Predicting Oral Drug Absorption: Mini Review on Physiologically-Based Pharmacokinetic Models
Abstract
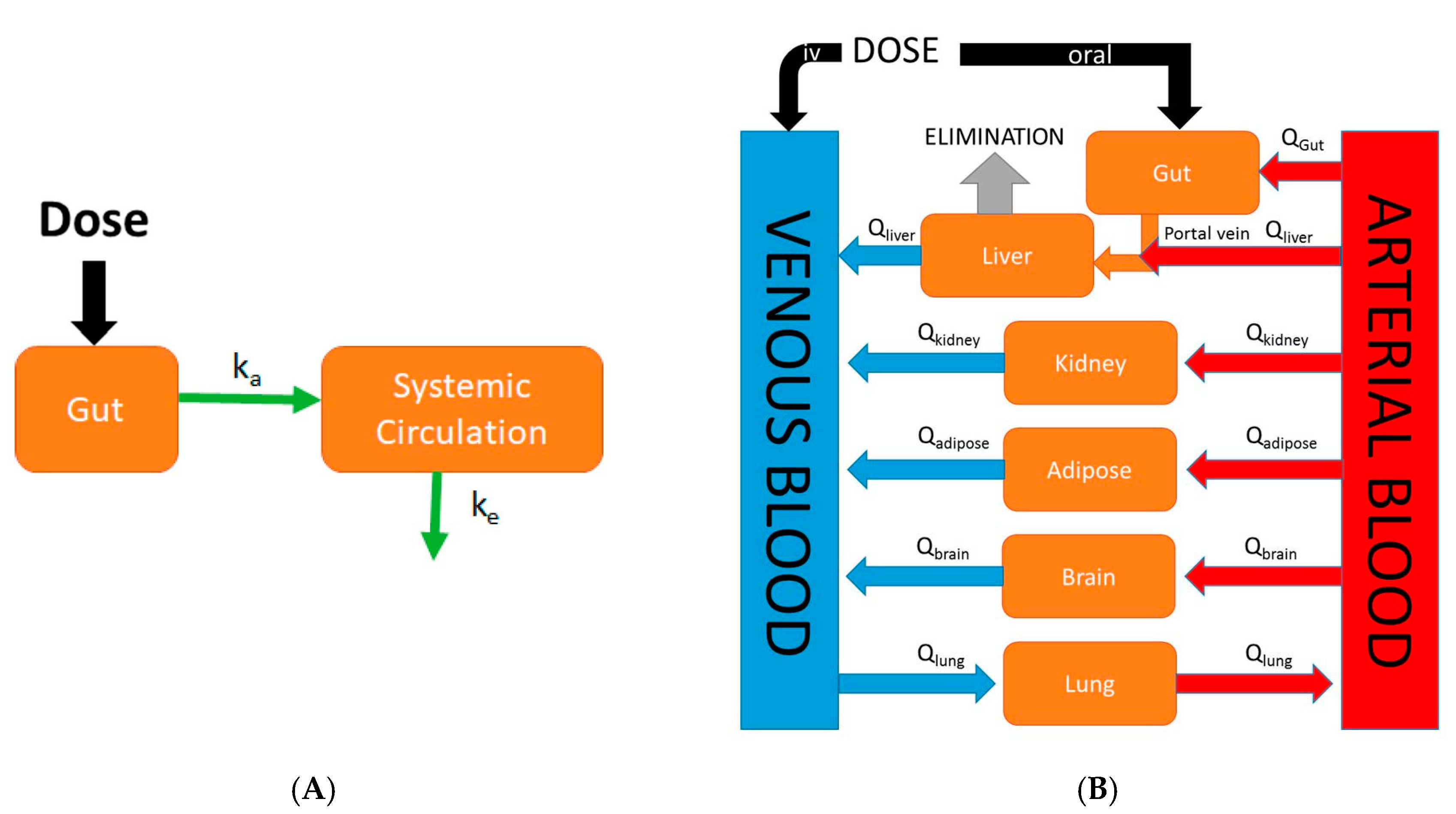
:1. Introduction
2. Fundamental Processes for Oral Absorption
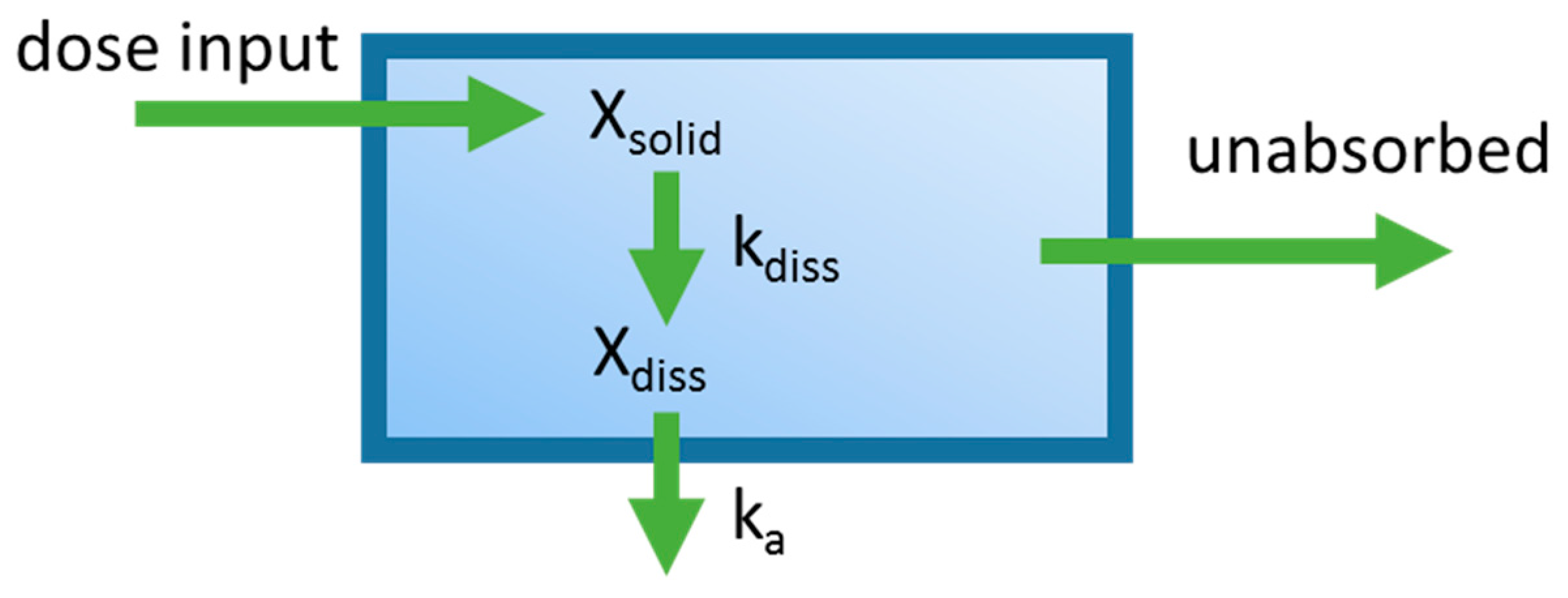
3. Mixing Tank Model
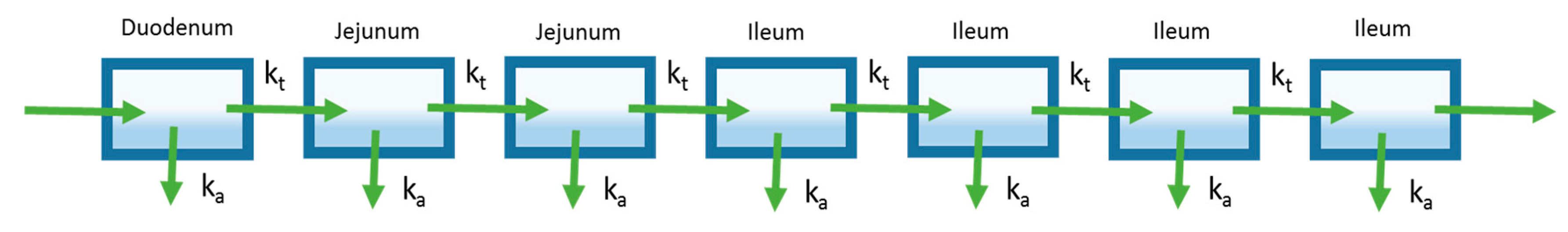
4. Compartmental Absorption and Transit Model
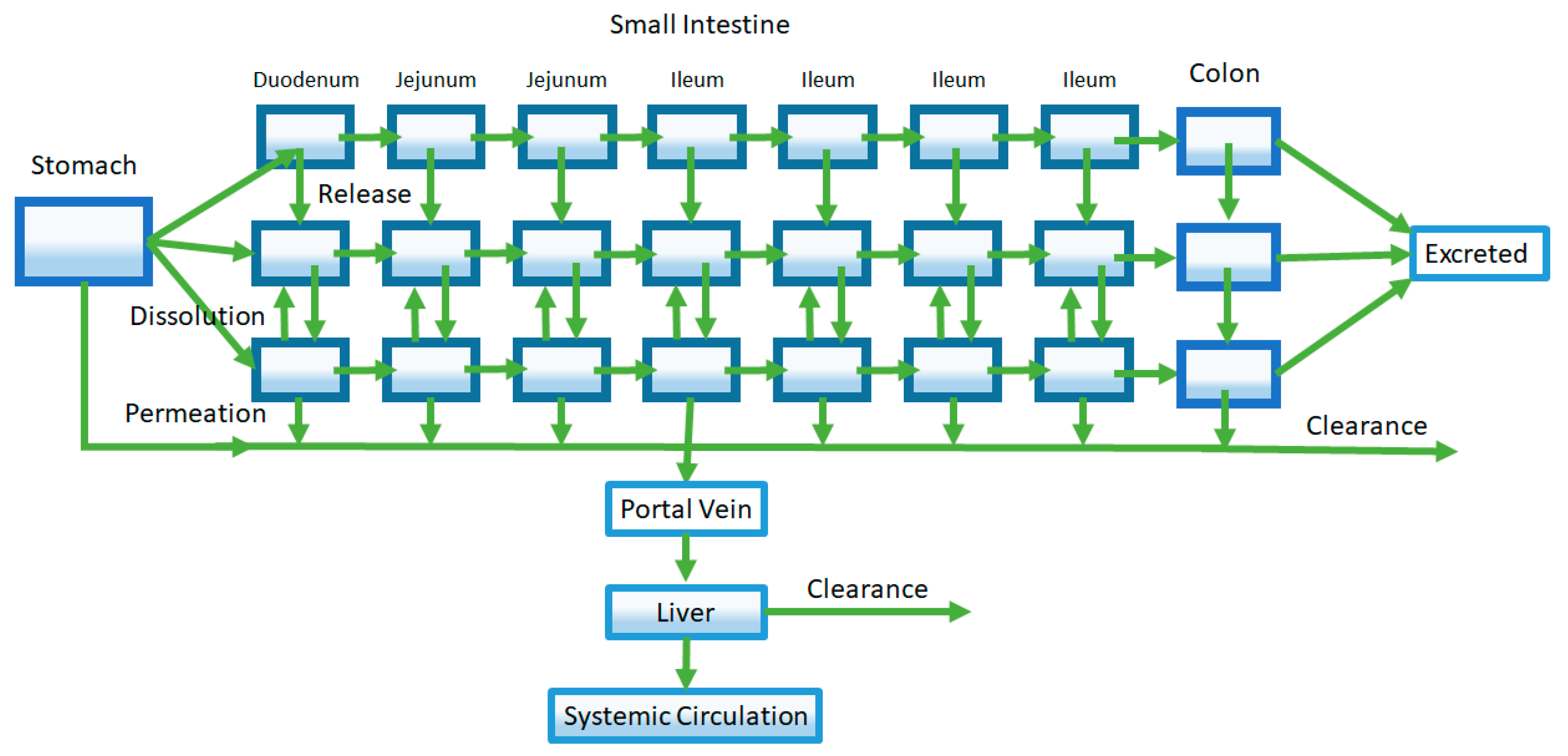
5. Advanced Compartmental Absorption and Transit Model
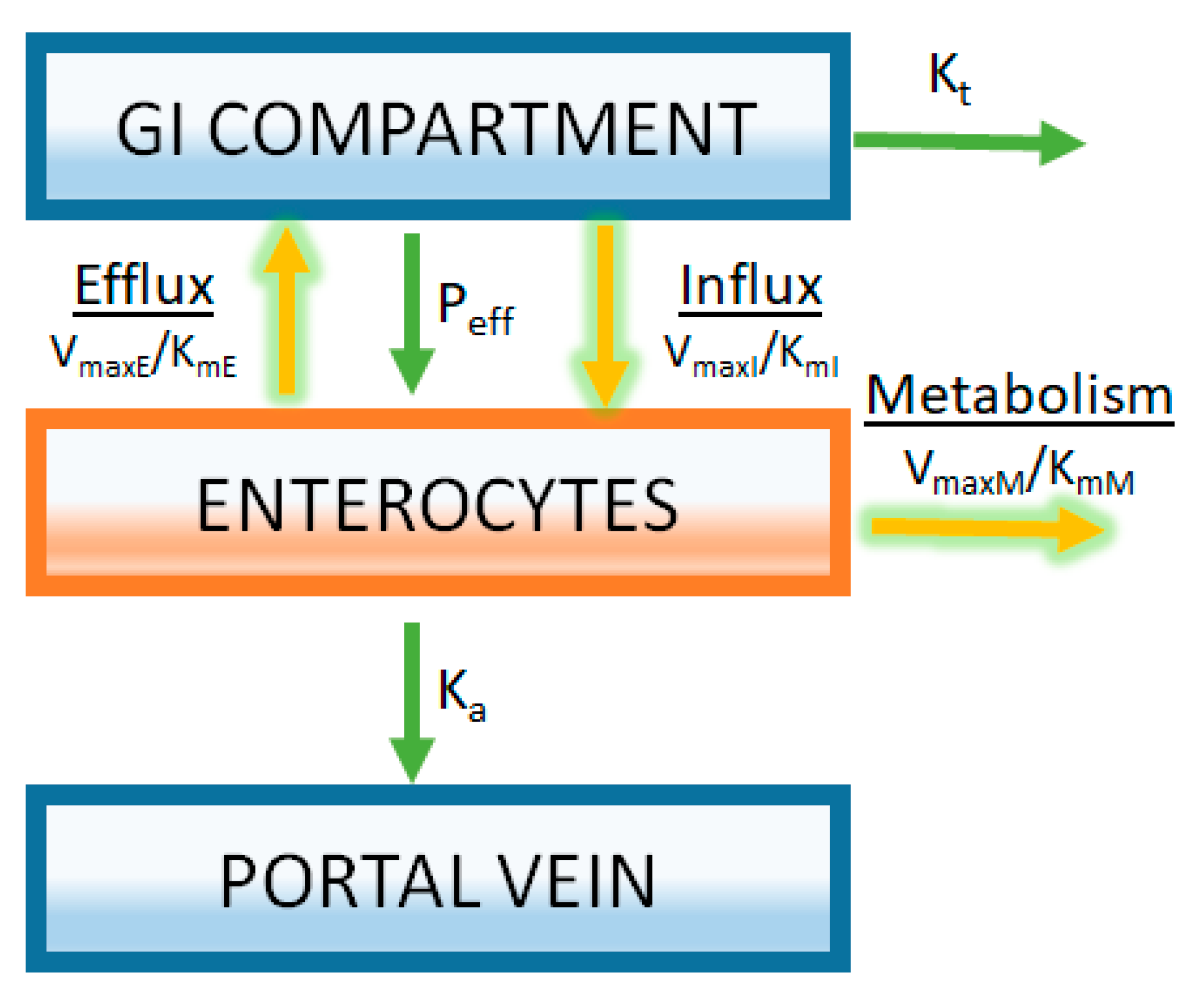
6. Incorporation of Saturable Processes: Metabolism and Drug Transporters
7. Applications of Oral PBPK Models
7.1. Drug Form Selection and Formulation Optimization
7.1.1. Salt Selection
7.1.2. Particle Size
7.2. Food and pH Effects
7.2.1. Investigating pH Effect
7.2.2. Investigating Mechanisms of Food Effect
7.3. Predicting Human Oral Pharmacokinetics
7.4. Performance of Various Applications of PBPK Models of Oral Absorption
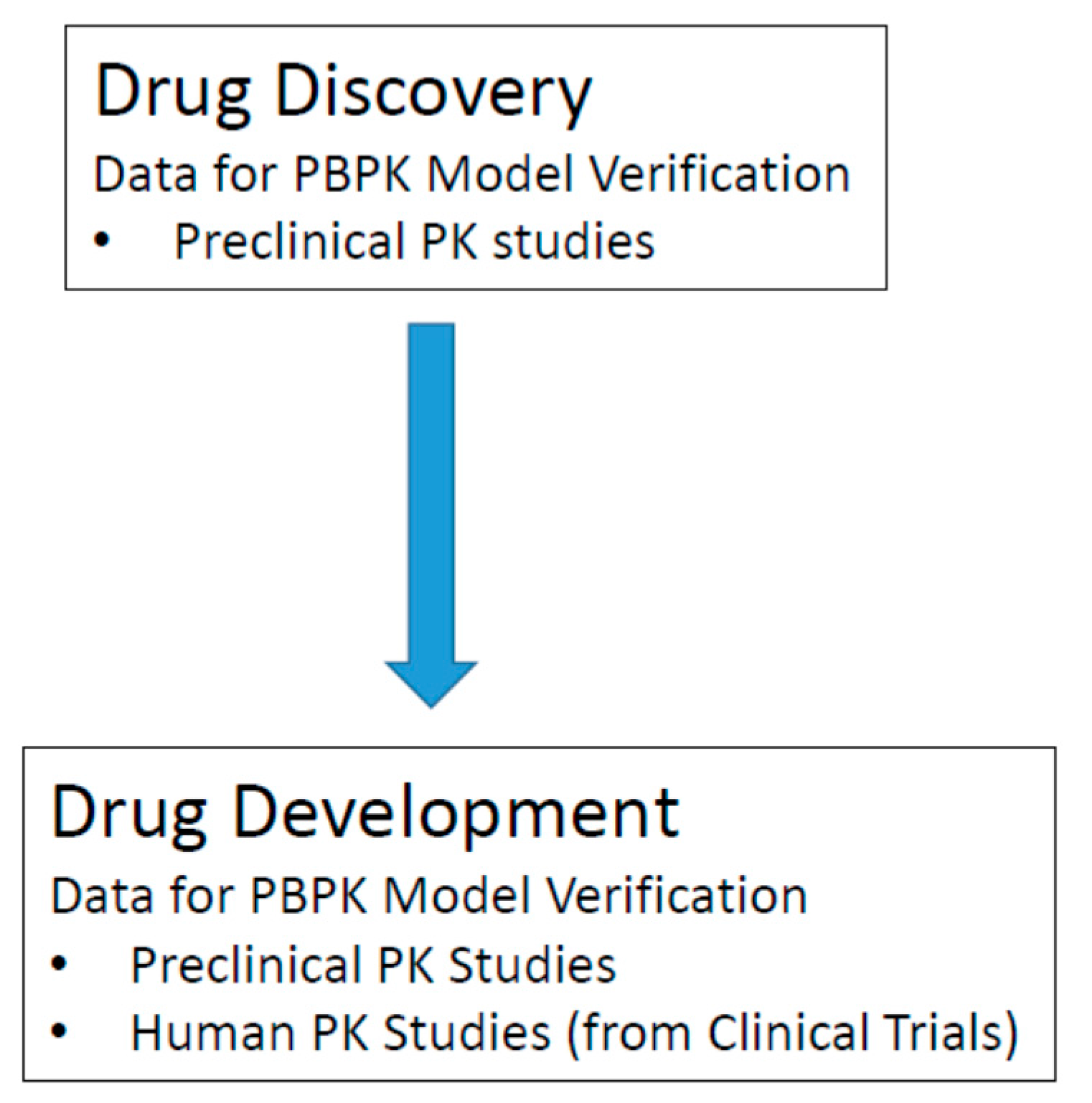
7.5. Model Verification/Validation
8. Conclusions
Author Contributions
Conflicts of Interest
References
- Sugihara, M.; Takeuchi, S.; Sugita, M.; Higaki, K.; Kataoka, M.; Yamashita, S. Analysis of intra- and intersubject variability in oral drug absorption in human bioequivalence studies of 113 generic products. Mol. Pharm. 2015, 12, 4405–4413. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.N.; Amidon, G.L. A mechanistic approach to understanding the factors affecting drug absorption: A review of fundamentals. J. Clin. Pharmacol. 2002, 42, 620–643. [Google Scholar] [CrossRef] [PubMed]
- Mudie, D.M.; Amidon, G.L.; Amidon, G.E. Physiological parameters for oral delivery and in vitro testing. Mol. Pharm. 2010, 7, 1388–1405. [Google Scholar] [CrossRef] [PubMed]
- Waring, M.J.; Arrowsmith, J.; Leach, A.R.; Leeson, P.D.; Mandrell, S.; Owen, R.M.; Pairaudeau, G.; Pennie, W.D.; Pickett, S.D.; Wang, J.; et al. An analysis of the attrition of drug candidates from four major pharmaceutical companies. Nat. Rev. Drug Discov. 2015, 14, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Ward, K.W. Optimizing pharmacokinetic properties and attaining candidate selection. In Reducing Drug Attrition; Topics in Medicinal Chemistry; Springer: Berlin, Germany, 2012; pp. 73–95. ISBN 978-3-662-43913-5. [Google Scholar]
- Chu, X.; Bleasby, K.; Evers, R. Species differences in drug transporters and implications for translating preclinical findings to humans. Expert Opin. Drug Metab. Toxicol. 2013, 9, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Chanteux, H.; Staelens, L.; Mancel, V.; Gerin, B.; Boucaut, D.; Prakash, C.; Nicolas, J.-M. Cross-species differences in the preclinical pharmacokinetics of CT7758, an α4β1/α4β7 integrin antagonist. Drug Metab. Dispos. Biol. Fate Chem. 2015, 43, 1381–1391. [Google Scholar] [CrossRef] [PubMed]
- Dressman, J.B.; Amidon, G.L.; Fleisher, D. Absorption potential: Estimating the fraction absorbed for orally administered compounds. J. Pharm. Sci. 1985, 74, 588–589. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.C.; Swindell, A.C. Guidance in the setting of drug particle size specifications to minimize variability in absorption. Pharm. Res. 1996, 13, 1795–1798. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Rose, J.P.; Van Gelder, J. Developability assessment of clinical drug products with maximum absorbable doses. Int. J. Pharm. 2012, 427, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Yu, L.X.; Hussain, M.A.; Wall, D.A.; Smith, R.L.; Amidon, G.L. In vitro testing of drug absorption for drug “developability” assessment: Forming an interface between in vitro preclinical data and clinical outcome. Curr. Opin. Drug Discov. Devel. 2004, 7, 75–85. [Google Scholar] [PubMed]
- Torsten, T. Kinetics of distribution of substances administered to the body, I: The extravascular modes of administration. Arch. Int. Pharmacodyn. Ther. 1937, 57, 205–225. [Google Scholar]
- Jones, H.M.; Parrott, N.; Jorga, K.; Lavé, T. A Novel strategy for physiologically based predictions of human pharmacokinetics. Clin. Pharmacokinet. 2006, 45, 511–542. [Google Scholar] [CrossRef] [PubMed]
- Noyes, A.A.; Whitney, W.R. The rate of solution of solid substances in their own solutions. J. Am. Chem. Soc. 1897, 19, 930–934. [Google Scholar] [CrossRef]
- Sugano, K.; Okazaki, A.; Sugimoto, S.; Tavornvipas, S.; Omura, A.; Mano, T. Solubility and dissolution profile assessment in drug discovery. Drug Metab. Pharmacokinet. 2007, 22, 225–254. [Google Scholar] [CrossRef] [PubMed]
- Dressman, J.B.; Fleisher, D. Mixing-tank model for predicting dissolution rate control or oral absorption. J. Pharm. Sci. 1986, 75, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.X.; Crison, J.R.; Amidon, G.L. Compartmental transit and dispersion model analysis of small intestinal transit flow in humans. Int. J. Pharm. 1996, 140, 111–118. [Google Scholar] [CrossRef]
- Dressman, J.B.; Fleisher, D.; Amidon, G.L. Physicochemical model for dose-dependent drugabsorption. J. Pharm. Sci. 1984, 73, 1274–1279. [Google Scholar] [CrossRef] [PubMed]
- Oberle, R.L.; Amidon, G.L. The influence of variable gastric emptying and intestinal transit rates on the plasma level curve of cimetidine; an explanation for the double peak phenomenon. J. Pharmacokinet. Biopharm. 1987, 15, 529–544. [Google Scholar] [CrossRef] [PubMed]
- Luner, P.E.; Amidon, G.L. Description and simulation of a multiple mixing tank model to predict the effect of bile sequestrants on bile salt excretion. J. Pharm. Sci. 1993, 82, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.M.; Shafer, S.L. A simple analytical solution to the three-compartment pharmacokinetic model suitable for computer-controlled infusion pumps. IEEE Trans. Biomed. Eng. 1991, 38, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.X. An integrated model for determining causes of poor oral drug absorption. Pharm. Res. 1999, 16, 1883–1887. [Google Scholar] [CrossRef] [PubMed]
- Agoram, B.; Woltosz, W.S.; Bolger, M.B. Predicting the impact of physiological and biochemical processes on oral drug bioavailability. Adv. Drug Deliv. Rev. 2001, 50 (Suppl. 1), S41–S67. [Google Scholar] [CrossRef]
- Jambhekar, S.S.; Breen, P.J. Drug dissolution: Significance of physicochemical properties and physiological conditions. Drug Discov. Today 2013, 18, 1173–1184. [Google Scholar] [CrossRef] [PubMed]
- Dressman, J.B.; Vertzoni, M.; Goumas, K.; Reppas, C. Estimating drug solubility in the gastrointestinal tract. Adv. Drug Deliv. Rev. 2007, 59, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Mouly, S.; Paine, M.F. P-glycoprotein increases from proximal to distal regions of human small intestine. Pharm. Res. 2003, 20, 1595–1599. [Google Scholar] [CrossRef] [PubMed]
- Ungell, A.L.; Nylander, S.; Bergstrand, S.; Sjöberg, A.; Lennernäs, H. Membrane transport of drugs in different regions of the intestinal tract of the rat. J. Pharm. Sci. 1998, 87, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Paine, M.F.; Khalighi, M.; Fisher, J.M.; Shen, D.D.; Kunze, K.L.; Marsh, C.L.; Perkins, J.D.; Thummel, K.E. Characterization of interintestinal and intraintestinal variations in human CYP3A-dependent metabolism. J. Pharmacol. Exp. Ther. 1997, 283, 1552–1562. [Google Scholar] [PubMed]
- Sawamoto, T.; Haruta, S.; Kurosaki, Y.; Higaki, K.; Kimura, T. Prediction of the plasma concentration profiles of orally administered drugs in rats on the basis of gastrointestinal transit kinetics and absorbability. J. Pharm. Pharmacol. 1997, 49, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Jamei, M.Y.J. A Novel Physiologically-Based Mechanistic Model for Predicting Oral Drug Absorption: The Advanced Dissolution, Absorption, and Metabolism (ADAM) Model. Available online: https://www.escholar.manchester.ac.uk/uk-ac-man-scw:108992 (accessed on 12 September 2017).
- Wang, J.; Flanagan, D.R. General solution for diffusion-controlled dissolution of spherical particles. 1. Theory. J. Pharm. Sci. 1999, 88, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Takano, M. Intestinal efflux transporters and drug absorption. Expert Opin. Drug Metab. Toxicol. 2008, 4, 923–939. [Google Scholar] [CrossRef] [PubMed]
- Hurst, S.; Loi, C.-M.; Brodfuehrer, J.; El-Kattan, A. Impact of physiological, physicochemical and biopharmaceutical factors in absorption and metabolism mechanisms on the drug oral bioavailability of rats and humans. Expert Opin. Drug Metab. Toxicol. 2007, 3, 469–489. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.M.S.; Cooper, A.E.; Dudley, A.L.J.; Ford, D.; Hirst, B.H. P-glycoprotein potentiates CYP3A4-mediated drug disappearance during Caco-2 intestinal secretory detoxification. J. Drug Target. 2004, 12, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Wacher, V.J.; Silverman, J.A.; Zhang, Y.; Benet, L.Z. Role of P-glycoprotein and cytochrome P450 3A in limiting oral absorption of peptides and peptidomimetics. J. Pharm. Sci. 1998, 87, 1322–1330. [Google Scholar] [CrossRef] [PubMed]
- Lown, K.S.; Mayo, R.R.; Leichtman, A.B.; Hsiao, H.L.; Turgeon, D.K.; Schmiedlin-Ren, P.; Brown, M.B.; Guo, W.; Rossi, S.J.; Benet, L.Z.; et al. Role of intestinal P-glycoprotein (MDR1) in interpatient variation in the oral bioavailability of cyclosporine. Clin. Pharmacol. Ther. 1997, 62, 248–260. [Google Scholar] [CrossRef]
- Wacher, V.J.; Wu, C.Y.; Benet, L.Z. Overlapping substrate specificities and tissue distribution of cytochrome P450 3A and P-glycoprotein: Implications for drug delivery and activity in cancer chemotherapy. Mol. Carcinog. 1995, 13, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Samuel, K.; Subramanian, R.; Braun, M.P.; Stearns, R.A.; Chiu, S.-H.L.; Evans, D.C.; Baillie, T.A. Extrapolation of diclofenac clearance from in vitro microsomal metabolism data: Role of Acyl glucuronidation and sequential oxidative metabolism of the Acyl glucuronide. J. Pharmacol. Exp. Ther. 2002, 303, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Kusuhara, H.; Sugiyama, Y. In vitro-in vivo extrapolation of transporter-mediated clearance in the liver and kidney. Drug Metab. Pharmacokinet. 2009, 24, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, S.; Maeda, K.; Sugiyama, Y. Recent progresses in the experimental methods and evaluation strategies of transporter functions for the prediction of the pharmacokinetics in humans. Naunyn-Schmiedebergs Arch. Pharmacol. 2008, 377, 617. [Google Scholar] [CrossRef] [PubMed]
- Gertz, M.; Houston, J.B.; Galetin, A. Physiologically based pharmacokinetic modeling of intestinal first-pass metabolism of CYP3A substrates with high intestinal extraction. Drug Metab. Dispos. 2011, 39, 1633–1642. [Google Scholar] [CrossRef] [PubMed]
- Chiang, P.-C.; Wong, H. Incorporation of physiologically based pharmacokinetic modeling in the evaluation of solubility requirements for the salt selection process: A case study using phenytoin. AAPS J. 2013, 15, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Parrott, N.; Lave, T. Applications of physiologically based absorption models in drug discovery and development. Mol. Pharm. 2008, 5, 760–775. [Google Scholar] [CrossRef] [PubMed]
- Abuhelwa, A.Y.; Williams, D.B.; Upton, R.N.; Foster, D.J.R. Food, gastrointestinal pH, and models of oral drug absorption. Eur. J. Pharm. Biopharm. 2017, 112, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shi, L.; Tang, X.; Wang, Q.; Zhou, L.; Song, W.; Feng, Z.; Ge, J.; Li, J.K.; Yang, L.; et al. Mechanistic prediction of food effects for Compound A tablet using PBPK model. Saudi J. Biol. Sci. 2017, 24, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.; Alvarez-Nunez, F.; Chow, V.; Daurio, D.; Davis, J.; Dodds, M.; Emery, M.; Litwiler, K.; Paccaly, A.; Peng, J.; et al. Utilizing physiologically based pharmacokinetic modeling to inform formulation and clinical development for a Compound with pH-dependent solubility. J. Pharm. Sci. 2015, 104, 1522–1532. [Google Scholar] [CrossRef] [PubMed]
- Welling, P.G. Effects of food on drug absorption. Annu. Rev. Nutr. 1996, 16, 383–415. [Google Scholar] [CrossRef] [PubMed]
- Glomme, A.; März, J.; Dressman, J.B. Predicting the intestinal solubility of poorly soluble drugs. In Pharmacokinetic Profiling in Drug Research; Testa, B., Krämer, S.D., Wunderli-Allenspach, H., Folkers, G., Eds.; Wiley-VCH: Weinheim, Germany, 2006; pp. 259–280. ISBN 978-3-906390-46-8. [Google Scholar]
- Andreas, C.J.; Pepin, X.; Markopoulos, C.; Vertzoni, M.; Reppas, C.; Dressman, J.B. Mechanistic investigation of the negative food effect of modified release zolpidem. Eur. J. Pharm. Sci. 2017, 102, 284–298. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xia, B.; Sheng, J.; Heimbach, T.; Lin, T.-H.; He, H.; Wang, Y.; Novick, S.; Comfort, A. Application of physiologically based absorption modeling to formulation development of a low solubility, low permeability weak base: Mechanistic investigation of food effect. AAPS PharmSciTech 2014, 15, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhuang, X.; Yang, C.; Li, Z.; Xiong, S.; Zhang, Z.; Li, J.; Lu, C.; Zhang, Z. Characterization of preclinical in vitro and in vivo ADME properties and prediction of human PK using a physiologically based pharmacokinetic model for YQA-14, a new dopamine D3 receptor antagonist candidate for treatment of drug addiction. Biopharm. Drug Dispos. 2014, 35, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Poulin, P.; Jones, R.D.O.; Jones, H.M.; Gibson, C.R.; Rowland, M.; Chien, J.Y.; Ring, B.J.; Adkison, K.K.; Ku, M.S.; He, H.; et al. PHRMA CPCDC initiative on predictive models of human pharmacokinetics, Part 5: Prediction of plasma concentration-time profiles in human by using the physiologically-based pharmacokinetic modeling approach. J. Pharm. Sci. 2011, 100, 4127–4157. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.D.; Trevaskis, N.L.; Charman, S.A.; Shanker, R.M.; Charman, W.N.; Pouton, C.W.; Porter, C.J.H. Strategies to address low drug solubility in discovery and development. Pharmacol. Rev. 2013, 65, 315–499. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, L.; Wong, H. Predicting Oral Drug Absorption: Mini Review on Physiologically-Based Pharmacokinetic Models. Pharmaceutics 2017, 9, 41. https://doi.org/10.3390/pharmaceutics9040041
Lin L, Wong H. Predicting Oral Drug Absorption: Mini Review on Physiologically-Based Pharmacokinetic Models. Pharmaceutics. 2017; 9(4):41. https://doi.org/10.3390/pharmaceutics9040041
Chicago/Turabian StyleLin, Louis, and Harvey Wong. 2017. "Predicting Oral Drug Absorption: Mini Review on Physiologically-Based Pharmacokinetic Models" Pharmaceutics 9, no. 4: 41. https://doi.org/10.3390/pharmaceutics9040041



