An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery
Abstract
:1. Introduction
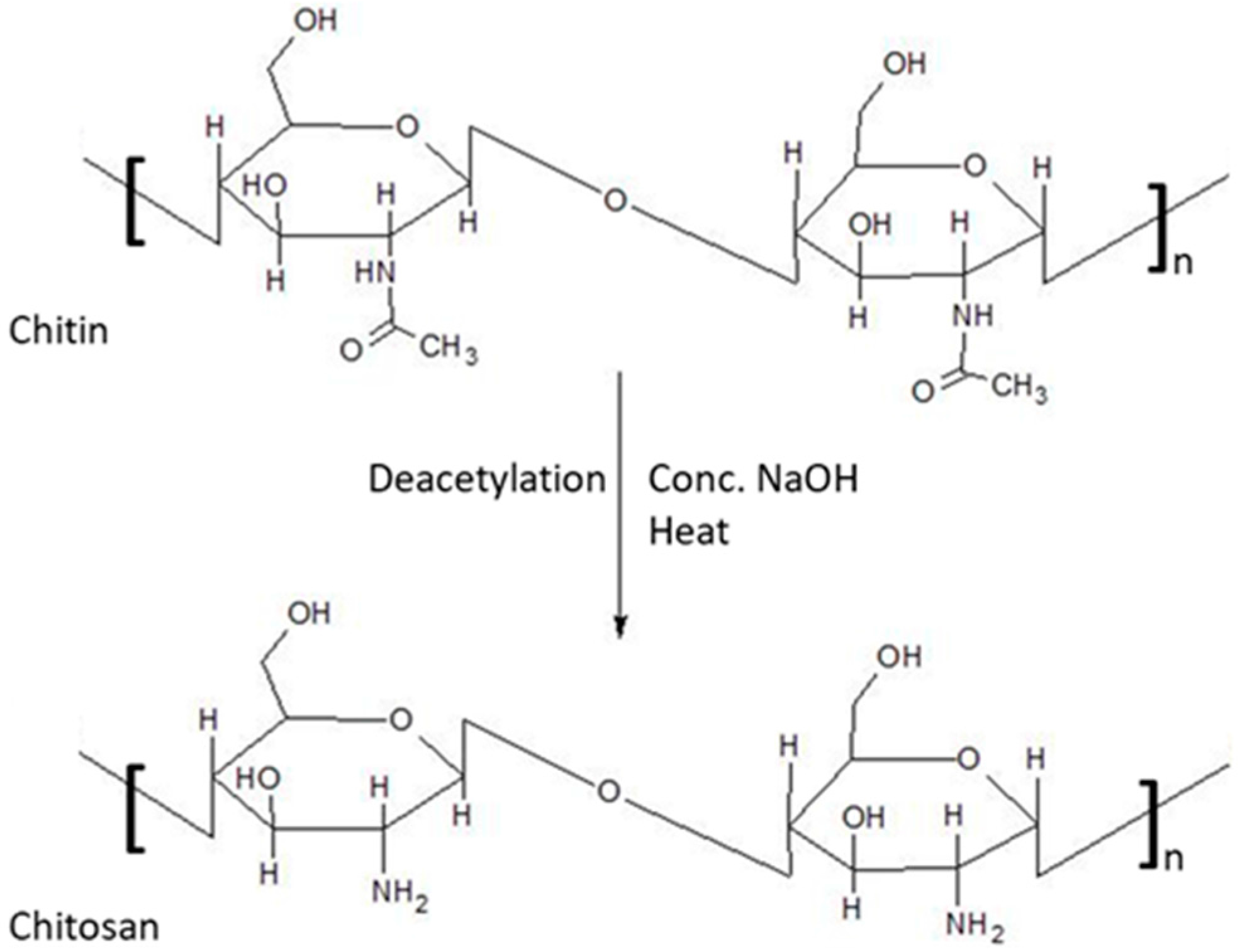
Chitosan
2. Modification of Chitosan
2.1. Physical Modification
2.2. Chemical Modification
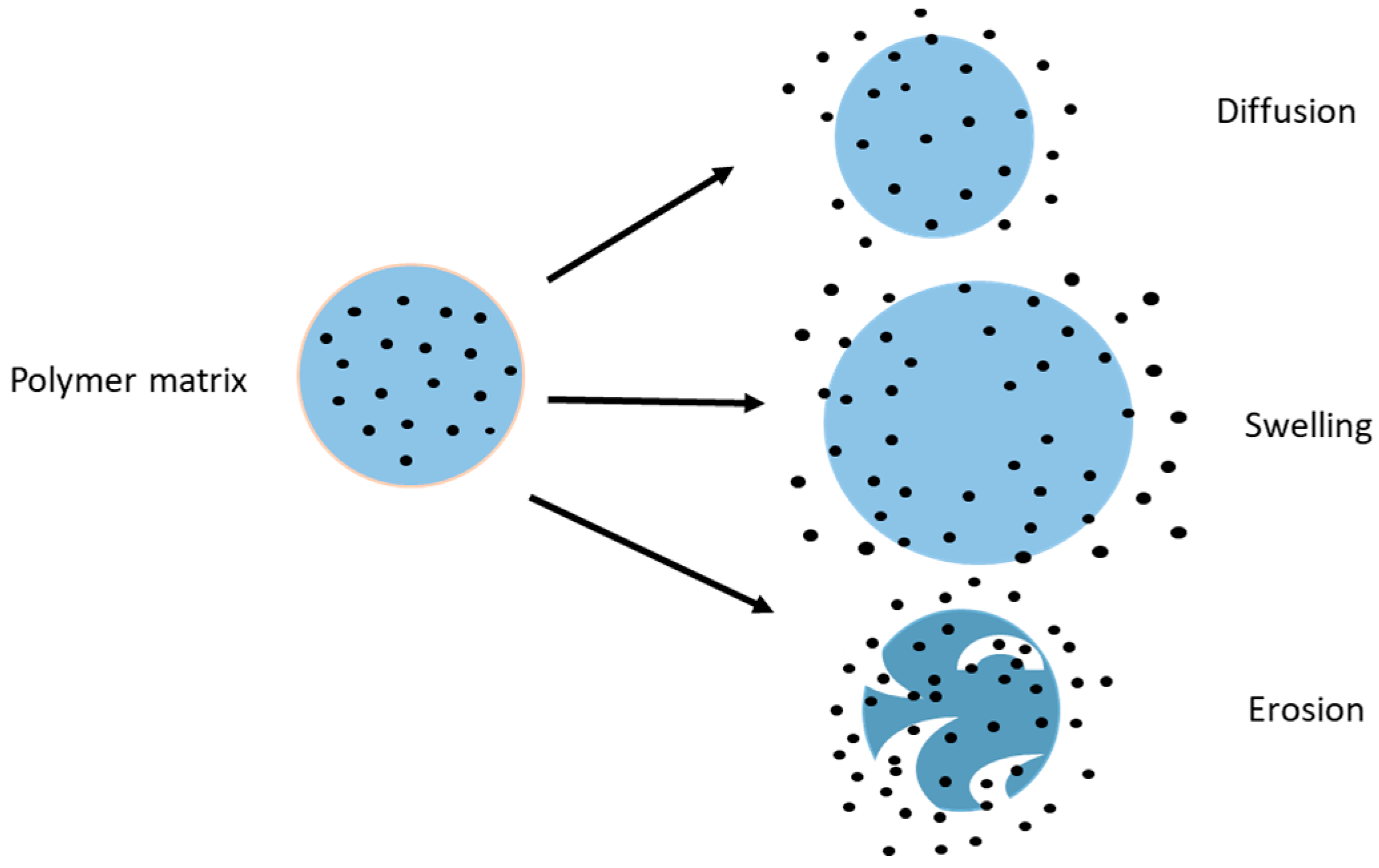
3. Drug Release from Chitosan Nanoparticles
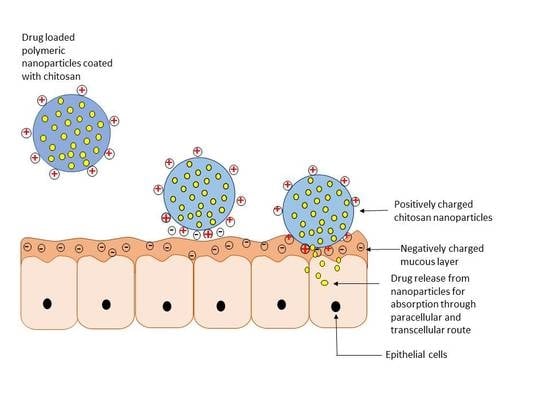
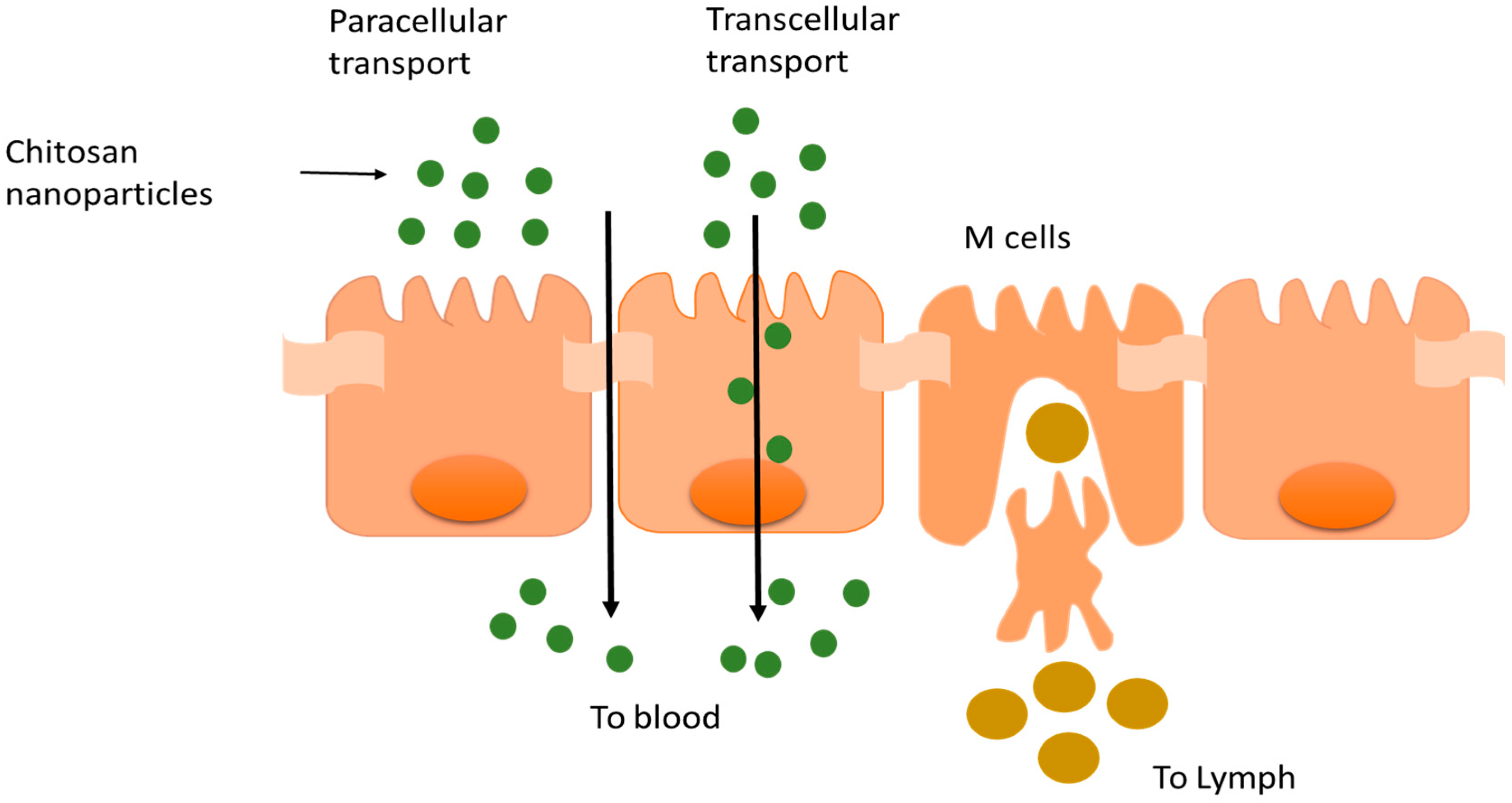
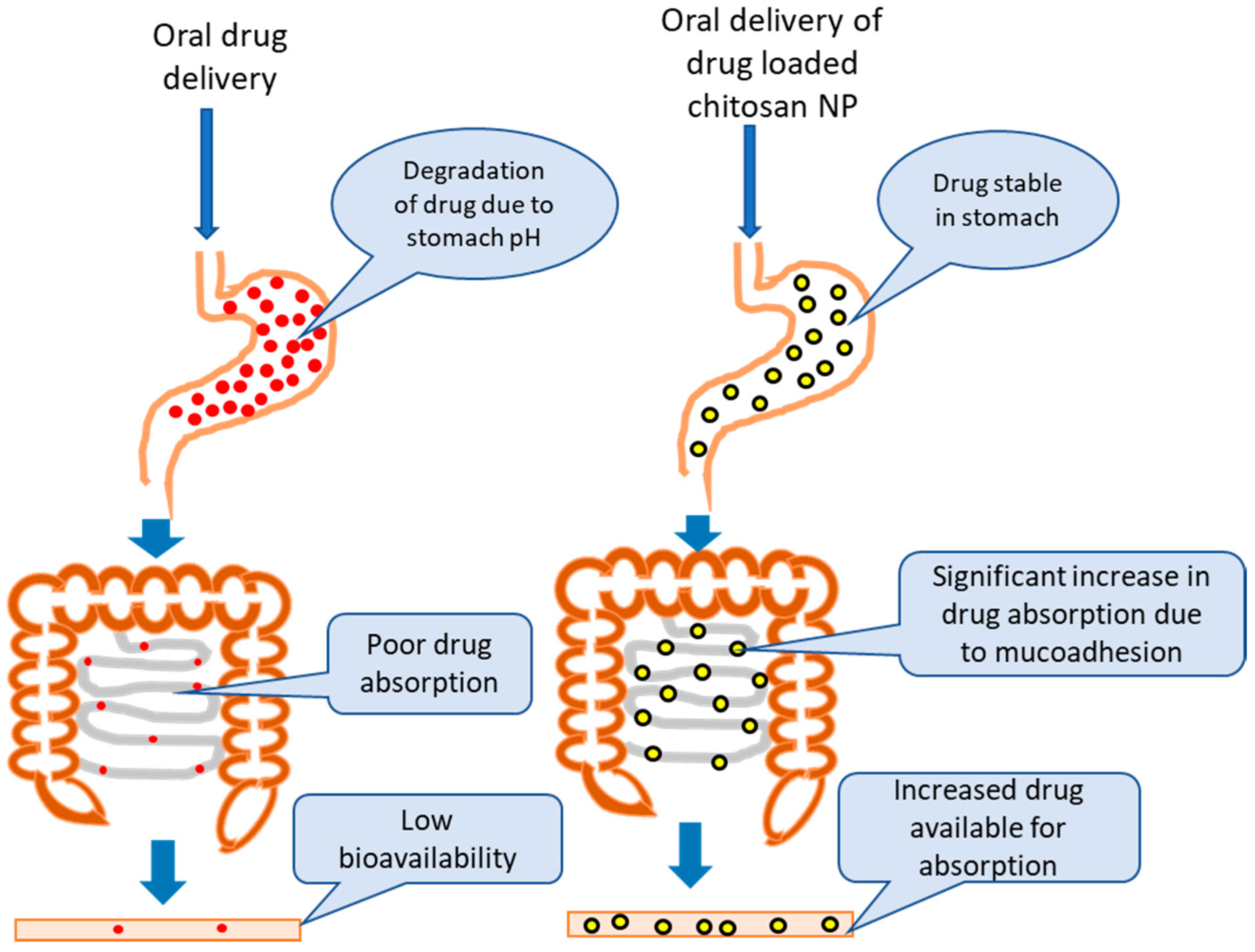
4. Chitosan in Oral Drug Delivery
5. Chitosan in Nasal Drug Delivery
6. Chitosan in Pulmonary Drug Delivery
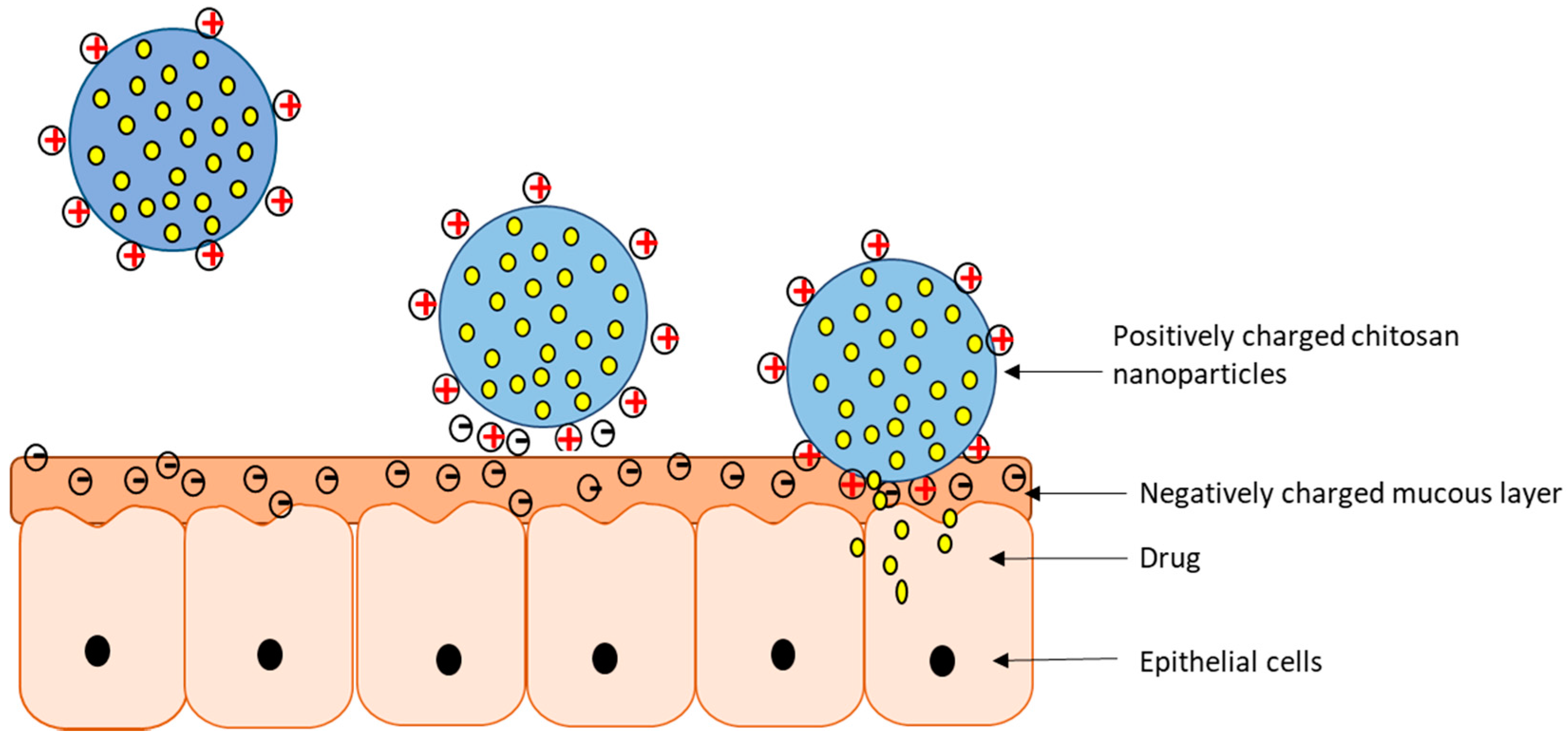
7. Mucoadhesion
7.1. Buccal Drug Delivery
7.2. Site Specific Delivery in the GIT
8. Pharmacokinetics (PK) of Chitosan Based Formulations
9. Toxicity and Safety of Chitosan
10. Clinical Vaccine Trials of Chitosan Based Formulations
11. Preparation of Chitosan Nanoparticles
11.1. Ionotropic Gelation
11.2. Complex Coacervation Method
11.3. Coprecipitation Method
11.4. Microemulsion Method
11.5. Emulsification Solvent Diffusion Method
11.6. Emulsion Based Solvent Evaporation Method
11.7. Reverse Micellar Method
12. Limitations
13. Conclusions and Future Work
Author Contributions
Conflicts of Interest
References
- Rampino, A.; Borgogna, M.; Blasi, P.; Bellich, B.; Cesàro, A. Chitosan nanoparticles: Preparation, size evolution and stability. Int. J. Pharm. 2013, 455, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, K.; Singh, S.K.; Mishra, D.N. Chitosan Nanoparticles: A Promising System in Novel Drug Delivery. Chem. Pharm. Bull. 2010, 58, 1423–1430. [Google Scholar] [CrossRef] [PubMed]
- Sorlier, P.; Denuzière, A.; Viton, C.; Domard, A. Relation between the Degree of Acetylation and the Electrostatic Properties of Chitin and Chitosan. Biomacromolecules 2001, 2, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Vårum, K.M.; Ottøy, M.H.; Smidsrød, O. Water-solubility of partially N-acetylated chitosans as a function of pH: Effect of chemical composition and depolymerisation. Carbohydr. Polym. 1994, 25, 65–70. [Google Scholar] [CrossRef]
- Van den Broek, L.A.M.; Knoop, R.J.I.; Kappen, F.H.J.; Boeriu, C.G. Chitosan films and blends for packaging material. Carbohydr. Polym. 2015, 116 (Suppl. C), 237–242. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.C.; Mi, F.L.; Liao, Z.X.; Hsiao, C.W.; Sonaje, K.; Chung, M.F.; Hsu, L.W.; Sung, H.W. Recent advances in chitosan-based nanoparticles for oral delivery of macromolecules. Adv. Drug Deliv. Rev. 2013, 65, 865–879. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.K.; Mishra, A.K.; Arotiba, O.A.; Mamba, B.B. Chitosan-based nanomaterials: A state-of-the-art review. Int. J. Biol. Macromol. 2013, 59, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Strobl, G.R. The Physics of Polymers; Springer: Berlin/Heidelberg, Germany, 2007; ISBN 978-3-540-25278-8. [Google Scholar]
- Park, S.Y.; Jun, S.T.; Marsh, K.S. Physical properties of PVOH/chitosan-blended films cast from different solvents. Food Hydrocoll. 2001, 15, 499–502. [Google Scholar] [CrossRef]
- Mima, S.; Miya, M.; Iwamoto, R.; Yoshikawa, S. Highly deacetylated chitosan and its properties. J. Appl. Polym. Sci. 1983, 28, 1909–1917. [Google Scholar] [CrossRef]
- Risbud, M.V.; Hardikar, A.A.; Bhat, S.V.; Bhonde, R.R. pH-sensitive freeze-dried chitosan–polyvinyl pyrrolidone hydrogels as controlled release system for antibiotic delivery. J. Control. Release 2000, 68, 23–30. [Google Scholar] [CrossRef]
- Kolhe, P.; Kannan, R.M. Improvement in Ductility of Chitosan through Blending and Copolymerization with PEG: FTIR Investigation of Molecular Interactions. Biomacromolecules 2003, 4, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Koul, V.; Mohamed, R.; Kuckling, D.; Adler, H.-J.P.; Choudhary, V. Interpenetrating polymer network (IPN) nanogels based on gelatin and poly(acrylic acid) by inverse miniemulsion technique: Synthesis and characterization. Colloids Surf. B Biointerfaces 2011, 83, 204–213. [Google Scholar] [CrossRef] [PubMed]
- El-Hefian, E.A.; Yahaya, A.H. Rheological study of chitosan and its blends: An overview. Maejo Int. J. Sci. Technol. 2010, 4, 210–220. [Google Scholar]
- Dambies, L.; Vincent, T.; Domard, A.; Guibal, E. Preparation of Chitosan Gel Beads by Ionotropic Molybdate Gelation. Biomacromolecules 2001, 2, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Al-Qadi, S.; Grenha, A.; Carrión-Recio, D.; Seijo, B.; Remuñán-López, C. Microencapsulated chitosan nanoparticles for pulmonary protein delivery: In vivo evaluation of insulin-loaded formulations. J. Control. Release 2012, 157, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Thanou, M.M.; Kotze, A.F.; Scharringhausen, T.; Lueßen, H.L.; De Boer, A.G.; Verhoef, J.C.; Junginger, H.E. Effect of degree of quaternization of N-trimethyl chitosan chloride for enhanced transport of hydrophilic compounds across intestinal Caco-2 cell monolayers. J. Control. Release 2000, 64, 15–25. [Google Scholar] [CrossRef]
- Bernkop-Schnürch, A.; Hornof, M.; Zoidl, T. Thiolated polymers—Thiomers: Synthesis and in vitro evaluation of chitosan–2-iminothiolane conjugates. Int. J. Pharm. 2003, 260, 229–237. [Google Scholar] [CrossRef]
- Sadeghi, A.M.M.; Dorkoosh, F.A.; Avadi, M.R.; Weinhold, M.; Bayat, A.; Delie, F.; Gurny, R.; Larijani, B.; Rafiee-Tehrani, M.; Junginger, H.E. Permeation enhancer effect of chitosan and chitosan derivatives: Comparison of formulations as soluble polymers and nanoparticulate systems on insulin absorption in Caco-2 cells. Eur. J. Pharm. Biopharm. 2008, 70, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Ding, J.; He, C.; Cui, L.; Tang, C.; Yin, C. Biomaterials Drug permeability and mucoadhesion properties of thiolated trimethyl chitosan nanoparticles in oral insulin delivery. Biomaterials 2009, 30, 5691–5700. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Qian, F.; Zhao, Z.; Yin, L.; Tang, C.; Yin, C. Preparation, Characterization and Oral Delivery of Insulin Loaded Carboxylated Chitosan Grafted Poly (methyl methacrylate) Nanoparticles. Biomacromolecules 2009, 10, 1253–1258. [Google Scholar] [CrossRef] [PubMed]
- Kurita, K.; Hashimoto, S.; Yoshino, H.; Ishii, S.; Nishimura, S.-I. Preparation of Chitin/Polystyrene Hybrid Materials by Efficient Graft Copolymerization Based on Mercaptochitin. Macromolecules 1996, 29, 1939–1942. [Google Scholar] [CrossRef]
- Furlani, F.; Sacco, P.; Marsich, E.; Donati, I.; Paoletti, S. Highly monodisperse colloidal coacervates based on a bioactive lactose-modified chitosan: From synthesis to characterization. Carbohydr. Polym. 2017, 174 (Suppl. C), 360–368. [Google Scholar] [CrossRef] [PubMed]
- Sacco, S.; Paoletti, M.; Cok, F.; Asaro, M.; Abrami, M.; Grassi, I. Donati Insight into the ionotropic gelation of chitosan using tripolyphosphate and pyrophosphate as cross-linkers. Int. J. Biol. Macromol. 2016, 92, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yang, S.; Ho, P.C. Intranasal administration of carbamazepine-loaded carboxymethyl chitosan nanoparticles for drug delivery to the brain. Asian J. Pharm. Sci. 2017. [Google Scholar] [CrossRef]
- Singh, R.; Lillard, J.W., Jr. Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 2009, 86, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Ye, Y.; Gao, F.; Yuan, H.; Lan, M.; Lou, K.; Wang, W. Chitosan-graft-β-cyclodextrin nanoparticles as a carrier for controlled drug release. Int. J. Pharm. 2013, 446, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Miladi, K.; Sfar, S.; Fessi, H.; Elaissari, A. Enhancement of alendronate encapsulation in chitosan nanoparticles. J. Drug Deliv. Sci. Technol. 2015, 30, 391–396. [Google Scholar] [CrossRef]
- Siafaka, P.I.; Titopoulou, A.; Koukaras, E.N.; Kostoglou, M.; Koutris, E.; Karavas, E.; Bikiaris, D.N. Chitosan derivatives as effective nanocarriers for ocular release of timolol drug. Int. J. Pharm. 2015, 495, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, J.; Siepmann, F. Modeling of diffusion controlled drug delivery. J. Control. Release 2012, 161, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Santos, B.; Chorilli, M. An overview of carboxymethyl derivatives of chitosan: Their use as biomaterials and drug delivery systems. Mater. Sci. Eng. C 2017, 77, 1349–1362. [Google Scholar] [CrossRef] [PubMed]
- Göpferich, A. Mechanisms of polymer degradation and erosion. Biomaterials 1996, 17, 103–114. [Google Scholar] [CrossRef]
- Pawar, D.; Mangal, S.; Goswami, R.; Jaganathan, K.S. Development and characterization of surface modified PLGA nanoparticles for nasal vaccine delivery: Effect of mucoadhesive coating on antigen uptake and immune adjuvant activity. Eur. J. Pharm. Biopharm. 2013, 85, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Manca, M.L.; Loy, G.; Zaru, M.; Fadda, A.M.; Antimisiaris, S.G. Release of rifampicin from chitosan, PLGA and chitosan-coated PLGA microparticles. Colloids Surf. B Biointerfaces 2008, 67, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, Y.; Feng, J.; Gu, T.; Dong, Q.; Yang, X.; Sun, Y.; Wu, Y.; Chen, Y.; Kong, W. Preparation, characterization and in vitro and in vivo investigation of chitosan-coated poly (d,l-lactide-co-glycolide) nanoparticles for intestinal delivery of exendin-4. Int. J. Nanomed. 2013, 8, 1141–1154. [Google Scholar] [CrossRef]
- Bowman, K.; Leong, K.W. Chitosan Nanoparticles for Oral Drug and Gene Delivery. Int. J. Nanomed. 2006, 1, 117–128. [Google Scholar] [CrossRef]
- Wang, J.J.; Zeng, Z.W.; Xiao, R.Z.; Xie, T.; Zhou, G.L.; Zhan, X.R.; Wang, S.L. Recent advances of chitosan nanoparticles as drug carriers. Int. J. Nanomed. 2011, 6, 765–774. [Google Scholar] [CrossRef]
- Palacio, J.; Agudelo, N.A.; Lopez, B.L. PEGylation of PLA nanoparticles to improve mucus-penetration and colloidal stability for oral delivery systems. Curr. Opin. Chem. Eng. 2016, 11, 14–19. [Google Scholar] [CrossRef]
- Dube, A.; Nicolazzo, J.A.; Larson, I. Chitosan nanoparticles enhance the intestinal absorption of the green tea catechins (+)-catechin and (−)-epigallocatechin gallate. Eur. J. Pharm. Sci. 2010, 41, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, S.; Buttini, F.; Rossi, A.; Bettini, R.; Colombo, P.; Ponchel, G.; Sonvico, F. Ex vivo permeation of tamoxifen and its 4-OH metabolite through rat intestine from lecithin/chitosan nanoparticles. Int. J. Pharm. 2015, 491, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Wang, Z.; Jiang, C.; Kong, M.; Zhou, X.; Li, Y.; Cheng, X.; Chen, X. Chitosan/o-carboxymethyl chitosan nanoparticles for efficient and safe oral anticancer drug delivery: In vitro and in vivo evaluation. Int. J. Pharm. 2013, 457, 158–167. [Google Scholar] [CrossRef] [PubMed]
- John, J.; Sangeetha, D.; Gomathi, T. Sunitinib loaded chitosan nanoparticles formulation and its evaluation. Int. J. Biol. Macromol. 2016, 82, 952–958. [Google Scholar] [CrossRef]
- Diop, M.; Auberval, N.; Viciglio, A.; Langlois, A.; Bietiger, W.; Mura, C.; Peronet, C.; Bekel, A.; David, D.J.; Zhao, M.; et al. Design, characterisation and bioefficiency of insulin-chitosan nanoparticles after stabilisation by freeze-drying or cross-linking. Int. J. Pharm. 2015, 491, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Hu, S.; Lu, Y.; Zhang, Y.; Jiang, X.; An, S.; Guo, Y.; Zhou, X.; Hou, H.; Jiang, C. Development of chitosan nanoparticles as drug delivery system for a prototype capsid inhibitor. Int. J. Pharm. 2015, 495, 771–782. [Google Scholar] [CrossRef] [PubMed]
- El-Shabouri, M.H. Positively charged nanoparticles for improving the oral bioavailability of cyclosporin-A. Int. J. Pharm. 2002, 249, 101–108. [Google Scholar] [CrossRef]
- Gao, P.; Xia, G.; Bao, Z.; Feng, C.; Cheng, X.; Kong, M.; Liu, Y.; Chen, X. Chitosan based nanoparticles as protein carriers for efficient oral antigen delivery. Int. J. Biol. Macromol. 2016, 91, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Bagre, A.P.; Jain, K.; Jain, N.K. Alginate coated chitosan core shell nanoparticles for oral delivery of enoxaparin: In vitro and in vivo assessment. Int. J. Pharm. 2013, 456, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tan, J.; Luo, J.; Huang, P.; Zhou, W.; Chen, L.; Long, L.; Zhang, L.; Zhu, B.; Yang, L.; et al. Enhancement of scutellarin oral delivery efficacy by vitamin B12-modified amphiphilic chitosan derivatives to treat type II diabetes induced-retinopathy. J. Nanobiotechnol. 2017, 15. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-C.; Chen, J.-K.; Lam, U.-I.; Chen, S.-Y. Preparing, characterizing and evaluating chitosan/fucoidan nanoparticles as oral delivery carriers. J. Polym. Res. 2014, 21, 415. [Google Scholar] [CrossRef]
- Shi, Y.; Xue, J.; Jia, L.; Du, Q.; Niu, J.; Zhang, D. Surface-modified PLGA nanoparticles with chitosan for oral delivery of tolbutamide. Colloids Surf. B Biointerfaces 2018, 161, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Derakhshandeh, K.; Fathi, S. Role of chitosan nanoparticles in the oral absorption of Gemcitabine. Int. J. Pharm. 2012, 437, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Maity, S.; Mukhopadhyay, P.; Kundu, P.P.; Chakraborti, A.S. Alginate coated chitosan core-shell nanoparticles for efficient oral delivery of naringenin in diabetic animals—An in vitro and in vivo approach. Carbohydr. Polym. 2017, 170, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Yan, H.; Yang, H.-J.; Kim, H.W.; Wan, X.; Lee, J.; Ko, S. Synthesis and controlled-release properties of chitosan/β-Lactoglobulin nanoparticles as carriers for oral administration of epigallocatechin gallate. Food Sci. Biotechnol. 2016, 25, 1583–1590. [Google Scholar] [CrossRef]
- Aluani, D.; Tzankova, V.; Kondeva-Burdina, M.; Yordanov, Y.; Nikolova, E.; Odzhakov, F.; Apostolov, A.; Markova, T.; Yoncheva, K. Evaluation of biocompatibility and antioxidant efficiency of chitosan-alginate nanoparticles loaded with quercetin. Int. J. Biol. Macromol. 2017, 103, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Van der Lubben, I.M.; Verhoef, J.C.; Borchard, G.; Junginger, H.E. Chitosan for mucosal vaccination. Adv. Drug Deliv. Rev. 2001, 52, 139–144. [Google Scholar] [CrossRef]
- Casettari, L.; Illum, L. Chitosan in nasal delivery systems for therapeutic drugs. J. Control. Release 2014, 190, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Lisbeth, I. Nasal drug delivery—Possibilities, problems and solutions. J. Control. Release 2003, 87, 187–198. [Google Scholar]
- Shahnaz, G.; Vetter, A.; Barthelmes, J.; Rahmat, D.; Laffleur, F.; Iqbal, J.; Perera, G.; Schlocker, W.; Dünnhaput, S.; Augustijns, P.; et al. Thiolated chitosan nanoparticles for the nasal administration of leuprolide: Bioavailability and pharmacokinetic characterization. Int. J. Pharm. 2012, 428, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Ruge, C.A.; Kirch, J.; Lehr, C.-M. Pulmonary drug delivery: From generating aerosols to overcoming biological barriers—Therapeutic possibilities and technological challenges. Lancet Respir. Med. 2013, 1, 402–413. [Google Scholar] [CrossRef]
- Lytting, E.; Nguyen, J.; Wang, X.; Kissel, T. Biodegradable polymeric nanocarriers for pulmonary drug delivery Biodegradable polymeric nanocarriers for pulmonary drug delivery. Expert Opin. Drug Deliv. 2008, 56, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Islam, N.; Ferro, V. Recent Advances in Chitosan-Based Nanoparticulate Pulmonary Drug Delivery. Nanoscale 2016, 14341–14358. [Google Scholar] [CrossRef] [PubMed]
- Rawal, T.; Parmar, R.; Tyagi, R.K.; Butani, S. Rifampicin loaded chitosan nanoparticle dry powder presents an improved therapeutic approach for alveolar tuberculosis. Colloids Surf. B Biointerfaces 2017, 154, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Jafarinejad, S.; Gilani, K.; Moazeni, E.; Ghazi-Khansari, M.; Najafabadi, A.R.; Mohajel, N. Development of chitosan-based nanoparticles for pulmonary delivery of itraconazole as dry powder formulation. Powder Technol. 2012, 222, 65–70. [Google Scholar] [CrossRef]
- Ni, S.; Liu, Y.; Tang, Y.; Chen, J.; Li, S.; Pu, J.; Han, L. GABA B receptor ligand-directed trimethyl chitosan/tripolyphosphate nanoparticles and their pMDI formulation for survivin siRNA pulmonary delivery. Carbohydr. Polym. 2017, 179, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Trapani, A.; Di Gioia, S.; Ditaranto, N.; Cioffi, N.; Goycoolea, F.M.; Carbone, A.; Garcia-Fuentes, M.; Conese, M.; Alonso, M.J. Systemic heparin delivery by the pulmonary route using chitosan and glycol chitosan nanoparticles. Int. J. Pharm. 2013, 447, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-W.; Shirley, S.A.; Lockey, R.F.; Mohapatra, S.S. Thiolated chitosan nanoparticles enhance anti-inflammatory effects of intranasally delivered theophylline. Respir. Res. 2006, 7. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chi, N.; Tang, X. Preparation of estradiol chitosan nanoparticles for improving nasal absorption and brain targeting. Eur. J. Pharm. Biopharm. 2008, 70, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Janes, K.; Behrens, I.; Kissel, T.; Vila, A.; Sa, A.; Vila, L. Low molecular weight chitosan nanoparticles as new carriers for nasal vaccine delivery in mice. Eur. J. Pharm. Biopharm. 2004, 57, 123–131. [Google Scholar] [CrossRef]
- Van der Lubben, I.M.; Verhoef, J.C.; Borchard, G.; Junginger, H.E. Chitosan and its derivatives in mucosal drug and vaccine delivery. Eur. J. Pharm. Sci. 2001, 14, 201–207. [Google Scholar] [CrossRef]
- Marguerite, R. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- George, M.; Abraham, T.E. Polyionic hydrocolloids for the intestinal delivery of protein drugs: Alginate and chitosan—A review. J. Control. Release 2006, 114, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Soares, P.I.P.; Isabel, A.; Carvalho, J.; Ferreira, I.M.M.; Novo, C.M.M.; Paulo, J. Chitosan-based nanoparticles as drug delivery systems for doxorubicin: Optimization and modelling. Carbohydr. Polym. 2016, 147, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Yostawonkul, J.; Surassmo, S.; Iempridee, T.; Pimtong, W.; Suktham, K.; Sajomsang, W.; Gonil, P.; Ruktanonchai, U.R. Surface modification of nanostructure lipid carrier (NLC) by oleoyl-quaternized-chitosan as a mucoadhesive nanocarrier. Colloids Surf. B Biointerfaces 2017, 149, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Mu, Y.; Xu, M.; Xia, G.; Wang, J.; Liu, Y.; Chen, X. Preparation and characterization of mucosal adhesive and two-step drug releasing cetirizine-chitosan nanoparticle. Carbohydr. Polym. 2017, 173, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Costa Idos, S.; Abranches, R.P.; Garcia, M.T.; Pierre, M.B. Chitosan-based mucoadhesive films containing 5-aminolevulinic acid for buccal cancer treatment. J. Photochem. Photobiol. B Biol. 2014, 140, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Icia-Mazzarino, L.; Borsali, R.; Lemos-senna, E. Mucoadhesive Films Containing Chitosan-Coated Nanoparticles: A New Strategy for Buccal Curcumin Release. J. Pharm. Sci. 2014, 103, 3764–3771. [Google Scholar] [CrossRef] [PubMed]
- Al-Kassas, R.; Wen, J.; Cheng, A.E.; Kim, A.M.; Liu, S.S.M.; Yu, J. Transdermal delivery of propranolol hydrochloride through chitosan nanoparticles dispersed in mucoadhesive gel. Carbohydr. Polym. 2016, 153, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.C.D.; Rescignano, N.; Boff, L.; Reginatto, F.H.; Simões, C.M.O.; de Campos, A.M.; Mijangos, C.U. Manufacture and characterization of chitosan/PLGA nanoparticles nanocomposite buccal films. Carbohydr. Polym. 2017, 173, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Pistone, S.; Goycoolea, F.M.; Young, A.; Smistad, G.; Hiorth, M. Formulation of polysaccharide-based nanoparticles for local administration into the oral cavity. Eur. J. Pharm. Biopharm. 2017, 96, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Giovino, C.; Ayensu, I.; Tetteh, J.; Boateng, J.S. Development and characterisation of chitosan films impregnated with insulin loaded PEG-b-PLA nanoparticles (NPs): A potential approach for buccal delivery of macromolecules. Int. J. Pharm. 2012, 428, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Vimal, A.; Kumar, A. Why Chitosan, From properties to perspective of mucosal drug delivery.pdf. Int. J. Biol. Macromol. 2016, 91, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Campisi, G.; Paderni, C.; Saccone, R.; Fede, O.; Wolff, A.; Giannola, L. Human Buccal Mucosa as an Innovative Site of Drug Delivery. Curr. Pharm. Des. 2010, 16, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Giovino, C.; Ayensu, I.; Tetteh, J.; Boateng, J.S. An integrated buccal delivery system combining chitosan films impregnated with peptide loaded PEG-b-PLA nanoparticles. Colloids Surf. B Biointerfaces 2013, 112, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Mazzarino, L.; Travelet, C.; Ortega-Murillo, S.; Otsuka, I.; Pignot-Paintrand, I.; Lemos-Senna, E.; Borsali, R. Elaboration of chitosan-coated nanoparticles loaded with curcumin for mucoadhesive applications. J. Colloid Interface Sci. 2012, 370, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Suvannasara, P.; Juntapram, K.; Praphairaksit, N.; Siralertmukul, K.; Muangsin, N. Mucoadhesive 4-carboxybenzenesulfonamide-chitosan with antibacterial properties.pdf. Carbohydr. Polym. 2013, 94, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.K.; Tiwari, A. Carbohydrate polymers: Applications and recent advances in delivering drugs to the colon. Carbohydr. Polym. 2012, 88, 399–416. [Google Scholar] [CrossRef]
- Wang, Q.-S.; Wang, G.-F.; Zhou, J.; Gao, L.-N.; Cui, Y.-L. Colon targeted oral drug delivery system based on alginate-chitosan microspheres loaded with icariin in the treatment of ulcerative colitis. Int. J. Pharm. 2016, 515, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Cerchiara, T.; Abruzzo, A.; Di Cagno, M.; Bigucci, F.; Bauer-Brandl, A.; Parolin, C.; Vitali, B.; Gallucci, M.C.; Luppi, B. Chitosan based micro- and nanoparticles for colon-targeted delivery of vancomycin prepared by alternative processing methods. Eur. J. Pharm. Biopharm. 2015, 92, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Coco, R.; Plapied, L.; Pourcelle, V.; Jérôme, C.; Brayden, D.J.; Schneider, Y.-J.; Préat, V. Drug delivery to inflamed colon by nanoparticles: Comparison of different strategies. Int. J. Pharm. 2013, 440, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Saboktakin, M.R.; Tabatabaie, R.M.; Maharramov, A.; Ramazanov, M.A. Synthesis and in vitro evaluation of carboxymethyl starch chitosan nanoparticles as drug delivery system to the colon. Int. J. Biol. Macromol. 2010, 48, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; Marks, E.; Schneider, J.J.; Keely, S. Advances in oral nano-delivery systems for colon targeted drug delivery in inflammatory bowel disease: Selective targeting to diseased versus healthy tissue. Nanomedicine 2015, 11, 1117–1132. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Urrusuno, R.; Calvo, P.; Remunan-Lopez, C.; Vila-Jato, J.L.; Alonso, M.J. Enhancement of nasal absorption of insulin using chitosan nanoparticles. Pharm. Res. 1999, 16, 1576–1581. [Google Scholar] [CrossRef] [PubMed]
- Baptista da Silva, S.; Ferreira, D.; Pintado, M.; Sarmento, B. Chitosan-based nanoparticles for rosmarinic acid ocular delivery—In vitro tests. Int. J. Biol. Macromol. 2016, 84, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Frank, L.; Chaves, P.; D’Amore, C.; Contri, R.; Frank, A.; Beck, R.; Pohlman, A.; Buffon, A.; Guterres, S. The use of chitosan as cationic coating or gel vehicle for polymeric nanocapsules: Increasing penetration and adhesion of imiquimod in vaginal tissue. Eur. J. Pharm. Biopharm. 2017, 114, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Aragao-Santiago, L.; Hillaireau, H.; Grabowski, N.; Mura, S.; Nascimento, T.L.; Dufort, S.; Coll, J.-L.; Tsapis, N.; Fattal, E. Compared in vivo toxicity in mice of lung delivered biodegradable and non-biodegradable nanoparticles. Nanotoxicology 2016, 10, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Grenha, A.; Grainger, C.I.; Dailey, L.A.; Seijo, B.; Martin, G.P.; Remuñán-López, C.; Forbes, B. Chitosan nanoparticles are compatible with respiratory epithelial cells in vitro. Eur. J. Pharm. Sci. 2007, 31, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.H.G.; Cosgrove, C.; McNeela, E.A.; Sexton, A.; Giemza, R.; Jabbal-Gill, I.; Church, A.; Lin, W.; Illum, L.; Podda, A.; et al. Protective levels of diphtheria-neutralizing antibody induced in healthy volunteers by unilateral priming-boosting intranasal immunization associated with restricted ipsilateral mucosal secretory immunoglobulin A. Infect. Immun. 2003, 71, 726–732. [Google Scholar] [CrossRef] [PubMed]
- El-Kamary, S.S.; Pasetti, M.F.; Mendelman, P.M.; Frey, S.E.; Bernstein, D.I.; Treanor, J.J.; Ferreira, J.; Chen, W.H.; Sublett, R.; Richardson, C.; et al. Adjuvanted Intranasal Norwalk Virus-Like Particle Vaccine Elicits Antibodies and Antibody-Secreting Cells That Express Homing Receptors for Mucosal and Peripheral Lymphoid Tissues. J. Infect. Dis. 2010, 202, 1649–1658. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, K.; Wahid, R.; Richardson, C.; Bargatze, R.F.; El-Kamary, S.S.; Sztein, M.B.; Pasetti, M.F. Intranasal vaccination with an adjuvanted Norwalk virus-like particle vaccine elicits antigen-specific B memory responses in human adult volunteers. Clin. Immunol. 2012, 144, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Alishahi, A.; Mirvaghefi, A.; Tehrani, M.R.; Farahmand, H.; Koshio, S.; Dorkoosh, F.A.; Elsabee, M.Z. Chitosan nanoparticle to carry vitamin C through the gastrointestinal tract and induce the non-specific immunity system of rainbow trout (Oncorhynchus mykiss). Carbohydr. Polym. 2011, 86, 142–146. [Google Scholar] [CrossRef]
- Hembram, K.C.; Prabha, S.; Chandra, R.; Ahmed, B.; Nimesh, S. Advances in preparation and characterization of chitosan nanoparticles for therapeutics. Artif. Cells Nanomed. Biotechnol. 2014, 1401, 1–10. [Google Scholar] [CrossRef]
- Gonçalves, I.C.; Henriques, P.C.; Seabra, C.L.; Martins, M.C.L. The potential utility of chitosan micro/nanoparticles in the treatment of gastric infection. Expert Rev. Anti. Infect. Ther. 2014, 12, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Shi, X.; Zhao, Y.; Wei, H.; Sun, Q.; Huang, T.; Zhang, X.; Wang, Y. Preparation and immunological effectiveness of a swine influenza DNA vaccine encapsulated in chitosan nanoparticles. Vaccine 2011, 29, 8549–8556. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Y.; Han, J.; Tang, L.; Liao, N.; Gui, G.-F.; Chai, Y.-Q.; Yuan, R. Quenching of the emission of peroxydisulfate system by ferrocene functionalized chitosan nanoparticles: A sensitive “signal off” electrochemiluminescence immunosensor. Sens. Actuators B Chem. 2014, 192, 791–795. [Google Scholar] [CrossRef]
- Chen, Y.; Mohanraj, V.J.; Wang, F.; Benson, H.A.E. Designing Chitosan–Dextran Sulfate Nanoparticles Using Charge Ratios. AAPS PharmSciTech 2007, 8, E98. [Google Scholar] [CrossRef] [PubMed]
- Leong, K.W.; Mao, H.-Q.; Truong-Le, V.L.; Roy, K.; Walsh, S.M.; August, J.T. DNA-polycation nanospheres as non-viral gene delivery vehicles. J. Control. Release 1998, 53, 183–193. [Google Scholar] [CrossRef]
- Tiyaboonchai, W. Chitosan Nanoparticles: A Promising System for Drug Delivery. Naresuan Univ. J. 2003, 11, 51–66. [Google Scholar] [CrossRef]
- Huang, M.; Fong, C.-W.; Khor, E.; Lim, L.-Y. Transfection efficiency of chitosan vectors: Effect of polymer molecular weight and degree of deacetylation. J. Control. Release 2005, 106, 391–406. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, N.; Ramay, H.R.; Chou, S.-H.; Zhang, M. Chitosan and lactic acid-grafted chitosan nanoparticles as carriers for prolonged drug delivery. Int. J. Nanomed. 2006, 1, 181–187. [Google Scholar] [CrossRef]
- Maitra, A.; Ghosh, P.K.; De, T.K.; Sahoo, S.K. Process for the Preparation of Highly Monodispersed Polymeric Hydrophilic Nanoparticles. US 5874111 A, 7 January 1999. [Google Scholar]
- Wang, Y.; Wang, X.; Luo, G.; Dai, Y. Adsorption of bovin serum albumin (BSA) onto the magnetic chitosan nanoparticles prepared by a microemulsion system. Bioresour. Technol. 2008, 99, 3881–3884. [Google Scholar] [CrossRef] [PubMed]
- Niwa, T.; Takeuchi, H.; Hino, T.; Kunou, N.; Kawashima, Y. Preparations of biodegradable nanospheres of water-soluble and insoluble drugs with d,l-lactide/glycolide copolymer by a novel spontaneous emulsification solvent diffusion method and the drug release behavior. J. Control. Release 1993, 25, 89–98. [Google Scholar] [CrossRef]
- Vila, A.; Sanchez, A.; Tobío, M.; Calvo, P.; Alonso, M.J. Design of Biodegradable Partilces for Protein Delivery. J. Control. Release 2002, 78, 15–24. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, Z.-L.; Wei, X.-H.; Liu, J.-H. Preparation and in vitro and in vivo characterization of cyclosporin A-loaded, PEGylated chitosan-modified, lipid-based nanoparticles. Int. J. Nanomed. 2013, 8, 601–610. [Google Scholar] [CrossRef]
- Zeinab Sadat, S.; Hamed, S.-K.; Mohammad, I.; Mohammad, A.; Azizollah, N. Exploring the effect of formulation parameters on the particle size of carboxymethyl chitosan nanoparticles prepared via reverse micellar crosslinking. J. Microencapsul. 2017, 34, 270–279. [Google Scholar] [CrossRef]
- Malmsten, M. Surfactants and Polymers in Drug Delivery; Marcel Dekker: New York, NY, USA, 2002; ISBN 9780824708047. [Google Scholar]
- Banerjee, T.; Mitra, S.; Kumar-Singh, A.; Kumar-Sharma, R.; Maitra, A. Preparation, characterization and biodistribution of ultrafine chitosan nanoparticles. Int. J. Pharm. 2002, 243, 93–105. [Google Scholar] [CrossRef]
- Liu, C.; Tan, Y.; Liu, C.; Chen, X.; Yu, L. Preparations, characterizations and applications of Chitosan-based nanoparticles. J. Ocean Univ. China 2007, 6, 237–243. [Google Scholar] [CrossRef]
- Mitra, S.; Gaur, U.; Ghosh, P.C.; Maitra, A.N. Tumour targeted delivery of encapsulated dextran–doxorubicin conjugate using chitosan nanoparticles as carrier. J. Control. Release 2001, 74, 317–323. [Google Scholar] [CrossRef]
- Bellich, B.; D’Agostino, I.; Semeraro, S.; Gamini, A.; Cesàro, A. “The good, the bad and the ugly” of chitosans. Mar. Drugs 2016, 14, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Key, J.; Park, K. Multicomponent, Tumor-Homing Chitosan Nanoparticles for Cancer Imaging and Therapy. Int. J. Mol. Sci. 2017, 18, E594. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, X.; Ji, J.; Liu, A.; Zhai, G. Tumor targeting strategies for chitosan-based nanoparticles. Colloids Surf. B Biointerfaces 2016, 148, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.A.; Aljaeid, B.M. Preparation, characterization and potential application of chitosan, chitosan derivatives and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug Des. Devel. Ther. 2016, 10, 483–507. [Google Scholar] [CrossRef] [PubMed]
- Swierczewska, M.; Han, H.S.; Kim, K.; Park, J.H.; Lee, S. Polysaccharide-based nanoparticles for theranostic nanomedicine. Adv. Drug Deliv. Rev. 2016, 99 Pt A, 70–84. [Google Scholar] [CrossRef] [PubMed]
| Drug | Composition | Purpose | Research Findings | In Vivo | Reference |
|---|---|---|---|---|---|
| Tamoxifen citrate | Chitosan, soybean lecithin NP | Intestinal permeation of tamoxifen in lecithin/chitosan NPs through the rat intestinal wall. | Ex vivo experiment using a jejunum chamber from the small intestine of male Wistar rats. Lecithin/chitosan nanoparticles improved the non-metabolized drug passage across the rat intestinal tissue. | [40] | |
| Sunitinib | Chitosan | Biophysical characterization | 1. In vitro biodegradation was improved in the presence of lysozyme. 2. In vitro controlled release was demonstrated with 69% released within 72 h. | [42] | |
| Hydro phobic Bay41-4109 | LMW chitosan | Solubility and bioavailability enhancement. | 1. In vitro drug release studies showed a sustained release profile. 2. Low cytotoxicity was demonstrated in a liver cell line. | In vivo studies were performed in rats. The absolute bioavailability of Bay41-4109 NPs was >70%, nearly 4-fold higher than other formulations | [44] |
| Insulin | Chitosan, TPP | Preparation, characterization and stabilization of insulin—chitosan NPs. | 1. In vitro validation-Caco2 and co-culture: Caco-2 cells internalized the NPs effectively. | In vivo studies where decreased glycaemia was observed in diabetic rats after insulin NP administration. | [43] |
| Cyclosporin-A | Chitosan HCl, Poloxamer 188, sodium glycolate, gelatin, soya lecithin | To develop and characterize Cy-A as positive charge NPs to improve GI uptake, bioavailability | Cy-A encapsulation efficiency was very high at 88–94%. | In vivo studies in beagle dogs showed relative bioavailability of Cy-A was significantly increased by NPs. | [45] |
| Extra cellular products (ECPs) of Vibrio anguillarum (Protein carrier) | Chitosan carboxymethyl chitosan | To study the efficacy of chitosan NPs as a vehicle for oral antigen delivery in fish vaccination. | In vitro release study was performed in Tris-buffer (pH 2.0, 4.5) & PBS (pH 7.4); the highest cumulative release was 58% at pH 7.4 followed by 37% at pH 2.0. | Biodistribution showed NP uptake in spleen and kidney. Lysozyme and complement evaluation. It showed elevated specific antibody and higher concentrations of lysozyme activity and complement | [46] |
| Alendro-nate sodium | Chitosan LMW, sodium tripolyphosphate (TPP), fluorenyl-methyloxycarbonyl chloride (FMOC) | To study the influence of physical parameters on drug encapsulation efficiency | In vitro drug release was performed in 0.1N HCl and PBS (pH 6.8). The NPs released alendronate faster in 0.1N HCl compared to PBS. Encapsulation efficiency was ~80%. | [28] | |
| Catechins (+) catechin (C) and (−) epigallocatechingallate (EGCg) | Chitosan LMW, sodium tripolyphos-phate, tris[2-carboxyethyl] phosphine hydrochloride (TCEP) | To enhance the intestinal absorption by encapsulation of C, EGCg in chitosan NPs | Ex vivo study using a jejunum chamber from mice. The cumulative amounts transported after encapsulation were significantly higher for C and EGCg, respectively. | [39] | |
| Enoxaparin | Chitosan, STPP, sodium alginate | Alginate coated chitosan-NPs containing enoxaparin for oral controlled release | In vitro drug release showed only 2% drug release in SGF (pH 1.2) but 60% in SIF (pH 6.5). | In vivo studies were performed in albino rats: the oral bioavailability of enoxaparin in Alg-CS-NPs bioavailability was significantly higher compared to enoxaparin solution. A 60% reduction was seen in thrombus formation in rat venous thrombosis model. | [47] |
| Scutellarin | Chitosan, deoxycholic acid, vitamin B12 | Enhancement of scutellarin oral delivery efficacy by Vit B12 modified amphiphilic chitosan derivatives to treat type II diabetes-induced retinopathy | Cytotoxicity study of Chit-DC and Chit-DC-VB12 displayed low cytotoxicity in Caco-2 cells. | In vivo studies: 1. Zebra fish embryo: development of the embryo was unaffected 2. Sprague-Dawleys rats: the AUC of scutellarin NP was 2–3 fold higher than free scutellarin and efficacy was achieved | [48] |
| Fucoidan | Chitosan, Tc-methylene di-phosphonate | To prepare and evaluate pH sensitivity of CS/F NPs | In vitro drug release of Tc-MDP from CS/F NPs rose as the pH levels changed from 2.5 to 7.4. CS/F NPs was stable into the stomach and decompose in the intestine. | [49] | |
| Tolbut-amide | Chitosan, PLGA, streptozotocin | To prepare PLGA NPs modified with chitosan to form TOL-CS-PLGA NPs to improve bioavailability and reduce dose frequency. | In vitro drug release of TOL-CS-PLGA NPs showed sustained release in PBS, pH 7.4. Cytotoxicity study of TOL-CS-PLGA NPs were non-toxic in HePG2 cells. | In vivo study was performed in adult Sprague-Dawley rats: the TOL-CS-PLGA NPS showed a long-acting hypoglycemic effect over 8 h, significantly longer than metformin tablets. | [50] |
| Gemcita-bine | Chitosan LMW, penta sodium tripolyphos-phate | To prepare gemcitabine-loaded chitosan NPs (Gem-CS-NP) for oral bio-availability enhancement | 1. In vitro drug release of Gem-CS-NPs showed controlled release by a two-phase process. 2. Absorption studies in an intestinal sac model: the absorption of Gem-CS-NPs intestinal transport increased 3–5-fold compared to free drug. | [51] | |
| Naringenin | Sodium alginate, chitosan, streptozotocin | To prepare alginate coated chitosan core shell NPs for effective oral delivery | In vitro drug release from NPs was 15% in SGF (pH 1.2) and 90% in pH 7.4 in slow, sustained fashion. | In vivo study in rats showed lack of toxicity; and an anti-diabetic effect: naringenin NPs have better efficacy in lowering blood glucose levels compare to free drug. | [52] |
| Epigallocatechin gallate (EGCG) | N-carboxymethyl chitosan (MW = 61 kDa), chitosan hydrochloride | To develop EGCG-chitosan/β-Lg NPs to achieve a prolong release for oral administration in the GI tract | In vitro drug release and degradation of EGCG-chitosan/β-Lg NPs was slower in simulated stomach condition compare to control particles | [53] | |
| Quercetin | Chitosan, sodium alginate, sodium pyruvate, l-glutamine | To develop and evaluate quercetin-chitosan/alginate NPs to preserve its antioxidant property without causing systemic toxicity. | In vitro cytotoxicity of both empty NPs and quercetin NPs exhibited nontoxic behavior in HepG2 liver cells when exposed for a period up to 72 h. | In vivo toxicity study in Wistar rats displayed no change in body weight, rat liver weight, histology, hematology and biochemical parameters after oral administration of empty NPs and quercetin loaded NPs. | [54] |
| Drug | Composition | Purpose | Research Findings | In Vivo | Reference |
|---|---|---|---|---|---|
| Rifampicin | TPP, lactose, Tween 80 | Preparing CS-NPs dry powder to achieve local and sustained targeting of anti-tubercular drugs in order to reduce dosage and frequency | 1. In vitro release showed 90% release of RFM from CS-NPs within 24 h. 2. Cell viability of J774 macrophage cells showed negligible toxicity of RFM-NPs up to 12 h exposure at concentrations up to 0.5 mg/mL | In vivo study—male Wistar rats. A marked increase in Cmax, t1/2 and AUC was seen in RFM-NPs compared to other formulations. | [62] |
| Itraconazole | Hydroxypropyl-beta-cyclodextrin (HPβCD), mannitol, lactose, TPP, l-leucine | To develop chitosan NPs for pulmonary delivery of itraconazole as a dry powder formulation | 1. Encapsulation efficiency of 55% was obtained in 1:3 ratio of chitosan:TPP. 2. In vitro drug release was ~80% during the first 4 h and the remaining encapsulated drug was released over 48 h. | [63] | |
| Baclofen and siRNA | N,N,N-tri-methyl chitosan, TPP | To prepare and evaluate baclofen-trimethyl chitosan/TPP NPs (Bac-TMC/TPP NPs) in a dry powder formulation | 1. Low in vitro cytotoxicity of TMC and Bac-TMC in A549 cells when treated with four different polymers for 48 h. 2. Cellular uptake of Bac-TMC3/TPP/siRNA NPs was greatly enhanced by clathrin -mediated cellular uptake pathway. | [64] | |
| Heparin (LMWH) | Chitosan, lipoid S100, glycol chitosan | To prepare and evaluate LMWH chitosan and glycol chitosan NPs for enhancing the pulmonary absorption of LMW heparin. | In vitro drug release of lipoid S100-LMWH GCS NPs in SLF showed progressive LMWH release up to 6 h, followed by a plateau for 24 h. | In vivo studies were performed in mice: the aerosol-type administration of free LMWH and Lipoid S100-LMWH GCS NPs led to a significant elongation of the coagulation time | [65] |
| Theo-phylline | Chitosan thioglycolic acid, TPP | To develop and evaluate whether theophylline-thiolated chitosan NPs can enhance theophylline’s capacity to alleviate allergic asthma | In vitro mucoadhesive study of TCNs exhibited a gradual increase in mucin binding and adsorption for 12 h compared to unmodified chitosan | In vivo study was performed in mice and the anti-inflammatory effects of theophylline were markedly enhanced when the drug was delivered by TCNs compared to unmodified chitosan or theophylline alone. | [66] |
| Leuprolide | Thiolated chitosan | To prepare and evaluate leuprolide thiolated-CS-NPs to enhance the half-life and bioavailability of leuprolide via nasal administration | In vitro drug release of leuprolide from thiolated chitosan showed slow and sustained release of drug about 43% in 2 h. | In vivo study was performed in male Sprague-Dawley rats showed improved nasal bioavailability of leuprolide thiolated NPs calculated based on AUC (0–6) was about 19.6% as compared to leuprolide solution alone 2.8%. | [60] |
| Estradiol (E2) | Chitosan, methylated β-cyclodextrin, TPP | To prepare estradiol-chitosan NPs for improving nasal absorption and brain targeting | In vivo study was performed in male Wister rats: The plasma concentration of E2 from E2-CS-NPs was significantly lower in intranasal administration compare to IV but CSF concentrations of E2 from E2-CS-NPs was significantly higher for intranasal administration compare to IV. | [67] | |
| Tetanus toxoid (TT) | LMW Chitosan, TPP, trehalose | To prepare tetanus toxoid chitosan nanoparticles (TT-CS NPs) as a new long-term nasal vaccine delivery vehicle | In vitro drug release of the TT from CS-NPs showed a rapid release over first 2 h followed by slow release for up to 16 days. | Intranasal immunization with two doses of TT-CS NPs in mice: The results showed the titers were significantly higher for the TT-loaded particles than for the free toxoid and at post-administration of TT-CS NPs IgA levels were significantly higher than the fluid vaccine. | [68] |
| Drug | Composition | Purpose | Research Findings | In Vivo | Reference |
|---|---|---|---|---|---|
| Doxorubicin HCL (DOX) | Chitosan (MW = 400 kDa), 4-CBS, TPP, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide HCl (EDAC) | Preparation, characterization, in vitro drug release, Topo II inhibitor activity and evaluation of DOX-loaded 4-CBS-chitosan/PLA nanoparticles. | 1. In vitro drug release of DOX loaded 4-CBS-chitosan/PLA nanoparticles showed sustained release up to 26 days. 2. Cytotoxic study (MTT) of DOX loaded nanoparticles showed the lowest effect on the cell viability of HepG2 compared to SW620, KAT03 and CHAGO cell lines. 3. Over 72% inhibition of Topo II. | [72] | |
| Alpha-mangostin (AP) | Chitosan (MW = 600 kDa), methane-sulfonic acid, oleoyl chloride, sodium bicarbonate, glycidyl-trimethyl ammonium chloride | To develop a mucoadhesive oleoyl-quaternized chitosan coated nanostructure lipid carrier (NLC) for potential oral administration with enhanced mucoadhesion | In vitro cytotoxicity of AP-NLC and CS-AP-NLC exhibited higher toxicity against Caco-2 than Hela cells. | In vivo toxicology study was performed in zebrafish embryos. | [73] |
| Cetirizine | Chitosan, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC. HCl), N-hydroxyl succinimide | Preparation and characterization of mucosal adhesion and drug release of cetirizine-chitosan NP | 1. In vitro, the cumulative release of cedH from cedH:CS-NPs and cedH:CTZ-CS-1-NPs were 71% & 76% in absence of lysozyme and increased to 77% & 84% in presence of lysozyme. Burst release and lysozyme induced sustained release was achieved. 2. In vitro cytotoxicity of cedH:CTZ-CS-1 NPs showed non-toxicity in L929 cells and biocompatibility with RBCs. | [74] | |
| 5-amino levulinic acid | Chitosan, lactic acid. | To develop and characterize chitosan-based 5-ALA mucoadhesive film to enhance its retention in oral mucosa | In vitro permeation and retention of 5-ALA (1.0% or 10%) were increased. However, 10% 5-ALA exhibited highest values 4 and 17 times, respectively, compared to propylene glycol vehicle. | [75] | |
| Curcumin | Chitosan Low (CLS 50,000–190,000 Da), medium (CSM 190,000–310,00 Da), high (CSH 310,000 to >375,000 Da), polycaprolactone, glycerol | Preparation of mucoadhesive films containing curcumin-loaded NPs to prolong the residence time of the dosage form in the oral cavity and to increase drug absorption through the buccal mucosa | 1. Swelling studies for mucoadhesive films containing curcumin loaded NPs showed good hydration in simulated saliva. 2. In vitro release of CSHG5-NP and CSMG5-NP exhibited high release rate approximately 3.4% and 2.8% curcumin respectively, over 24 h. | [76] | |
| Propranolol HCl | Chitosan, TPP, Carbopol 940, poloxamer 407. | To develop a propranolol-chitosan NPs transdermal gels to improve the systemic bioavailability of the drug. | 1. In vitro drug release was performed in buffer solution, only 7% and 11% of propranolol was released in 24 h from nanoparticle suspension and gel. 2. Ex vivo drug release was performed in pig ear, skin showed a slow permeation rate from nanoparticles in gel over 24 h. | [77] | |
| C-glycosyl flavonoid enriched fractions of cecropia glaziovii (EFF-Cg) | Resomer PLGA, ploxamer 188, sorbitan monoaleate, chitosan | To develop and characterize EFF-Cg nanocomposites chitosan film containing PLGA NPs. | In vitro cytotoxicity study was performed in Vero cell line: chitosan film and nanocomposite film exhibited low toxicity. | [78] | |
| Alginate and pectin | Chitosan, TPP, Triton X-100 | Preparation of alginate and pectin chitosan NPs for oral drug delivery | 1. Cytotoxicity was performed in TR146 cells: Chit-NP showed cytocompatibility while Alg-NP, Pec-NP exhibited cytotoxicity at some concentrations. 2. Stability of NP in simulated salivary fluid: The Alg-NP were the most stable (a > 2 h), while Pec-NP and especially Chit-NP were unstable. | [79] | |
| Insulin | Chitosan MMW, PEG, PVP, trehalose | To develop and characterize chitosan films with insulin loaded PEG-b-PLA NPs | In vitro drug release of both insulin NPs showed pH dependent classic biphasic sustained release of protein over 5 weeks and insulin encapsulation efficiency of 30–70%. | [80] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics 2017, 9, 53. https://doi.org/10.3390/pharmaceutics9040053
Mohammed MA, Syeda JTM, Wasan KM, Wasan EK. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics. 2017; 9(4):53. https://doi.org/10.3390/pharmaceutics9040053
Chicago/Turabian StyleMohammed, Munawar A., Jaweria T. M. Syeda, Kishor M. Wasan, and Ellen K. Wasan. 2017. "An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery" Pharmaceutics 9, no. 4: 53. https://doi.org/10.3390/pharmaceutics9040053