The Role of PXR Genotype and Transporter Expression in the Placental Transport of Lopinavir in Mice
Abstract
:1. Introduction
2. Materials and Methods
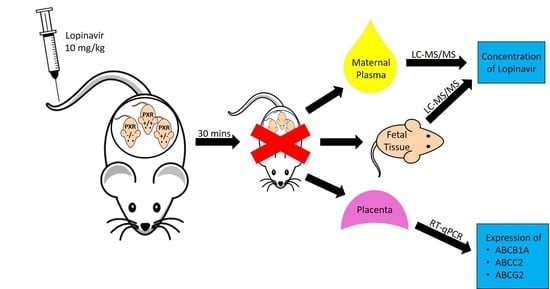
2.1. Animals and Experimental Design
2.2. Analysis of Transporter mRNA Expression
2.3. Lopinavir LC-MS/MS Analysis
2.4. Statistical Analysis
3. Results
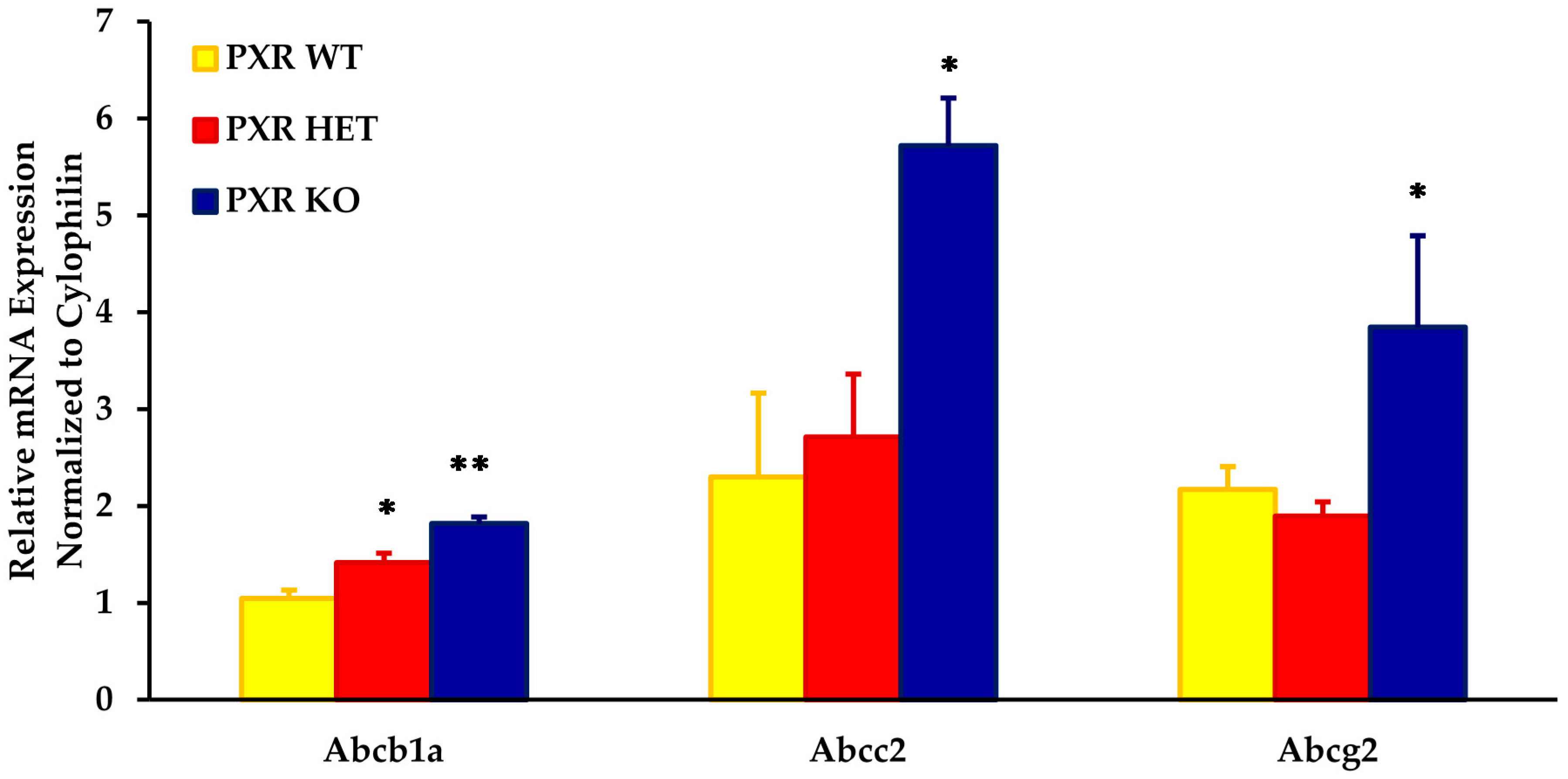
3.1. Impact of Fetal PXR Genotype on Transporter Expression
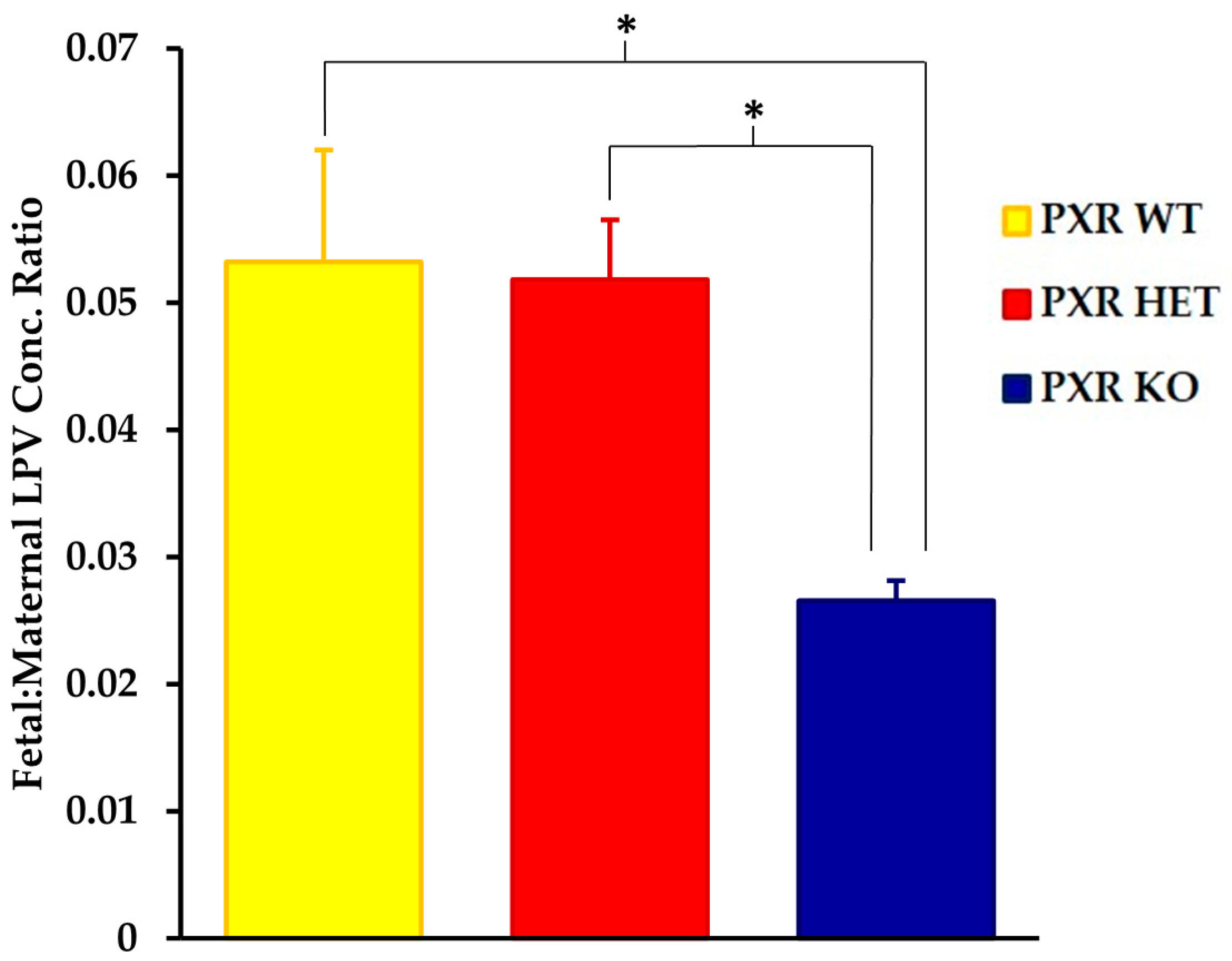
3.2. Impact of Fetal PXR Genotype on Fetal Accumulation of LPV
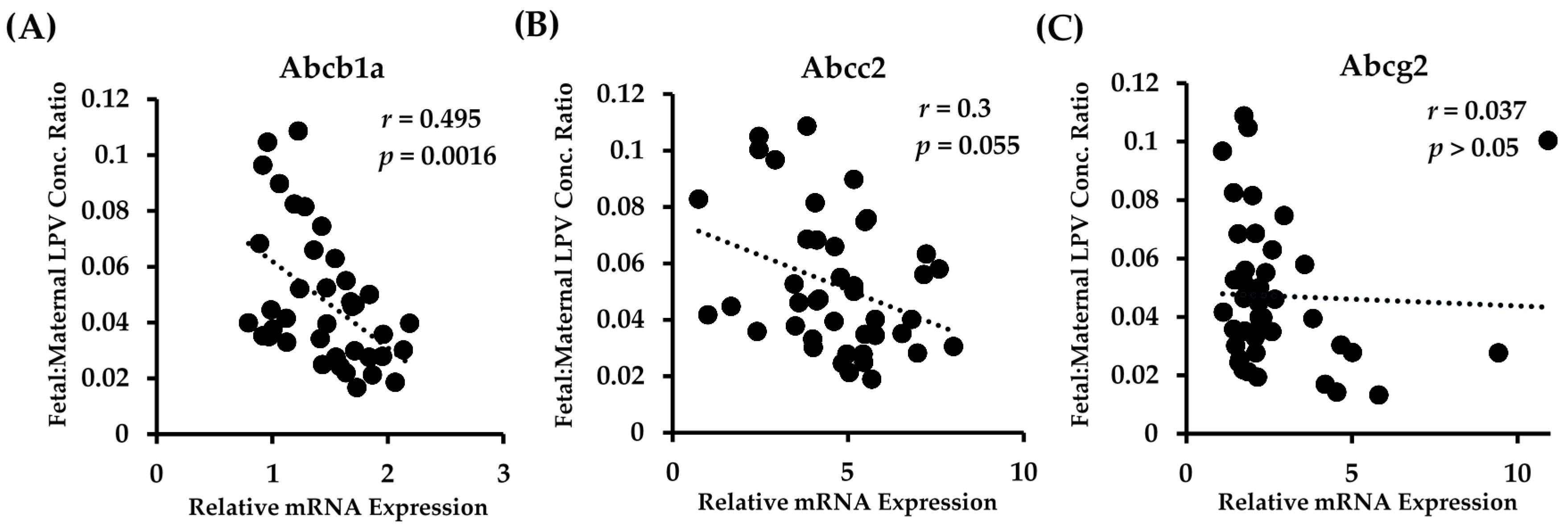
3.3. Relationship of Fetal Drug Accumulation and Transporter Expression
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ABC | ATP Binding Cassette |
| BCRP | Brest Cancer Resistance Protein |
| CNS | Central Nervous System |
| GD | Gestational Day |
| HAART | Highly Active Antiretroviral Therapy |
| HIV | Human Immunodeficiency Virus |
| I.V. | Intravenously |
| LC-MS/MS | Liquid Chromatography-Tandem Mass Spectrometry |
| LPV | Lopinavir |
| MRP | Multidrug Resistance Associated Protein |
| MTCT | Mother-to-Child Transmission |
| PI | Protease Inhibitor |
| PXR | Pregnane X Receptor |
| qRT-PCR | Quantitative Real Time Polymerase Chain Reaction |
References
- HIV. Gov. Available online: https://www.hiv.gov/federal-response/pepfar-global-aids/global-hiv-aids-overview (accessed on 27 September 2017).
- Sturt, A.S.; Dokubo, E.K.; Sint, T.T. Antiretroviral therapy (ART) for treating HIV infection in ART-eligible pregnant women. Cochrane Database Sys. Rev. 2010, 3, CD008440. [Google Scholar] [CrossRef]
- Van Dyke, R.B. Mother-to-child transmission of HIV-1 in the era prior to the availability of combination antiretroviral therapy: The role of drugs of abuse. Life Sci. 2011, 88, 922–925. [Google Scholar] [CrossRef] [PubMed]
- Prouillac, C.; Lecoeur, S. The role of the placenta in fetal exposure to xenobiotics: Importance of membrane transporters and human models for transfer studies. Drug Metab. Dispos. 2010, 38, 1623–1635. [Google Scholar] [CrossRef] [PubMed]
- Syme, M.R.; Paxton, J.W.; Keelan, J.A. Drug transfer and metabolism by the human placenta. Clin. Pharmacokinet. 2004, 43, 487–514. [Google Scholar] [CrossRef] [PubMed]
- Vähäkangas, K.; Myllynen, P. Drug transporters in the human blood–placental barrier. Br. J. Pharmacol. 2009, 158, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Mao, Q. ATP-binding cassette efflux transporters in human placenta. Curr. Pharm. Biotechnol. 2010, 12, 674–685. [Google Scholar] [CrossRef]
- Tomi, M.; Nishimura, T.; Nakashima, E. Mother-to-fetus transfer of antiviral drugs and the involvement of transporters at the placental barrier. J. Pharm. Sci. 2011, 100, 3708–3718. [Google Scholar] [CrossRef] [PubMed]
- Hutson, J.R.; Garcia-Bournissen, F.; Davis, A.; Koren, G. The human placental perfusion model: A systemic review and development of a model to predict in vivo transfer of therapeutic drugs. Clin. Pharmacol. Ther. 2011, 90, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Huisman, M.T.; Smit, J.W.; Wiltshire, H.R.; Hoetelmans, R.M.; Beijnen, J.H.; Schinkel, A.H. P-glycoprotein limits oral availability, brain, and fetal penetration of saquinavir even with high doses of ritonavir. Mol. Pharmacol. 2001, 59, 806–813. [Google Scholar] [PubMed]
- Navér, L.; Albert, J.; Belfrage, E.; Flamholc, L.; Gisslén, M.; Gyllensten, K.; Yilmaz, A. Prophylaxis and treatment of HIV-1 infection in pregnancy: Swedish recommendations 2010. Scand. J. Infect. Dis. 2011, 43, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Baroncelli, S.; Tamburrini, E.; Ravizza, M.; Dalzero, S.; Tibaldi, C.; Ferrazzi, E.; Anzidei, G.; Fiscon, M. Italian Group on Surveillance on Antiretroviral Treatment in Pregnancy. Antiretroviral treatment in pregnancy: A six-year perspective on recent trends in prescription patters, viral load suppression, and pregnancy outcomes. AIDS Patient Care STDS 2009, 23, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Janneh, O.; Jones, E.; Chandler, B.; Owen, A.; Khoo, S.H. Inhibition of P-glycoprotein and multidrug resistance-associated proteins modulates the intracellular concentration of lopinavir in cultured CD4 T cells and primary human lymphocytes. J. Antimicrob. Chemoth. 2007, 60, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Pal, D.; Mitra, A.K. Both P-gp and MRP2 mediate transport of lopinavir, a protease inhibitor. Int. J. Pharm. 2007, 339, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Gulati, A.; Gerk, P.M. Role of placental ABC transporters in antiretroviral therapy during pregnancy. J. Pharm. Sci. 2009, 98, 2317–2335. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.E.; Dintaman, J.M.; Waddell, D.S.; Silverman, J.A. Saquinavir, an HIV protease inhibitor, is transported by P-glycoprotein. J. Pharmacol. Exp. Ther. 1998, 286, 1439–1445. [Google Scholar] [PubMed]
- Van Waterschoot, R.A.; ter Heine, R.; Wagenaar, E.; van der Kruijssen, C.M.; Rooswinkel, R.W.; Huitema, A.D.; Schinkel, A.H. Effects of cytochrome P450 3A (CYP3A) and the drug transporters P-glycoprotein (MDR1/ABCB1) and MRP2 (ABCC2) on the pharmacokinetics of lopinavir. Br. J. Pharmacol. 2010, 160, 1224–1233. [Google Scholar] [CrossRef] [PubMed]
- Marzolini, C.; Rudin, C.; Decosterd, L.A.; Telenti, A.; Schreyer, A.; Biollaz, J. Swiss Mother + Child HIV Cohort Study. Transplacental passage of protease inhibitors at delivery. AIDS 2002, 16, 889–893. [Google Scholar] [CrossRef] [PubMed]
- Mirochnick, M.; Capparelli, E. Pharmacokinetics of antiretrovirals in pregnant women. Clin. Pharmacokinet. 2004, 43, 1071–1087. [Google Scholar] [CrossRef] [PubMed]
- Yeh, R.F.; Rezk, N.L.; Kashuba, A.D.; Dumond, J.B.; Tappouni, H.L.; Tien, H.C.; Patterson, K.B. Genital tract, cord blood, and amniotic fluid exposures of seven antiretroviral drugs during and after pregnancy in human immunodeficiency virus type 1-infected women. Antimicrob. Agents Chemother. 2009, 53, 2367–2374. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Kingdom, J.; Baczyk, D.; Lye, S.J.; Matthews, S.G.; Gibb, W. Expression of the multidrug resistance P-glycoprotein, (ABCB1) in the human placenta decreases with advancing gestation. Placenta 2006, 27, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Gil, S.; Saura, R.; Forestier, F.; Farinotti, R. P-glycoprotein expression of the human placenta during pregnancy. Placenta 2005, 26, 268–270. [Google Scholar] [CrossRef] [PubMed]
- Yeboah, D.; Sun, M.; Kingdom, J.; Baczyk, D.; Lye, S.J.; Matthews, S.G.; Gibb, W. Expression of breast cancer resistance protein (BCRP/ABCG2) in human placenta throughout gestation and at term before and after labor. Can. J. Physiol. Pharmacol. 2006, 84, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- Camus, M.; Deloménie, C.; Didier, N.; Faye, A.; Gil, S.; Dauge, M.C.; Farinotti, R. Increased expression of MDR1 mRNA and P-glycoprotein in placentas from HIV-1 infected women. Placenta 2006, 27, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Kliewer, S.A.; Goodwin, B.; Willson, T.M. The nuclear Pregnane X Receptor: A key regulator of xenobiotic metabolism. Endocr. Rev. 2002, 23, 687–702. [Google Scholar] [CrossRef] [PubMed]
- Teng, S.; Piquette-Miller, M. Regulation of transporters by nuclear hormone receptors: Implications during inflammation. Mol. Pharmaceut. 2008, 5, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Teng, S.; Jekerle, V.; Piquette-Miller, M. Induction of ABCC3 (MRP3) by Pregnane X Receptor activators. Drug Metab. Dispos. 2003, 31, 1296–1299. [Google Scholar] [CrossRef] [PubMed]
- Anapolsky, A.; Teng, S.; Dixit, S.; Piquette-Miller, M. The role of Pregnane X Receptor in 2-acetylaminofluorene-mediated induction of drug transport and metabolizing enzymes in mice. Drug Metab. Dispos. 2006, 34, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.K.; Waxman, D.J. Synthetic drugs and natural products as modulators of constitutive androstane receptor (CAR) and Pregnane X Receptor (PXR). Drug Metab. Rev. 2006, 38, 51–73. [Google Scholar] [CrossRef] [PubMed]
- Kliewer, S.A.; Moore, J.T.; Wade, L.; Staudinger, J.L.; Watson, M.A.; Jones, S.A.; Lehmann, J.M. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell 1998, 92, 73–82. [Google Scholar] [CrossRef]
- Sandanaraj, E.; Lal, S.; Selvarajan, V.; Ooi, L.L.; Wong, Z.W.; Wong, N.S.; Ang, P.C.; Lee, E.J.; Chowbay, B. PXR pharmacogenetics: Association of haplotypes with hepatic CYP3A4 and ABCB1 messenger RNA expression and doxorubicin clearance in Asian breast cancer patients. Clin. Cancer Res. 2008, 14, 7116–7126. [Google Scholar] [CrossRef] [PubMed]
- Schipani, A.; Siccardi, M.; D’Avolio, A.; Baietto, L.; Simiele, M.; Bonora, S.; Rodríguez Novoa, S.; Cuenca, L.; Soriano, V.; Chierakul, N.; et al. Population pharmacokinetic modeling of the association between 63396C->T Pregnane X Receptor polymorphism and unboosted atazanavir clearance. Antimicrob. Agents Chemother. 2010, 54, 5242–5250. [Google Scholar] [CrossRef] [PubMed]
- Gahir, S.S.; Piquette-Miller, M. Gestational and Pregnane X Receptor-mediated regulation of placental ATP-binding cassette drug transporters in mice. Drug Metab. Dispos. 2011, 39, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Daud, A.; Bergman, J.; Bakker, M.; Wang, H.; de Walle, H.; Plosch, T. Pharmacogenetics of drug-induced birth defects; the role of polymorphisms of placental transport proteins. Pharmacogenomics 2014, 15, 1029–1041. [Google Scholar] [CrossRef] [PubMed]
- Teng, S.; Piquette-Miller, M. The involvement of the Pregnane X Receptor in hepatic gene regulation during inflammation in mice. J. Pharmacol. Exp. Ther. 2005, 312, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Anger, G.; Piquette-Miller, M. Mechanisms of reduced maternal and fetal lopinavir exposure in a rat model of gestational diabetes. Drug Metab. Dispos. 2011, 39, 1850–1859. [Google Scholar] [CrossRef] [PubMed]
- Ceccaldi, P.F.; Gavard, L.; Mandelbrot, L.; Rey, E.; Farinotti, R.; Treluyer, J.M.; Gil, S. Functional role of P-glycoprotein and binding protein effect on the placental transfer of lopinavir/ritonavir in the ex vivo human perfusion model. Obstet. Gynecol. Int. 2009, 2009, 726593. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, V.; Piquette-Miller, M. Polyinosinic/Polycytidylic Acid-mediated changes in maternal and fetal disposition of lopinavir in rats. Drug Metab. Dispos. 2015, 43, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Schinkel, A.H.; Mayer, U.; Wagenaar, E.; Mol, C.A.; van Deemter, L.; Smit, J.J.; van der Valk, M.A.; Voordouw, A.C.; Spits, H.; van Tellingen, O.; et al. Normal viability and altered pharmacokinetics in mice lacking mdr1-type (drug-transporting) P-glycoproteins. Proc. Natl. Acad. Sci. USA 1997, 94, 4028–4033. [Google Scholar] [CrossRef] [PubMed]
- Lankas, G.R.; Wise, L.D.; Cartwright, M.E.; Pippert, T.; Umbenhauer, D.R. Placental P-glycoprotein deficiency enhances susceptibility to chemically induced birth defects in mice. Reprod. Toxicol. 1998, 12, 457–463. [Google Scholar] [CrossRef]
- May, K.; Minarikova, V.; Linnemann, K.; Zygmunt, M.; Kroemer, H.K.; Fusch, C.; Siegmund, W. Role of the multidrug transporter proteins ABCB1 and ABCC2 in the placental transport of talinolol in the term human placenta. Drug Metab. Dispos. 2008, 36, 740–744. [Google Scholar] [CrossRef] [PubMed]
- Hahnova-Cygalova, L.; Ceckova, M.; Staud, F. Fetoprotective activity of breast cancer resistance protein (BCRP, ABCG2): Expression and function throughout pregnancy. Drug Metab. Rev. 2011, 43, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.; Rose, J.; Storch, C.H.; Ketabi-Kiyanvash, N.; Sauer, A.; Haefeli, W.E.; Efferth, T. Modulation of human BCRP (ABCG2) activity by anti-HIV drugs. J. Antimicrob. Chemother. 2007, 59, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, J.; Xie, W.; Rosenfeld, J.M.; Barwick, J.L.; Guzelian, P.S.; Evans, R.M. Regulation of a xenobiotic sulfonation cascade by nuclear Pregnane X Receptor (PXR). Prac. Natl. Acad. Sci. USA 2002, 99, 13801–13806. [Google Scholar] [CrossRef] [PubMed]
- Kliewar, S.A.; Lehmann, J.M.; Milburn, M.V.; Willson, T.M. The PPARs and PXRs: Nuclear xenobiotic receptors that define novel hormone signaling pathways. Recent Prog. Horm. Res. 1999, 54, 345–367. [Google Scholar]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gahir, S.S.; Piquette-Miller, M. The Role of PXR Genotype and Transporter Expression in the Placental Transport of Lopinavir in Mice. Pharmaceutics 2017, 9, 49. https://doi.org/10.3390/pharmaceutics9040049
Gahir SS, Piquette-Miller M. The Role of PXR Genotype and Transporter Expression in the Placental Transport of Lopinavir in Mice. Pharmaceutics. 2017; 9(4):49. https://doi.org/10.3390/pharmaceutics9040049
Chicago/Turabian StyleGahir, Sarabjit S., and Micheline Piquette-Miller. 2017. "The Role of PXR Genotype and Transporter Expression in the Placental Transport of Lopinavir in Mice" Pharmaceutics 9, no. 4: 49. https://doi.org/10.3390/pharmaceutics9040049