oil with zein using spray and freeze drying. LWT Food Sci. Technol. 2011, 44, 1880–1887. [Google Scholar] [CrossRef]
- Ko, J.A.; Park, H.J.; Park, Y.S.; Hwang, S.J.; Park, J.B. Chitosan microparticle preparation for controlled drug release by response surface methodology. J. Microencapsul. 2003, 20, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Khamanga, S.M.; Walker, R.B. The use of response surface methodology in the evaluation of captopril microparticles manufactured using an oil in oil solvent evaporation technique. J. Microencapsul. 2012, 29, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Elbary, A.A.; Aboelwafa, A.A.; Al Sharabi, I.M. Once daily, high-dose mesalazine controlled-release tablet for colonic delivery: Optimisation of formulation variables using box-behnken design. AAPS PharmSciTech 2011, 12, 1454–1464. [Google Scholar] [CrossRef] [PubMed]
- Sastry, S.V.; Reddy, I.K.; Khan, M.A. Atenolol gastrointestinal therapeutic system: Optimization of formulation variables using response surface methodology. J. Control. Release 1997, 45, 121–130. [Google Scholar] [CrossRef]
- Chaudhary, H.; Kohli, K.; Amin, S.; Rathee, P.; Kumar, V. Optimization and formulation design of gels of diclofenac and curcumin for transdermal drug delivery by box-behnken statistical design. J. Pharm. Sci. 2011, 100, 580–593. [Google Scholar] [CrossRef] [PubMed]
- Ben-Gal, I.; Bukchin, J. The ergonomic design of workstations using virtual manufacturing and repsonse surface methodology. IIE Trans. 2002, 34, 375–391. [Google Scholar] [CrossRef]
- Lau, E.T.L.; Johnson, S.K.; Stanley, R.A.; Mereddy, R.; Mikkelsen, D.; Halley, P.J.; Steadman, K.J. Formulation and characterization of drug-loaded microparticles using distiller’s dried grain kafirin. Cereal Chem. 2015, 92, 246–253. [Google Scholar] [CrossRef]
- Lau, E.T.L.; Giddings, S.J.; Mohammed, S.G.; Dubois, P.; Johnson, S.K.; Stanley, R.A.; Halley, P.J.; Steadman, K.J. Encapsulation of hydrocortisone and mesalazine in zein microparticles. Pharmaceutics 2013, 5, 277–293. [Google Scholar] [CrossRef] [PubMed]
- Lau, E.T.L.; Johnson, S.K.; Mikkelsen, D.; Halley, P.J.; Steadman, K.J. Preparation and in vitro release of zein microparticles loaded with prednisolone for oral delivery. J. Microencapsul. 2012, 29, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Nwabueze, T.U. Basic steps in adapting response surface methodology as mathematical modelling for bioprocess optimisation in the food systems. Int. J. Food Sci. Technol. 2010, 45, 1768–1776. [Google Scholar] [CrossRef]
- Flander, L.; Salmenkallio-Marttila, M.; Suortti, T.; Autio, K. Optimization of ingredients and baking process for improved wholemeal oat bread quality. LWT Food Sci. Technol. 2006, 40, 860–870. [Google Scholar] [CrossRef]
- Baş, D.; Boyacı, İ.H. Modeling and optimization i: Usability of response surface methodology. J. Food Eng. 2007, 78, 836–845. [Google Scholar] [CrossRef]
- Chauhan, O.P.; Raju, P.S.; Ravi, N.; Roopa, N.; Bawa, A.S. Studies on retention of antioxidant activity, phenolics and flavonoids in high pressure processed black grape juice and their modelling. Int. J. Food Sci. Technol. 2011, 46, 2562–2568. [Google Scholar] [CrossRef]
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline: Stability Testing of New Drug Subtances and Products Q1A(R2). Available online: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q1A_R2/Step4/Q1A_R2__Guideline.pdf (accessed on 6 February 2003).
- United States Pharmacopeial Convention. The United States Pharmacopeia and National Formulary (Usp 30-Nsp 25); United States Pharmacopeial Convention: Rockville, MD, USA, 2007. [Google Scholar]
- British Pharmacopoeia Commission. Appendix XII b. Dissolution annex: Recommendations on dissolution testing. In The British Pharmacopoeia 2012; TSO: Norwich, UK, 2012; Volume 5. [Google Scholar]
- Vertzoni, M.; Fotaki, N.; Kostewicz, E.; Stippler, E.; Leuner, C.; Nicolaides, E.; Dressman, J.; Reppas, C. Dissolution media simulating the intralumenal composition of the small intestine: Physiological issues and practical aspects. J. Pharm. Pharmacol. 2004, 56, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Clarysse, S.; Brouwers, J.; Tack, J.; Annaert, P.; Augustijns, P. Intestinal drug solubility estimation based on simulated intestinal fluids: Comparison with solubility in human intestinal fluids. Eur. J. Pharm. Sci. 2011, 43, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Zughaid, H.; Forbes, B.; Martin, G.P.; Patel, N. Bile salt composition is secondary to bile salt concentration in determining hydrocortisone and progesterone solubility in intestinal mimetic fluids. Int. J. Pharm. 2012, 422, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.A.; Bosch, M.W.; Boer, H.; Verstegen, M.W.A.; Tamminga, S. An in vitro batch culture method to assess potential fermentability of feed ingredients for monogastric diets. Anim. Feed Sci. Technol. 2005, 123–124, 445–462. [Google Scholar] [CrossRef]
- Mikkelsen, D.; Gidley, M.J.; Williams, B.A. In vitro fermentation of bacterial cellulose composites as model dietry fibers. J. Agric. Food Chem. 2011, 59, 4025–4032. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, Q.; Wang, H.; Zhang, L.; Wang, J. Microspheres of corn protein, zein, for an ivermectin drug delivery system. Biomaterials 2005, 26, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Muthuselvi, L.; Dhathathreyan, A. Simple coacervates of zein to encapsulate gitoxin. Colloids Surf. B Biointerfaces 2006, 51, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Mehanna, M.M.; Motawaa, A.M.; Samaha, M.W. Tadalafil inclusion in microporous silica as effective dissolution enhancer: Optimization of loading procedure and molecular state characterization. J. Pharm. Sci. 2011, 100, 1805–1818. [Google Scholar] [CrossRef] [PubMed]
- British Pharmacopoeia Commission. Formulated preparations: Specific monographs—Prednisolone tablets. In The British Pharmacopoeia 2012; TSO: Norwich, UK, 2012; Volume 3. [Google Scholar]
- Taylor, J.; Taylor, J.R.N.; Dutton, M.F.; de Kock, S. Identification of kafirin film casting solvents. Food Chem. 2005, 90, 401–408. [Google Scholar] [CrossRef]
- Tsumoto, K.; Ejima, D.; Senczuk, A.M.; Kita, Y.; Arakawa, T. Effects of salts on protein-surface interactions: Applications for column chromatography. J. Pharm. Sci. 2006, 96, 1677–1690. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.H.; Le, J.; Abraham, M.H.; Hersey, A.; Eddershaw, P.J.; Luscombe, C.N.; Boutina, D.; Beck, G.; Sherborne, B.; Cooper, I.; et al. Evaluation of human intestinal absorption data and subsequent derivation of a quantitative structure-activity relationship (QSAR) with the abraham descriptors. J. Pharm. Sci. 2000, 90, 749–784. [Google Scholar] [CrossRef]
- Doh, M.J.; Jung, Y.J.; Kim, I.H.; Kong, H.S.; Kim, Y.M. Synthesis and in vitro properties of prednisolone 21-sulfate sodium as a colon-specific prodrug of prednisolone. Arch. Pharm. Res. 2003, 26, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Aspen Pharmacare Australia Pty Ltd. Panafcortelone(r) Prednisolone Consumer Medicine Information (CMI). Available online: http://www.nps.org.au/__data/assets/pdf_file/0007/81826/ascpanlo.pdf (accessed on 10 May 2012).

| Real Independent Variables | Coded Independent Variable Levels | ||||
| Corner Points | Centre Point | Star Points | |||
| –1.414 | –1.0 | 0 | +1.0 | +1.414 | |
| X1 = kafirin (mg) | 37.87 | 100 | 250 | 400 | 462.13 |
| X2 = prednisolone (mg) | 48.25 | 100 | 225 | 350 | 401.75 |
| Real dependent variables | - | ||||
| Y1 = prednisolone loading (%) | |||||
| Y2 = loading efficiency (%) | |||||
| Substrate Number | Substrate Classification | Description |
|---|---|---|
| 1 | Sample: replicate 1 | Kafirin microparticles loaded with prednisolone equivalent to 5 mg of prednisolone + fecal inoculum + 0.5 g glucose |
| 2 | Sample: replicate 2 | Kafirin microparticles loaded with prednisolone equivalent to 5 mg of prednisolone + fecal inoculum + 0.5 g glucose |
| 3 | Sample: replicate 3 | Kafirin microparticles loaded with prednisolone equivalent to 5 mg of prednisolone + fecal inoculum + 0.5 g glucose |
| 4 | Blank | Medium + fecal inoculum |
| 5 | Control 1: for glucose | Fecal inoculum + 0.5 g glucose |
| 6 | Control 2: for microparticles | Kafirin microparticles (no prednisolone) + fecal inoculum |
| 7 | Control 3: for microparticles | Commercially available 5 mg prednisolone tablets + fecal inoculum + 0.5 g glucose |
| 8 | Control 4: for microparticles and fecal inoculum | Commercially available 5 mg prednisolone tablets + autoclaved fecal inoculum |
| Response | Intercept | X1 | X2 | X22 |
|---|---|---|---|---|
| Y1 | 7.25 | –0.21 | 3.37 | - |
| p-value | - | 0.8301 | 0.0055 | - |
| Y2 | 14.67 | 5.98 | –1.38 | 5.99 |
| p-value | - | 0.0417 | 0.5964 | 0.0522 |
| Dependent variable | Predicted Value | Observed Value | Predicted Error (%) |
|---|---|---|---|
| Optimal independent variable combination: 400 mg kafirin and 350 mg prednisolone | |||
| Y1 = Prednisolone loading (%) | 10.414 | 13.53 | 29.9 |
| Y2 = Loading efficiency (%) | 25.269 | 30.80 | 21.9 |
| ′Non-optimal′ independent variable combination: 200 mg kafirin and 200 mg prednisolone | |||
| Y1 = Prednisolone loading (%) | 6.64 | 3.73 | –43.8 |
| Y2 = Loading efficiency (%) | 13.22 | 8.33 | –37.0 |
| Microparticles | d(v,0.1) (µm) | d(v,0.5) (µm) | d(v,0.9) (µm) | D(3,2) (µm) | D(4,3) (µm) |
|---|---|---|---|---|---|
| Empty | 3.317 | 12.535 | 33.519 | 7.632 | 17.205 |
| Prednisolone loaded | 2.534 | 8.820 | 34.159 | 5.946 | 15.334 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lau, E.T.L.; Johnson, S.K.; Williams, B.A.; Mikkelsen, D.; McCourt, E.; Stanley, R.A.; Mereddy, R.; Halley, P.J.; Steadman, K.J. Optimizing Prednisolone Loading into Distiller’s Dried Grain Kafirin Microparticles, and In vitro Release for Oral Delivery. Pharmaceutics 2017, 9, 17. https://doi.org/10.3390/pharmaceutics9020017
Lau ETL, Johnson SK, Williams BA, Mikkelsen D, McCourt E, Stanley RA, Mereddy R, Halley PJ, Steadman KJ. Optimizing Prednisolone Loading into Distiller’s Dried Grain Kafirin Microparticles, and In vitro Release for Oral Delivery. Pharmaceutics. 2017; 9(2):17. https://doi.org/10.3390/pharmaceutics9020017
Chicago/Turabian StyleLau, Esther T. L., Stuart K. Johnson, Barbara A. Williams, Deirdre Mikkelsen, Elizabeth McCourt, Roger A. Stanley, Ram Mereddy, Peter J. Halley, and Kathryn J. Steadman. 2017. "Optimizing Prednisolone Loading into Distiller’s Dried Grain Kafirin Microparticles, and In vitro Release for Oral Delivery" Pharmaceutics 9, no. 2: 17. https://doi.org/10.3390/pharmaceutics9020017