Revolutionizing Therapeutic Drug Monitoring with the Use of Interstitial Fluid and Microneedles Technology
Abstract
:1. Introduction
2. Principles of Therapeutic Drug Monitoring
3. Alternative Matrices for Therapeutic Drug Monitoring
4. Current Evidence Supporting the Use of Interstitial Fluid for Therapeutic Drug Monitoring in Clinical Models
4.1. Anti-Infectives
4.2. Anticonvulsants
4.3. Miscellaneous Agents
5. Microneedle Technologies for Interstitial Fluid Collection for Therapeutic Drug Monitoring
5.1. Interstitial Fluid Extraction Devices for Off-Device Analysis
5.2. Interstitial Fluid Extraction for On-Device Analysis
5.3. Continuous Monitoring Microneedle Devices
5.4. Other Methods for ISF Extraction
6. Future Directions
Acknowledgments
Conflicts of Interest
References
- Kiang, T.K.; Hafeli, U.O.; Ensom, M.H. A Comprehensive Review on the Pharmacokinetics of Antibiotics in Interstitial Fluid Spaces in Humans: Implications on Dosing and Clinical Pharmacokinetic Monitoring. Clin. Pharmacokinet. 2014, 53, 695–730. [Google Scholar] [CrossRef] [PubMed]
- Koka, S.; Beebe, T.J.; Merry, S.P.; DeJesus, R.S.; Berlanga, L.D.; Weaver, A.L.; Montori, V.M.; Wong, D.T. The Preferences of Adult Outpatients in Medical or Dental Care Settings for Giving Saliva, Urine or Blood for Clinical Testing. J. Am. Dent. Assoc. 2008, 139, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Pichini, S.; Altieri, I.; Zuccaro, P.; Pacifici, R. Drug Monitoring in Nonconventional Biological Fluids and Matrices. Clin. Pharmacokinet. 1996, 30, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Kiang, T.K.; Schmitt, V.; Ensom, M.H.; Chua, B.; Hafeli, U.O. Therapeutic Drug Monitoring in Interstitial Fluid: A Feasibility Study using a Comprehensive Panel of Drugs. J. Pharm. Sci. 2012, 101, 4642–4652. [Google Scholar] [CrossRef] [PubMed]
- Hafeli, U.O.; Ensom, M.H.; Kiang, T.K.; Stoeber, B.; Chua, B.A.; Pudek, M.; Schmitt, V. Comparison of Vancomycin Concentrations in Blood and Interstitial Fluid: A Possible Model for Less Invasive Therapeutic Drug Monitoring. Clin. Chem. Lab. Med. 2011, 49, 2123–2125. [Google Scholar] [CrossRef] [PubMed]
- Ranamukhaarachchi, S.A.; Padeste, C.; Dubner, M.; Hafeli, U.O.; Stoeber, B.; Cadarso, V.J. Integrated Hollow Microneedle-Optofluidic Biosensor for Therapeutic Drug Monitoring in Sub-Nanoliter Volumes. Sci. Rep. 2016, 6, 29075. [Google Scholar] [CrossRef] [PubMed]
- Ensom, M.H.; Davis, G.A.; Cropp, C.D.; Ensom, R.J. Clinical Pharmacokinetics in the 21st Century. Does the Evidence Support Definitive Outcomes? Clin. Pharmacokinet. 1998, 34, 265–279. [Google Scholar] [CrossRef] [PubMed]
- DiPiro, J.; Blouin, R.; Pruemer, J.; Spruill, W. Concepts in Clinical Pharmacokinetics: A Self-Instructional Course, 2nd ed.; American Society of Health-System Pharmacists Inc.: Bethesda, MD, USA, 1996; p. 200. [Google Scholar]
- Kiang, T.K.; Ensom, M.H. Therapeutic Drug Monitoring of Mycophenolate in Adult Solid Organ Transplant Patients: An Update. Expert Opin. Drug Metab. Toxicol. 2016, 12, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Kiang, T.K.; Ensom, M.H. A Comprehensive Review on the Predictive Performance of the Sheiner-Tozer and Derivative Equations for the Correction of Phenytoin Concentrations. Ann. Pharmacother. 2016, 50, 311–325. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Kiang, T.K.; Bring, P.; Ensom, M.H. Predictive Performance of the Winter-Tozer and Derivative Equations for Estimating Free Phenytoin Concentration. Can. J. Hosp. Pharm. 2016, 69, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Kiang, T.K.; Sherwin, C.M.; Spigarelli, M.G.; Ensom, M.H. Fundamentals of Population Pharmacokinetic Modelling: Modelling and Software. Clin. Pharmacokinet. 2012, 51, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Sherwin, C.M.; Kiang, T.K.; Spigarelli, M.G.; Ensom, M.H. Fundamentals of Population Pharmacokinetic Modelling: Validation Methods. Clin. Pharmacokinet. 2012, 51, 573–590. [Google Scholar] [CrossRef] [PubMed]
- Bring, P.; Ensom, M.H. Does Oxcarbazepine Warrant Therapeutic Drug Monitoring? A Critical Review. Clin. Pharmacokinet. 2008, 47, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Paquette, V.; Levine, M.; Ensom, M.H. Levetiracetam Clinical Pharmacokinetic Monitoring in Pediatric Patients with Epilepsy. Clin. Pharmacokinet. 2017. [Google Scholar] [CrossRef] [PubMed]
- Kuo, I.F.; Ensom, M.H. Role of Therapeutic Drug Monitoring of Voriconazole in the Treatment of Invasive Fungal Infections. Can. J. Hosp. Pharm. 2009, 62, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.; Mabasa, V.H.; Chow, I.; Ensom, M.H. Systematic Review of Efficacy, Pharmacokinetics, and Administration of Intraventricular Vancomycin in Adults. Neurocrit. Care 2014, 20, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Stenton, S.B.; Partovi, N.; Ensom, M.H. Sirolimus: The Evidence for Clinical Pharmacokinetic Monitoring. Clin. Pharmacokinet. 2005, 44, 769–786. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.C.; Leung, M.; Wright, A.J.; Ensom, M.H. Clinical Pharmacokinetic Monitoring of Leflunomide in Renal Transplant Recipients with BK Virus Reactivation: A Review of the Literature. Clin. Pharmacokinet. 2017, 56, 1015–1031. [Google Scholar] [CrossRef] [PubMed]
- Kendrick, J.G.; Carr, R.R.; Ensom, M.H. Pharmacokinetics and Drug Dosing in Obese Children. J. Pediatr. Pharmacol. Ther. 2010, 15, 94–109. [Google Scholar] [PubMed]
- Strong, D.K.; Lai, A.; Primmett, D.; White, C.T.; Lirenman, D.S.; Carter, J.E.; Hurley, R.M.; Virji, M.; Ensom, M.H. Limited Sampling Strategy for Cyclosporine (Neoral) Area under the Curve Monitoring in Pediatric Kidney Transplant Recipients. Pediatr. Transplant. 2005, 9, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Teng, J.F.; Mabasa, V.H.; Ensom, M.H. The Role of Therapeutic Drug Monitoring of Imatinib in Patients with Chronic Myeloid Leukemia and Metastatic or Unresectable Gastrointestinal Stromal Tumors. Ther. Drug Monit. 2012, 34, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Schwenger, E.; Dumontet, J.; Ensom, M.H. Does Olanzapine Warrant Clinical Pharmacokinetic Monitoring in Schizophrenia? Clin. Pharmacokinet. 2011, 50, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Seto, K.; Dumontet, J.; Ensom, M.H. Risperidone in Schizophrenia: Is there a Role for Therapeutic Drug Monitoring? Ther. Drug Monit. 2011, 33, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Raju, K.S.; Taneja, I.; Singh, S.P.; Wahajuddin. Utility of Noninvasive Biomatrices in Pharmacokinetic Studies. Biomed. Chromatogr. 2013, 27, 1354–1366. [Google Scholar] [CrossRef] [PubMed]
- Haeckel, R.; Hanecke, P. The Application of Saliva, Sweat and Tear Fluid for Diagnostic Purposes. Ann. Biol. Clin. 1993, 51, 903–910. [Google Scholar]
- Aps, J.K.; Martens, L.C. Review: The Physiology of Saliva and Transfer of Drugs into Saliva. Forensic Sci. Int. 2005, 150, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Nunes, L.A.; Mussavira, S.; Bindhu, O.S. Clinical and Diagnostic Utility of Saliva as a Non-Invasive Diagnostic Fluid: A Systematic Review. Biochem. Med. 2015, 25, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Mullangi, R.; Agrawal, S.; Srinivas, N.R. Measurement of Xenobiotics in Saliva: Is Saliva an Attractive Alternative Matrix? Case Studies and Analytical Perspectives. Biomed. Chromatogr. 2009, 23, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Anizan, S.; Huestis, M.A. The Potential Role of Oral Fluid in Antidoping Testing. Clin. Chem. 2014, 60, 307–322. [Google Scholar] [CrossRef] [PubMed]
- Haeckel, R. Factors Influencing the saliva/plasma Ratio of Drugs. Ann. N. Y. Acad. Sci. 1993, 694, 128–142. [Google Scholar] [CrossRef] [PubMed]
- Levy, G.; Ellis, E.F.; Koysooko, R. Indirect Plasma-Theophylline Monitoring in Asthmatic Children by Determination of Theophylline Concentration in Saliva. Pediatrics 1974, 53, 873–876. [Google Scholar] [PubMed]
- Jusko, W.J.; Gerbracht, L.; Golden, L.H.; Koup, J.R. Digoxin Concentrations in Serum and Saliva. Res. Commun. Chem. Pathol. Pharmacol. 1975, 10, 189–192. [Google Scholar] [PubMed]
- Neu, C.; DiMascio, A.; Williams, D. Saliva Lithium Levels: Clinical Applications. Am. J. Psychiatry 1975, 132, 66–68. [Google Scholar] [PubMed]
- McAuliffe, J.J.; Sherwin, A.L.; Leppik, I.E.; Fayle, S.A.; Pippenger, C.E. Salivary Levels of Anticonvulsants: A Practical Approach to Drug Monitoring. Neurology 1977, 27, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Kiang, T.K.; Ensom, M.H. A Qualitative Review on the Pharmacokinetics of Antibiotics in Saliva: Implications on Clinical Pharmacokinetic Monitoring in Humans. Clin. Pharmacokinet. 2016, 55, 313–358. [Google Scholar] [CrossRef] [PubMed]
- Patsalos, P.N.; Berry, D.J. Therapeutic Drug Monitoring of Antiepileptic Drugs by use of Saliva. Ther. Drug Monit. 2013, 35, 4–29. [Google Scholar] [CrossRef] [PubMed]
- Ter Heine, R.; Beijnen, J.H.; Huitema, A.D. Bioanalytical Issues in Patient-Friendly Sampling Methods for Therapeutic Drug Monitoring: Focus on Antiretroviral Drugs. Bioanalysis 2009, 1, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Pichini, S.; Papaseit, E.; Joya, X.; Vall, O.; Farre, M.; Garcia-Algar, O.; de laTorre, R. Pharmacokinetics and Therapeutic Drug Monitoring of Psychotropic Drugs in Pediatrics. Ther. Drug Monit. 2009, 31, 283–318. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, B.; Morgan, T.J.; Cohen, J. Interstitium: The Next Diagnostic and Therapeutic Platform in Critical Illness. Crit. Care Med. 2010, 38, S630–S636. [Google Scholar] [CrossRef] [PubMed]
- Wiig, H.; Swartz, M.A. Interstitial Fluid and Lymph Formation and Transport: Physiological Regulation and Roles in Inflammation and Cancer. Physiol. Rev. 2012, 92, 1005–1060. [Google Scholar] [CrossRef] [PubMed]
- Poulin, P. Drug Distribution to Human Tissues: Prediction and Examination of the Basic Assumption in in Vivo Pharmacokinetics-Pharmacodynamics (PK/PD) Research. J. Pharm. Sci. 2015, 104, 2110–2118. [Google Scholar] [CrossRef] [PubMed]
- Poulin, P. A Paradigm Shift in Pharmacokinetic-Pharmacodynamic (PKPD) Modeling: Rule of Thumb for Estimating Free Drug Level in Tissue Compared with Plasma to Guide Drug Design. J. Pharm. Sci. 2015, 104, 2359–2368. [Google Scholar] [CrossRef] [PubMed]
- Poulin, P.; Haddad, S. Advancing Prediction of Tissue Distribution and Volume of Distribution of Highly Lipophilic Compounds from a Simplified Tissue-Composition-Based Model as a Mechanistic Animal Alternative Method. J. Pharm. Sci. 2012, 101, 2250–2261. [Google Scholar] [CrossRef] [PubMed]
- Poulin, P.; Schoenlein, K.; Theil, F.P. Prediction of Adipose Tissue: Plasma Partition Coefficients for Structurally Unrelated Drugs. J. Pharm. Sci. 2001, 90, 436–447. [Google Scholar] [CrossRef]
- Konings, I.R.; Sleijfer, S.; Mathijssen, R.H.; de Bruijn, P.; Ghobadi Moghaddam-Helmantel, I.M.; van Dam, L.M.; Wiemer, E.A.; Verweij, J.; Loos, W.J. Increasing Tumoral 5-Fluorouracil Concentrations during a 5-Day Continuous Infusion: A Microdialysis Study. Cancer Chemother. Pharmacol. 2011, 67, 1055–1062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konings, I.R.; Engels, F.K.; Sleijfer, S.; Verweij, J.; Wiemer, E.A.; Loos, W.J. Application of Prolonged Microdialysis Sampling in Carboplatin-Treated Cancer Patients. Cancer Chemother. Pharmacol. 2009, 64, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Muller, M.; Mader, R.M.; Steiner, B.; Steger, G.G.; Jansen, B.; Gnant, M.; Helbich, T.; Jakesz, R.; Eichler, H.G.; Blochl-Daum, B. 5-Fluorouracil Kinetics in the Interstitial Tumor Space: Clinical Response in Breast Cancer Patients. Cancer Res. 1997, 57, 2598–2601. [Google Scholar] [PubMed]
- Muller, M.; Brunner, M.; Schmid, R.; Mader, R.M.; Bockenheimer, J.; Steger, G.G.; Steiner, B.; Eichler, H.G.; Blochl-Daum, B. Interstitial Methotrexate Kinetics in Primary Breast Cancer Lesions. Cancer Res. 1998, 58, 2982–2985. [Google Scholar] [PubMed]
- Blochl-Daum, B.; Muller, M.; Meisinger, V.; Eichler, H.G.; Fassolt, A.; Pehamberger, H. Measurement of Extracellular Fluid Carboplatin Kinetics in Melanoma Metastases with Microdialysis. Br. J. Cancer 1996, 73, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.F.; Siebert, G.A.; Anissimov, Y.G.; Smithers, B.M.; Doubrovsky, A.; Anderson, C.D.; Roberts, M.S. Microdialysis and Response during Regional Chemotherapy by Isolated Limb Infusion of Melphalan for Limb Malignancies. Br. J. Cancer 2001, 85, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Quist, S.R.; Quist, J.; Birkenmaier, J.; Stauch, T.; Gollnick, H.P. Pharmacokinetic Profile of Methotrexate in Psoriatic Skin Via the Oral Or Subcutaneous Route using Dermal Microdialysis Showing Higher Methotrexate Bioavailability in Psoriasis Plaques than in Non-Lesional Skin. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1537–1543. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Gallo, J.M. In Vivo Microdialysis for PK and PD Studies of Anticancer Drugs. AAPS J. 2005, 7, E659–E667. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.; Gallo, J.M. Application of Microdialysis to Characterize Drug Disposition in Tumors. Adv. Drug Deliv. Rev. 2000, 45, 243–253. [Google Scholar] [CrossRef]
- Roberts, J.A.; Udy, A.A.; Jarrett, P.; Wallis, S.C.; Hope, W.W.; Sharma, R.; Kirkpatrick, C.M.; Kruger, P.S.; Roberts, M.S.; Lipman, J. Plasma and Target-Site Subcutaneous Tissue Population Pharmacokinetics and Dosing Simulations of Cefazolin in Post-Trauma Critically Ill Patients. J. Antimicrob. Chemother. 2015, 70, 1495–1502. [Google Scholar] [CrossRef] [PubMed]
- Tegeder, I.; Schmidtko, A.; Brautigam, L.; Kirschbaum, A.; Geisslinger, G.; Lotsch, J. Tissue Distribution of Imipenem in Critically Ill Patients. Clin. Pharmacol. Ther. 2002, 71, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Dahyot, C.; Marchand, S.; Bodin, M.; Debeane, B.; Mimoz, O.; Couet, W. Application of Basic Pharmacokinetic Concepts to Analysis of Microdialysis Data: Illustration with Imipenem Muscle Distribution. Clin. Pharmacokinet. 2008, 47, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Varghese, J.M.; Jarrett, P.; Wallis, S.C.; Boots, R.J.; Kirkpatrick, C.M.; Lipman, J.; Roberts, J.A. Are Interstitial Fluid Concentrations of Meropenem Equivalent to Plasma Concentrations in Critically Ill Patients Receiving Continuous Renal Replacement Therapy? J. Antimicrob. Chemother. 2015, 70, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Housman, S.T.; Bhalodi, A.A.; Shepard, A.; Nugent, J.; Nicolau, D.P. Vancomycin Tissue Pharmacokinetics in Patients with Lower-Limb Infections Via in Vivo Microdialysis. J. Am. Podiatr. Med. Assoc. 2015, 105, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Hamada, Y.; Kuti, J.L.; Nicolau, D.P. Vancomycin Serum Concentrations do Not Adequately Predict Tissue Exposure in Diabetic Patients with Mild to Moderate Limb Infections. J. Antimicrob. Chemother. 2015, 70, 2064–2067. [Google Scholar] [CrossRef] [PubMed]
- Skhirtladze, K.; Hutschala, D.; Fleck, T.; Thalhammer, F.; Ehrlich, M.; Vukovich, T.; Muller, M.; Tschernko, E.M. Impaired Target Site Penetration of Vancomycin in Diabetic Patients Following Cardiac Surgery. Antimicrob. Agents Chemother. 2006, 50, 1372–1375. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Inagaki, Y.; Kobuchi, S.; Takada, K.; Sakaeda, T. Therapeutic Drug Monitoring of Vancomycin in Dermal Interstitial Fluid using Dissolving Microneedles. Int. J. Med. Sci. 2016, 13, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Linder, C.; Andersson, M.; Wide, K.; Beck, O.; Pohanka, A. A LC–MS/MS Method for Therapeutic Drug Monitoring of Carbamazepine, Lamotrigine and Valproic Acid in DBS. Bioanalysis 2015, 7, 2031–2039. [Google Scholar] [CrossRef] [PubMed]
- Stahle, L.; Alm, C.; Ekquist, B.; Lundquist, B.; Tomson, T. Monitoring Free Extracellular Valproic Acid by Microdialysis in Epileptic Patients. Ther. Drug Monit. 1996, 18, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Lindberger, M.; Tomson, T.; Stahle, L. Validation of Microdialysis Sampling for Subcutaneous Extracellular Valproic Acid in Humans. Ther. Drug Monit. 1998, 20, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Lindberger, M.; Tomson, T.; Wallstedt, L.; Stahle, L. Distribution of Valproate to Subdural Cerebrospinal Fluid, Subcutaneous Extracellular Fluid, and Plasma in Humans: A Microdialysis Study. Epilepsia 2001, 42, 256–261. [Google Scholar] [PubMed]
- Lindberger, M.; Tomson, T.; Stahle, L. Unbound Valproate Fraction in Plasma and Subcutaneous Microdialysate in Steady State and After a Single Dose in Humans. Ther. Drug Monit. 2003, 25, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Lindberger, M.; Tomson, T.; Lars, S. Microdialysis Sampling of Carbamazepine, Phenytoin and Phenobarbital in Subcutaneous Extracellular Fluid and Subdural Cerebrospinal Fluid in Humans: An In Vitro and In Vivo Study of Adsorption to the Sampling Device. Pharmacol. Toxicol. 2002, 91, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Lindberger, M.; Tomson, T.; Ohman, I.; Wallstedt, L.; Stahle, L. Estimation of Topiramate in Subdural Cerebrospinal Fluid, Subcutaneous Extracellular Fluid, and Plasma: A Single Case Microdialysis Study. Epilepsia 1999, 40, 800–802. [Google Scholar] [CrossRef] [PubMed]
- Boschmann, M.; Nussberger, J.; Engeli, S.; Danser, A.H.; Yeh, C.M.; Prescott, M.F.; Dahlke, M.; Jordan, J. Aliskiren Penetrates Adipose and Skeletal Muscle Tissue and Reduces Renin-Angiotensin System Activity in Obese Hypertensive Patients. J. Hypertens. 2012, 30, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Stahle, L.; Arner, P.; Ungerstedt, U. Drug Distribution Studies with Microdialysis. III: Extracellular Concentration of Caffeine in Adipose Tissue in Man. Life Sci. 1991, 49, 1853–1858. [Google Scholar] [CrossRef]
- Stetina, P.M.; Madai, B.; Kulemann, V.; Kirch, W.; Joukhadar, C. Pharmacokinetics of Scopolamine in Serum and Subcutaneous Adipose Tissue in Healthy Volunteers. Int. J. Clin. Pharmacol. Ther. 2005, 43, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Groenendaal, W.; von Basum, G.; Schmidt, K.A.; Hilbers, P.A.; van Riel, N.A. Quantifying the Composition of Human Skin for Glucose Sensor Development. J. Diabetes Sci. Technol. 2010, 4, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Ranamukhaarachchi, S. Skin Mechanics, Intradermal Delivery and Biosensing with Hollow Metallic Microneedles. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, December 2016. [Google Scholar]
- Wang, P.M.; Cornwell, M.; Prausnitz, M.R. Minimally Invasive Extraction of Dermal Interstitial Fluid for Glucose Monitoring using Microneedles. Diabetes Technol. Ther. 2005, 7, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, K.; Hirota, Y.; Hashimoto, N.; Ogawa, W.; Sato, T.; Okada, S.; Hagino, K.; Asakura, Y.; Kikkawa, Y.; Kojima, J.; et al. A Minimally Invasive System for Glucose Area Under the Curve Measurement using Interstitial Fluid Extraction Technology: Evaluation of the Accuracy and Usefulness with Oral Glucose Tolerance Tests in Subjects with and without Diabetes. Diabetes Technol. Ther. 2012, 14, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; Mooney, K.; Caffarel-Salvador, E.; Torrisi, B.M.; Eltayib, E.; McElnay, J.C. Microneedle-Mediated Minimally Invasive Patient Monitoring. Ther. Drug Monit. 2014, 36, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Caffarel-Salvador, E.; Brady, A.J.; Eltayib, E.; Meng, T.; Alonso-Vicente, A.; Gonzalez-Vazquez, P.; Torrisi, B.M.; Vicente-Perez, E.M.; Mooney, K.; Jones, D.S.; et al. Hydrogel-Forming Microneedle Arrays Allow Detection of Drugs and Glucose in Vivo: Potential for use in Diagnosis and Therapeutic Drug Monitoring. PLoS ONE 2015, 10, e0145644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romanyuk, A.V.; Zvezdin, V.N.; Samant, P.; Grenader, M.I.; Zemlyanova, M.; Prausnitz, M.R. Collection of Analytes from Microneedle Patches. Anal. Chem. 2014, 86, 10520–10523. [Google Scholar] [CrossRef] [PubMed]
- Mukerjee, E.V.; Collins, S.D.; Isseroff, R.R.; Smith, R.L. Microneedle Array for Transdermal Biological Fluid Extraction and in Situ Analysis. Sens. Actuators A Phys. 2004, 114, 267–275. [Google Scholar] [CrossRef]
- Strambini, L.M.; Longo, A.; Scarano, S.; Prescimone, T.; Palchetti, I.; Minunni, M.; Giannessi, D.; Barillaro, G. Self-Powered Microneedle-Based Biosensors for Pain-Free High-Accuracy Measurement of Glycaemia in Interstitial Fluid. Biosens. Bioelectron. 2015, 66, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Jina, A.; Tierney, M.J.; Tamada, J.A.; McGill, S.; Desai, S.; Chua, B.; Chang, A.; Christiansen, M. Design, Development, and Evaluation of a Novel Microneedle Array-Based Continuous Glucose Monitor. J. Diabetes Sci. Technol. 2014, 8, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Zheng, M.; Yu, X.; Than, A.; Seeni, R.Z.; Kang, R.; Tian, J.; Khanh, D.P.; Liu, L.; Chen, P.; et al. A Swellable Microneedle Patch to Rapidly Extract Skin Interstitial Fluid for Timely Metabolic Analysis. Adv. Mater. 2017, 37. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, S.; Fienbork, D.; Flounders, A.W.; Liepmann, D. In-Device Enzyme Immobilization: Wafer-Level Fabrication of an Integrated Glucose Sensor. Sens. Actuators B Chem. 2004, 99, 163–173. [Google Scholar] [CrossRef]
- Chua, B.; Desai, S.P.; Tierney, M.J.; Tamada, J.A.; Jina, A.N. Effect of Microneedles Shape on Skin Penetration and Minimally Invasive Continuous Glucose Monitoring In Vivo. Sens. Actuators A Phys. 2013, 203, 373–381. [Google Scholar] [CrossRef]
- Heinemann, L.; Glucose Monitoring Study Group. Continuous Glucose Monitoring by Means of the Microdialysis Technique: Underlying Fundamental Aspects. Diabetes Technol. Ther. 2003, 5, 545–561. [Google Scholar] [CrossRef] [PubMed]
- Tiessena, R.G.; Kapteinab, W.A.; Venemaa, K.; Korf, J. Slow Ultrafiltration for Continuous In Vivo Sampling: Application for Glucose and Lactate in Man. Anal. Chim. Acta 1999, 379, 327–335. [Google Scholar] [CrossRef]
- Tamada, J.A.; Bohannon, N.J.; Potts, R.O. Measurement of Glucose in Diabetic Subjects using Noninvasive Transdermal Extraction. Nat. Med. 1995, 1, 1198–1201. [Google Scholar] [CrossRef] [PubMed]
- Kost, J.; Mitragotri, S.; Gabbay, R.A.; Pishko, M.; Langer, R. Transdermal Monitoring of Glucose and Other Analytes using Ultrasound. Nat. Med. 2000, 6, 347–350. [Google Scholar] [CrossRef] [PubMed]
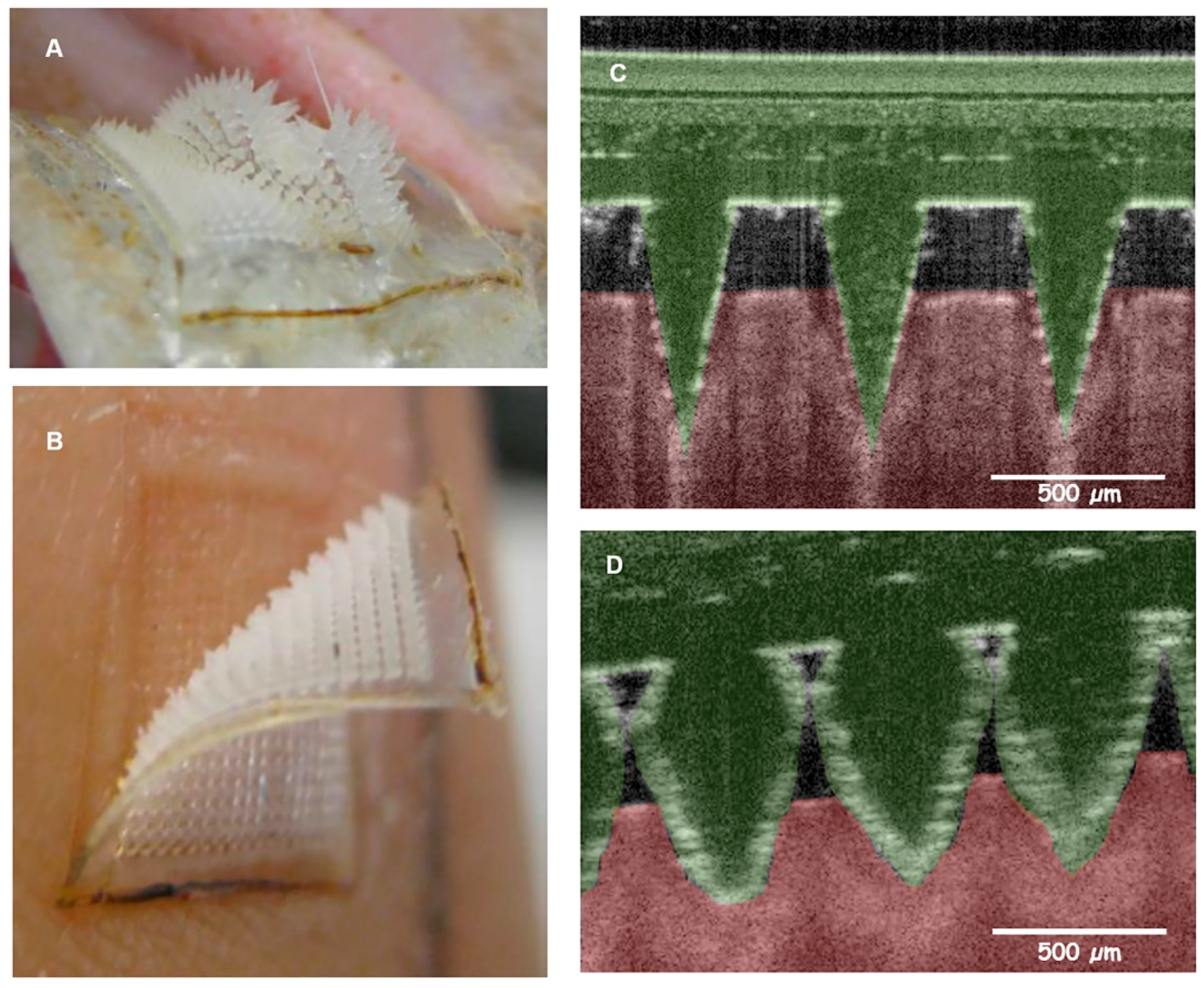
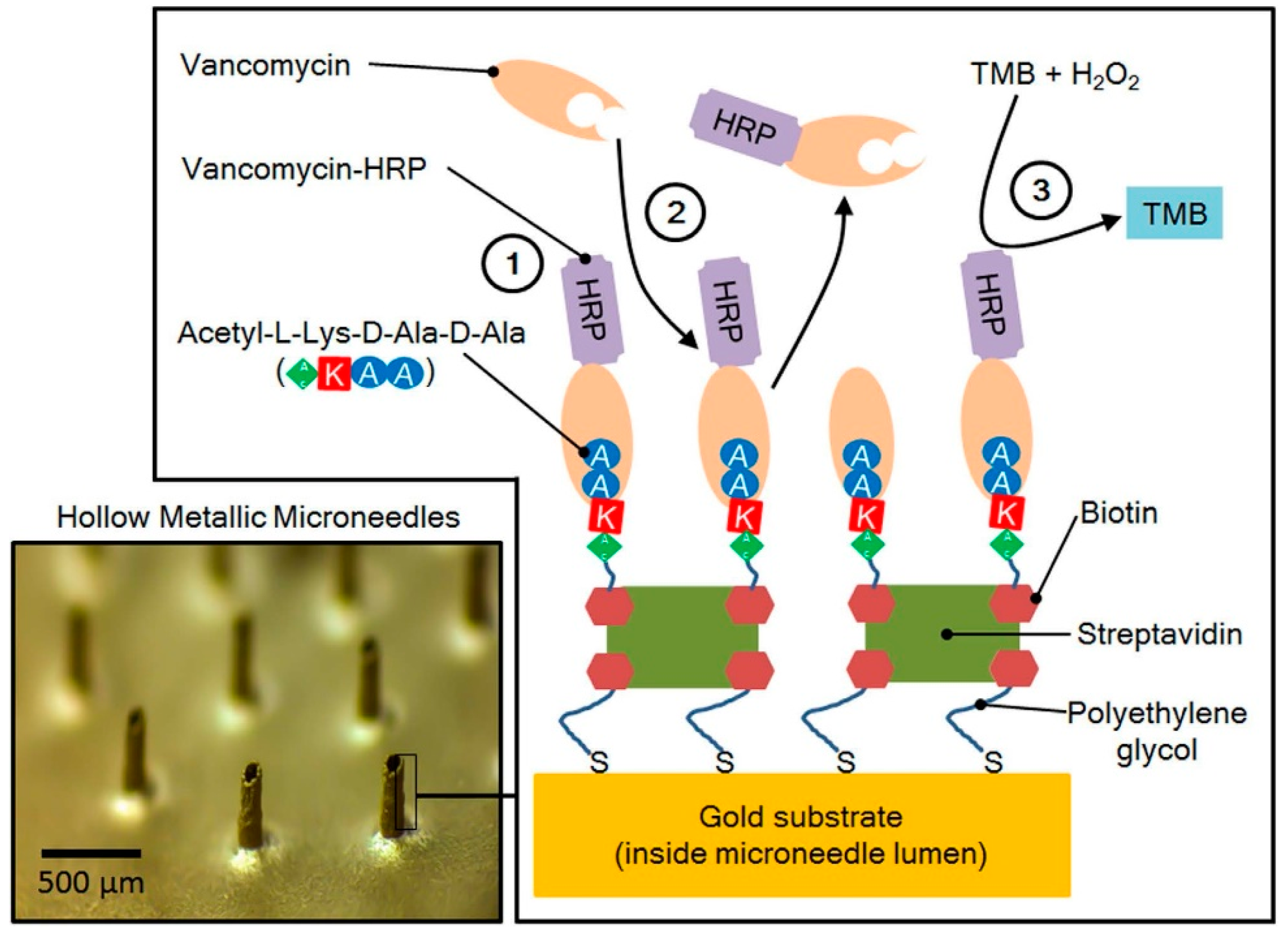
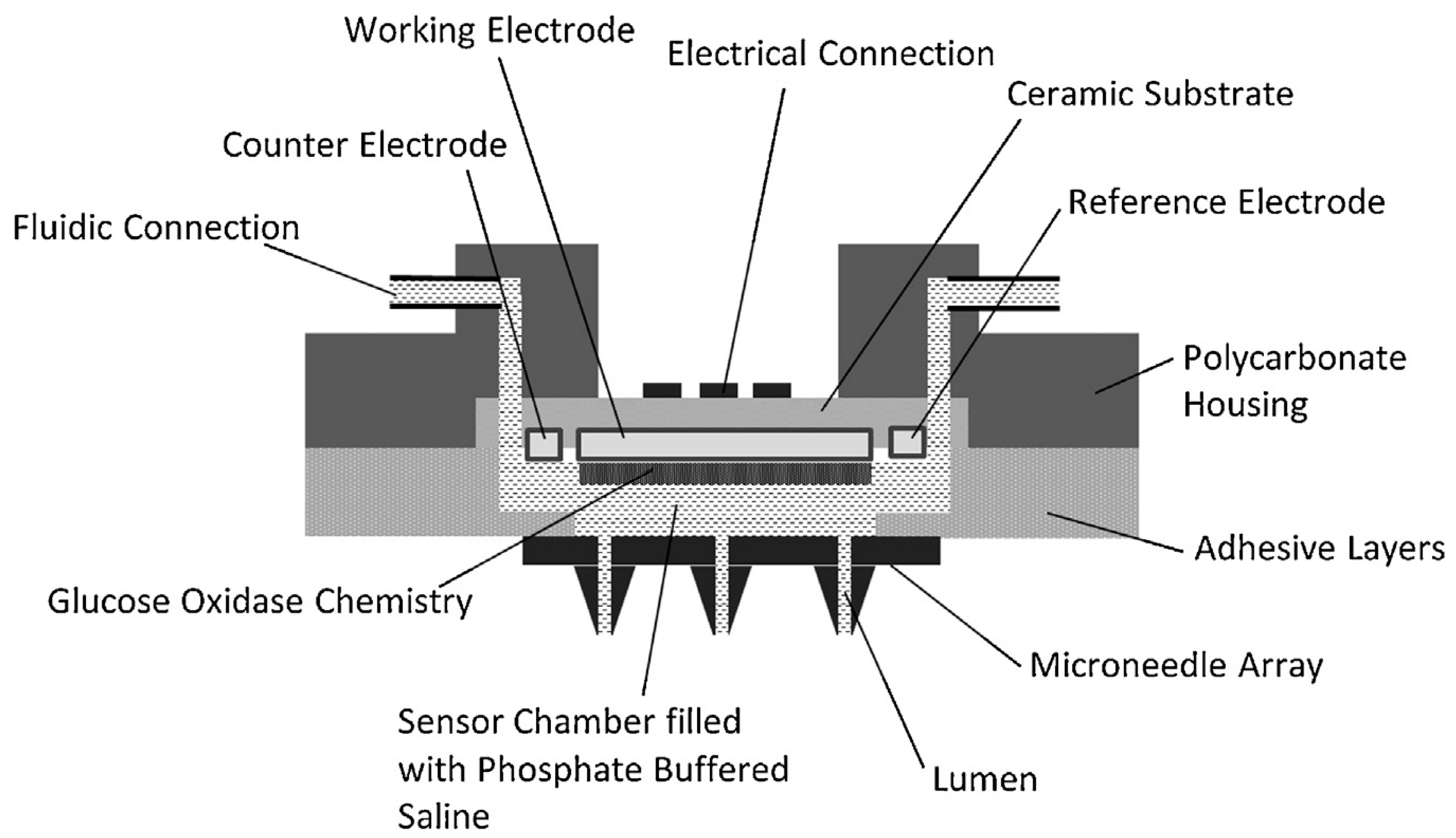
| Microneedle Device Strategy | Description | Indication | Testing Model | ISF Volume Collected/Required |
|---|---|---|---|---|
| ISF extraction and off-device analysis | Glass hollow microneedle (0.7–1.5 mm long) and vacuum-assisted ISF collection [75] | Glucose | Tail vein of rats, finger tips of humans | 1–10 µL |
| Dissolving microneedle “poke” followed by vacuum suction [62] | Vancomycin | Male Wistar rats | 2 µL | |
| Solid microneedle arrays “poked” the skin, and hydrogel “patch” collected ISF [76] | Glucose and sodium ion concentration | Human subjects | <10 µL | |
| Hydrogel forming microneedle array [77,78,79] | Theophylline, caffeine, glucose | Pigs, rats, and human subjects | - | |
| ISF extraction and on-device analysis | Hollow microneedles with integrated ISF collection reservoir [80] | Glucose | Human subject | - |
| Hollow microneedle array integrated with screen-printed enzyme sensor [81] | Glucose | In vitro bench testing | 10 µL | |
| Hollow microneedle integrated with optofluidic sensor [6] | Vancomycin | In vitro bench testing | 0.6 nL | |
| Continuous ISF monitoring | Hollow microneedle integrated with buffer-filled glucose sensor [82] | Glucose | Human subjects | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiang, T.K.L.; Ranamukhaarachchi, S.A.; Ensom, M.H.H. Revolutionizing Therapeutic Drug Monitoring with the Use of Interstitial Fluid and Microneedles Technology. Pharmaceutics 2017, 9, 43. https://doi.org/10.3390/pharmaceutics9040043
Kiang TKL, Ranamukhaarachchi SA, Ensom MHH. Revolutionizing Therapeutic Drug Monitoring with the Use of Interstitial Fluid and Microneedles Technology. Pharmaceutics. 2017; 9(4):43. https://doi.org/10.3390/pharmaceutics9040043
Chicago/Turabian StyleKiang, Tony K.L., Sahan A. Ranamukhaarachchi, and Mary H.H. Ensom. 2017. "Revolutionizing Therapeutic Drug Monitoring with the Use of Interstitial Fluid and Microneedles Technology" Pharmaceutics 9, no. 4: 43. https://doi.org/10.3390/pharmaceutics9040043





