Chemically Modified Cyclodextrins: An Attractive Class of Supramolecular Hosts for the Development of Aqueous Biphasic Catalytic Processes
Abstract
:1. Introduction
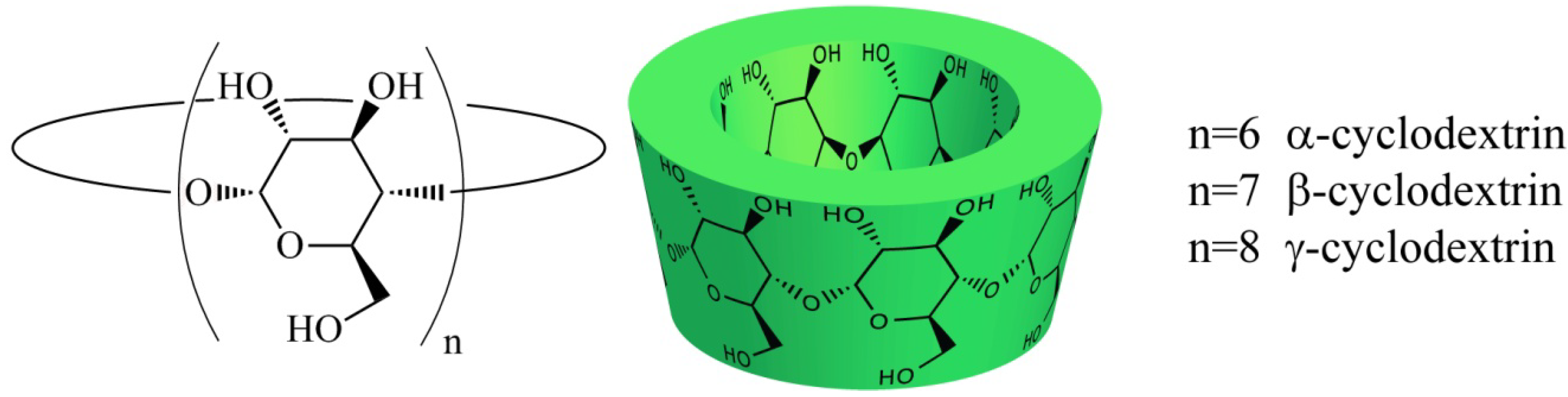
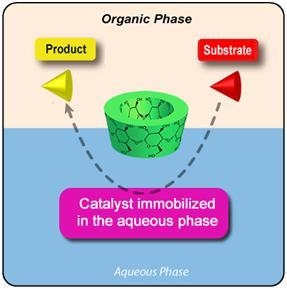
2. Results and Discussion
2.1. Cyclodextrins as Mass Transfer Additives in Aqueous Organometallic Catalysis
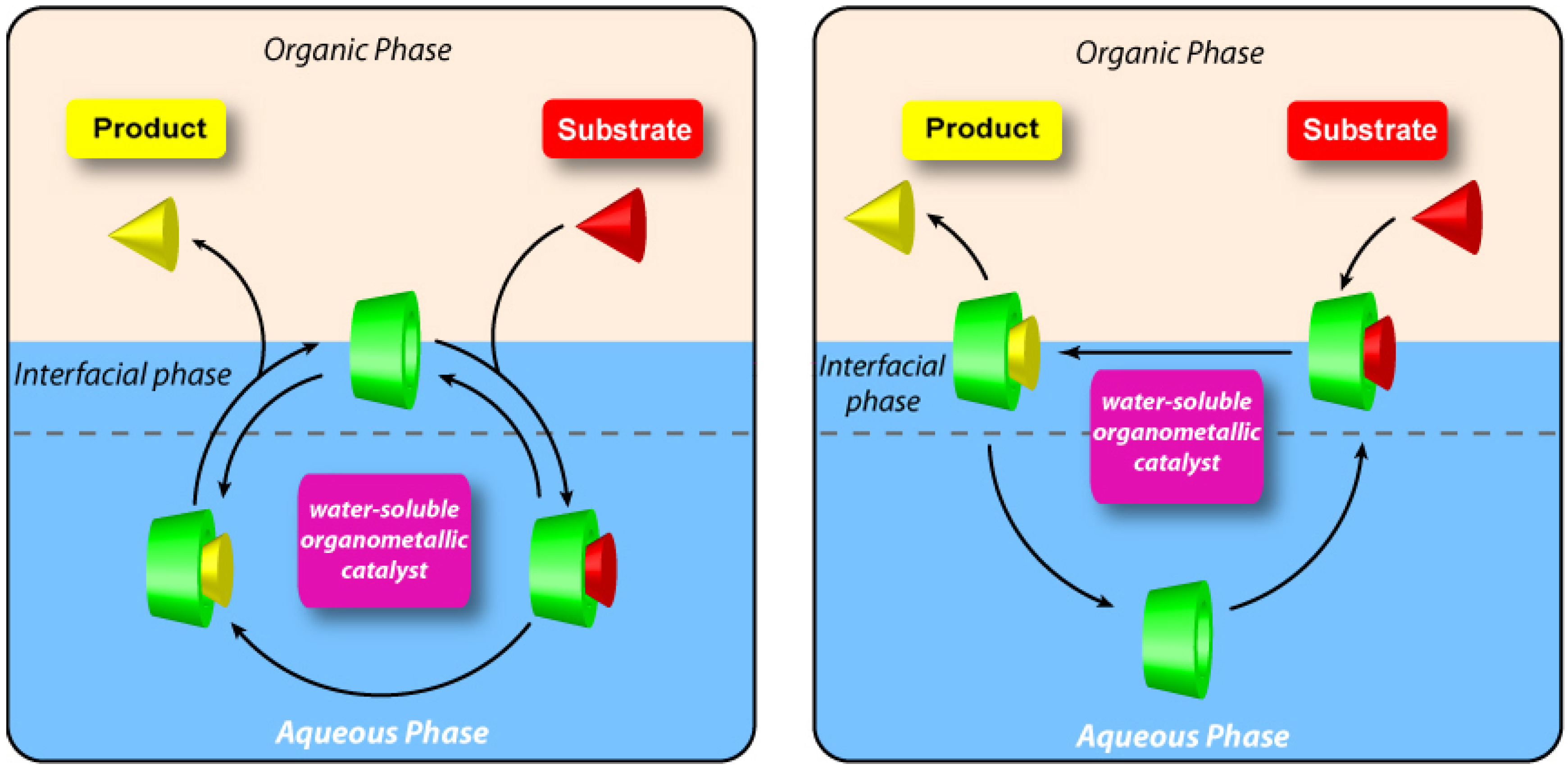
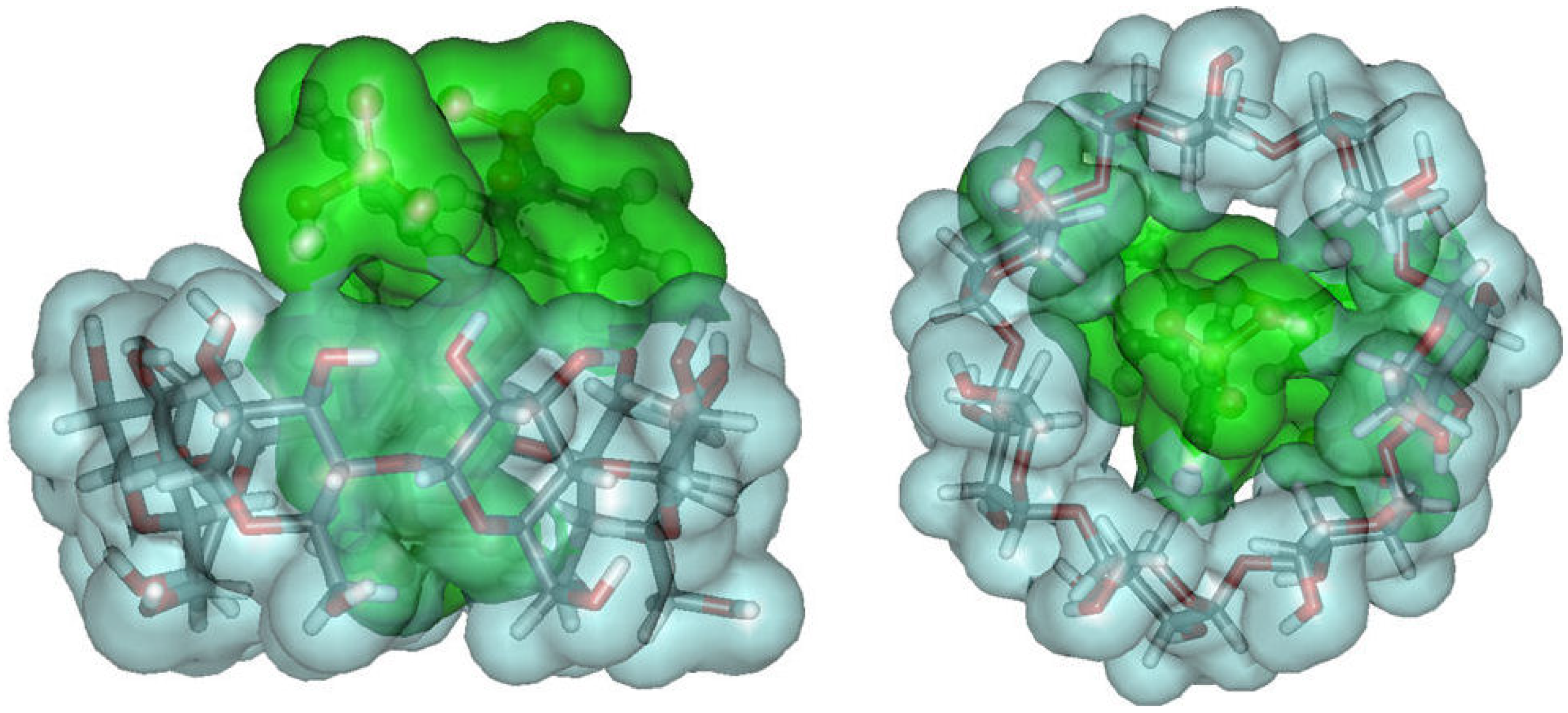
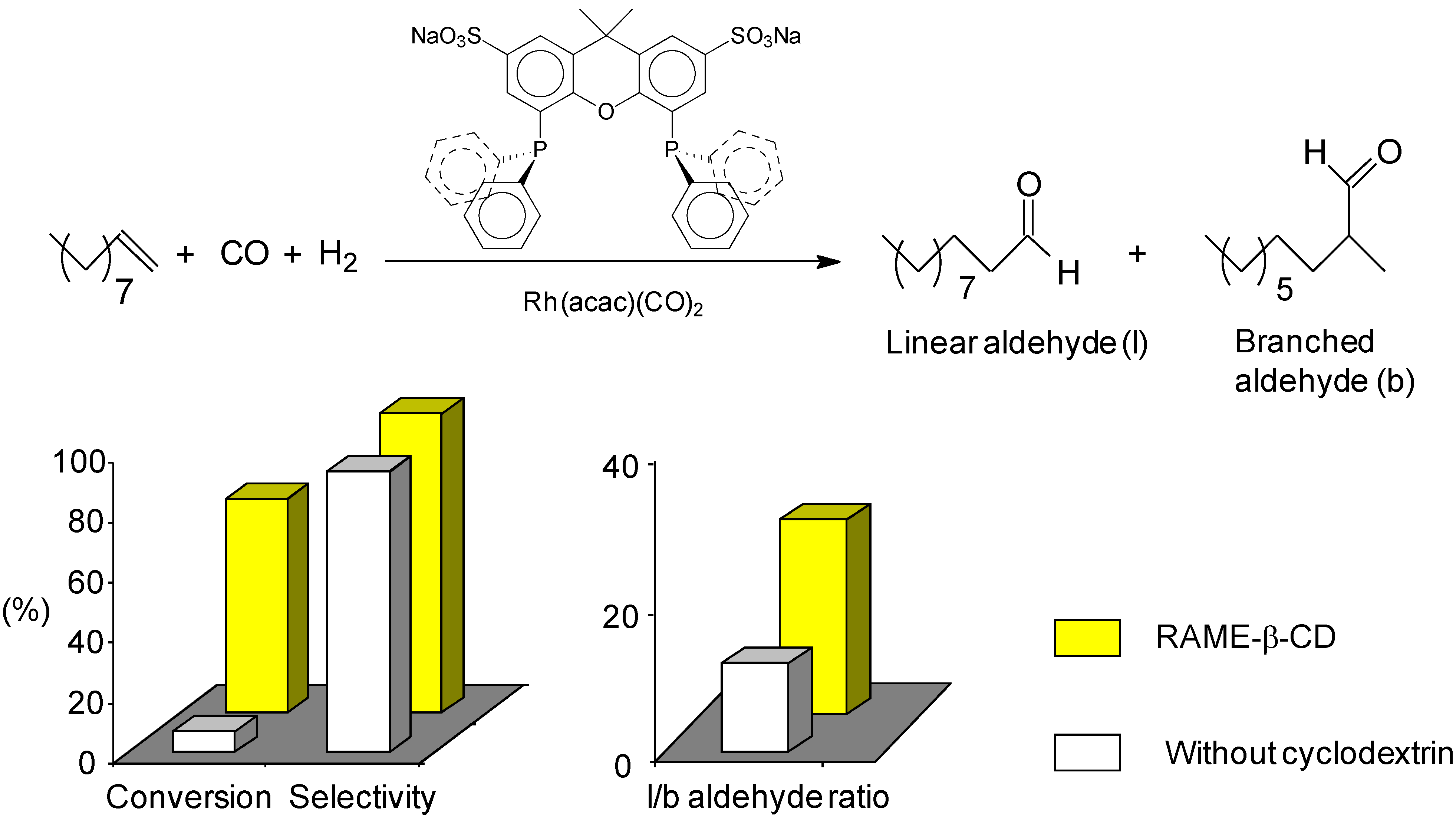

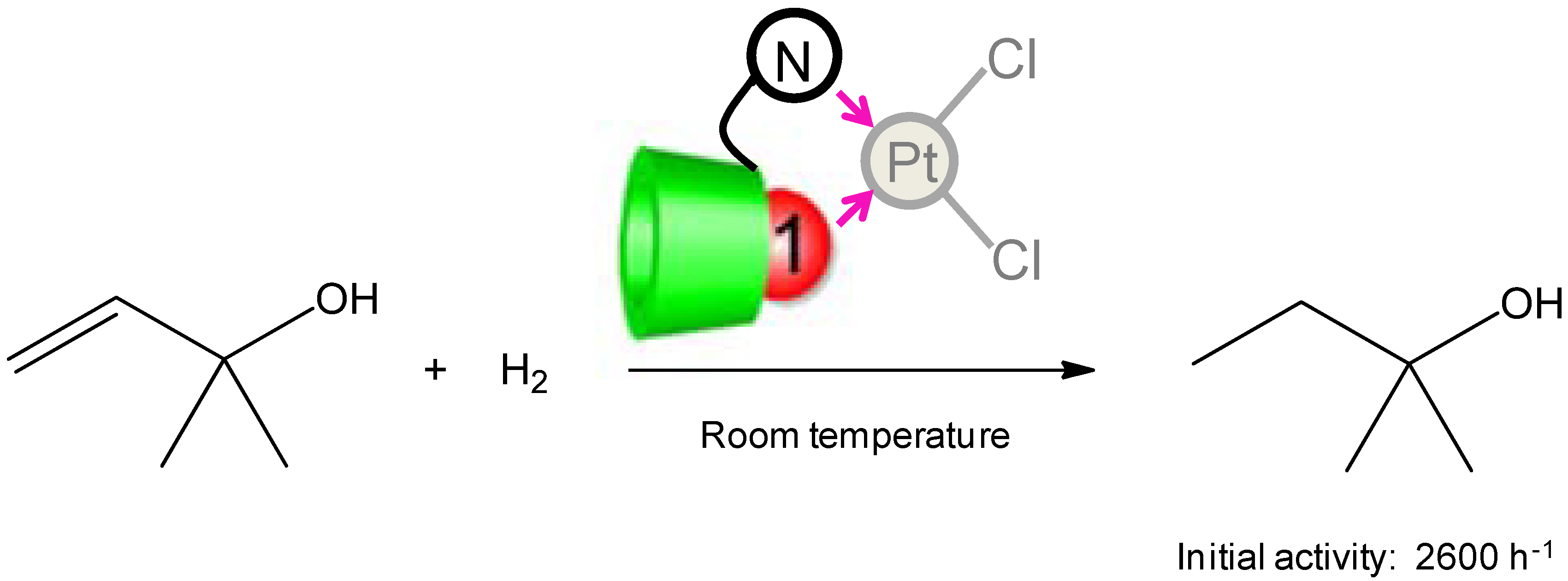
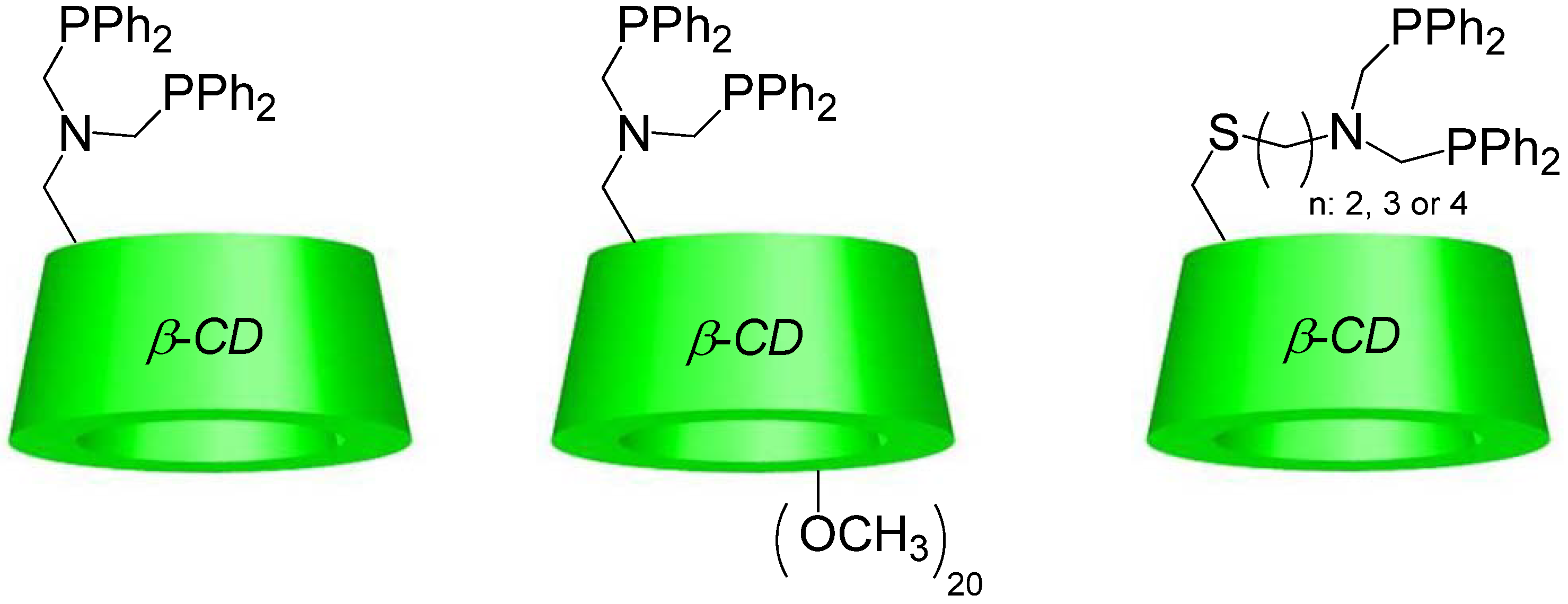
2.2. New Water-Soluble Catalysts Based on Cyclodextrins
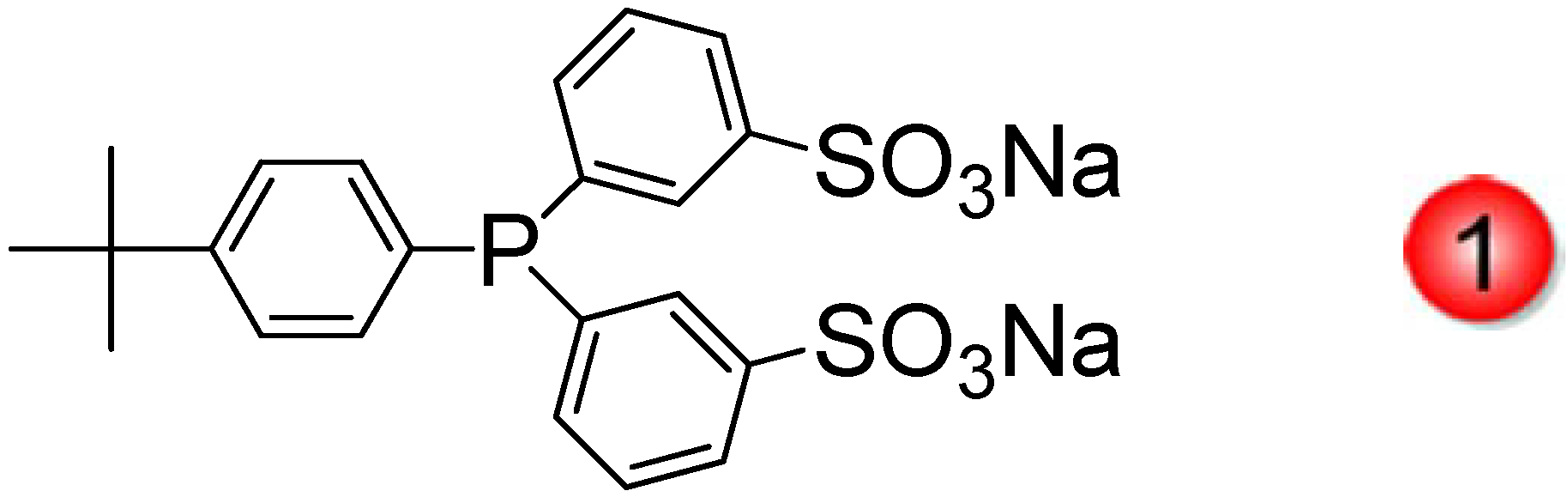
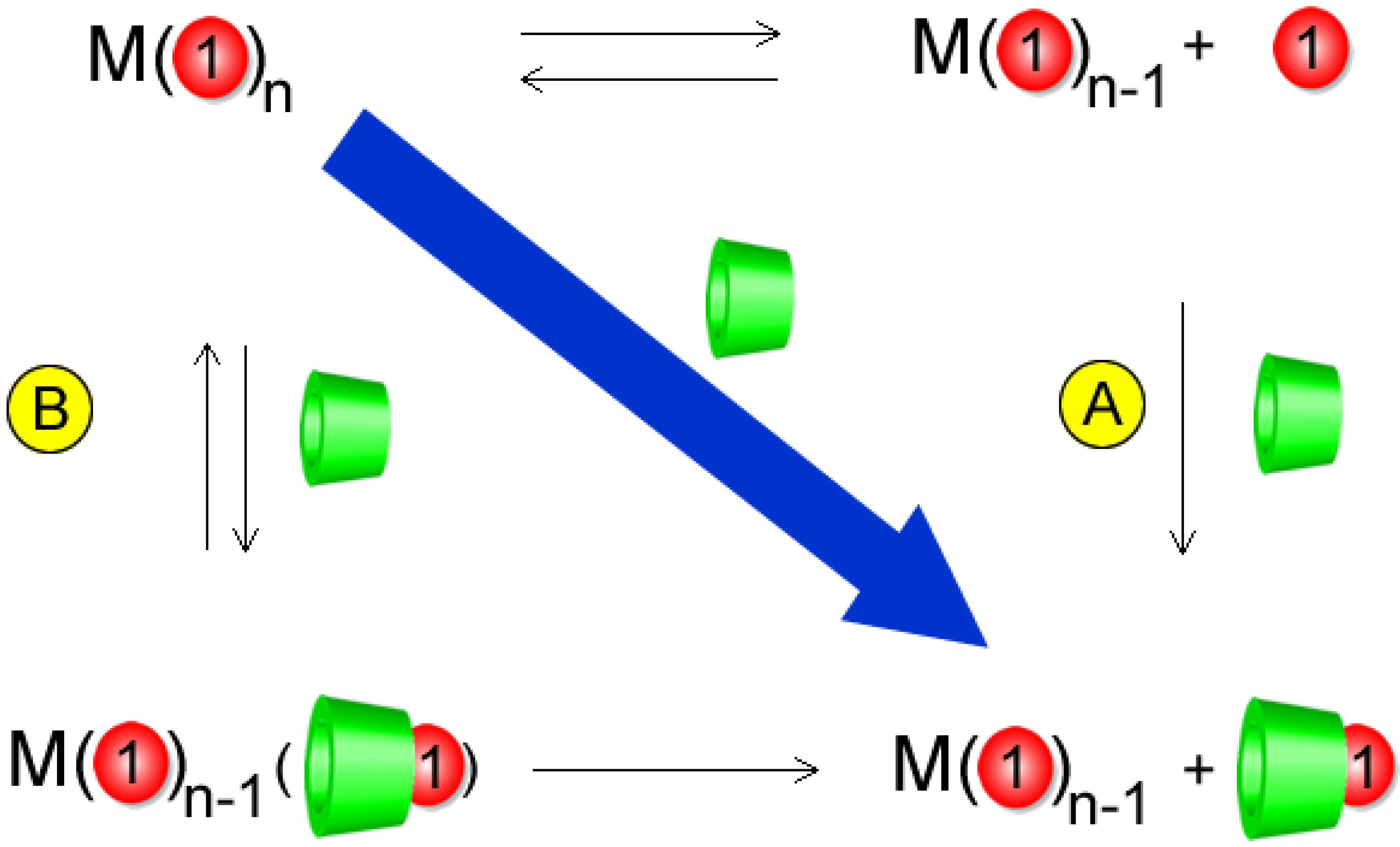


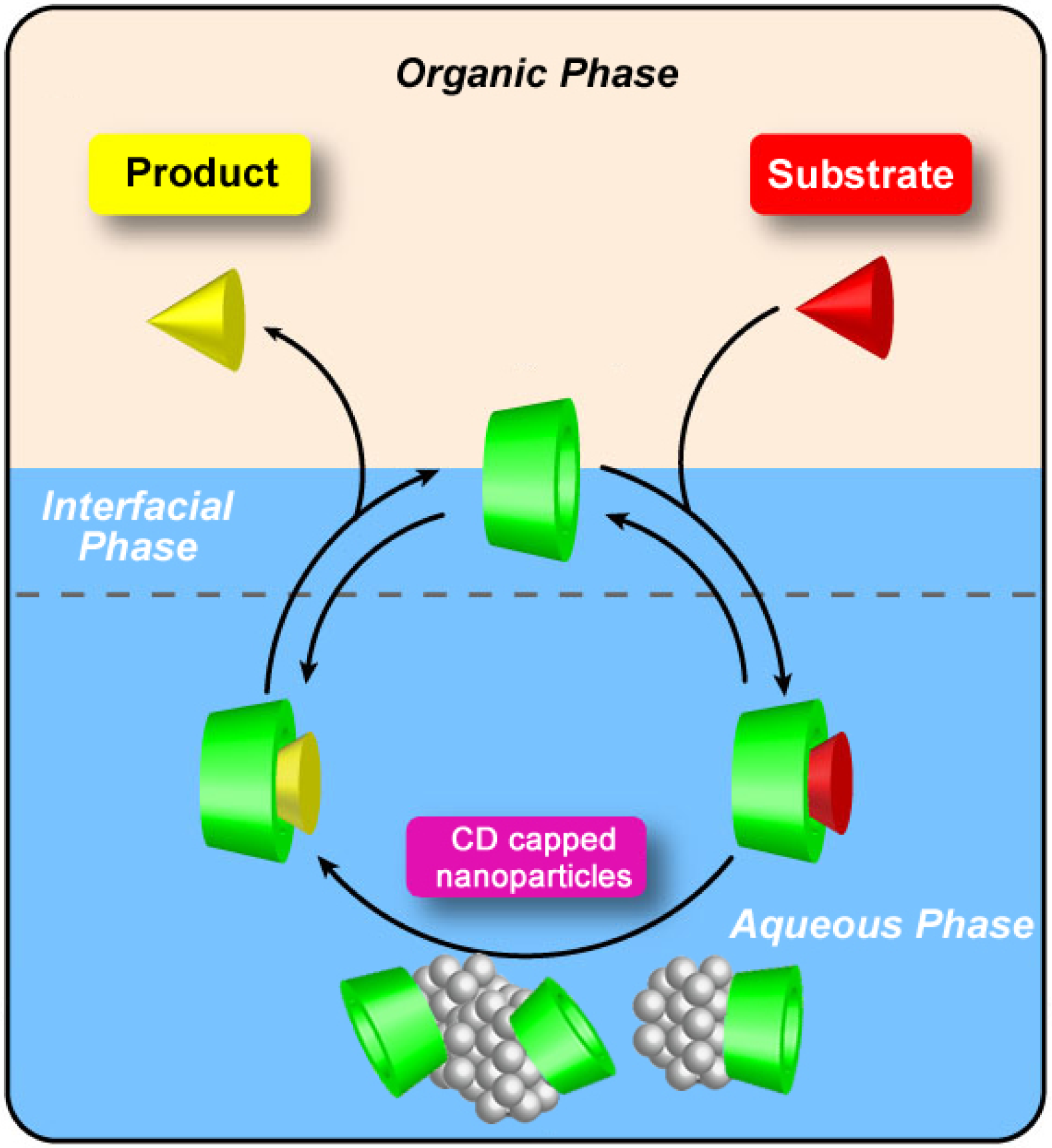
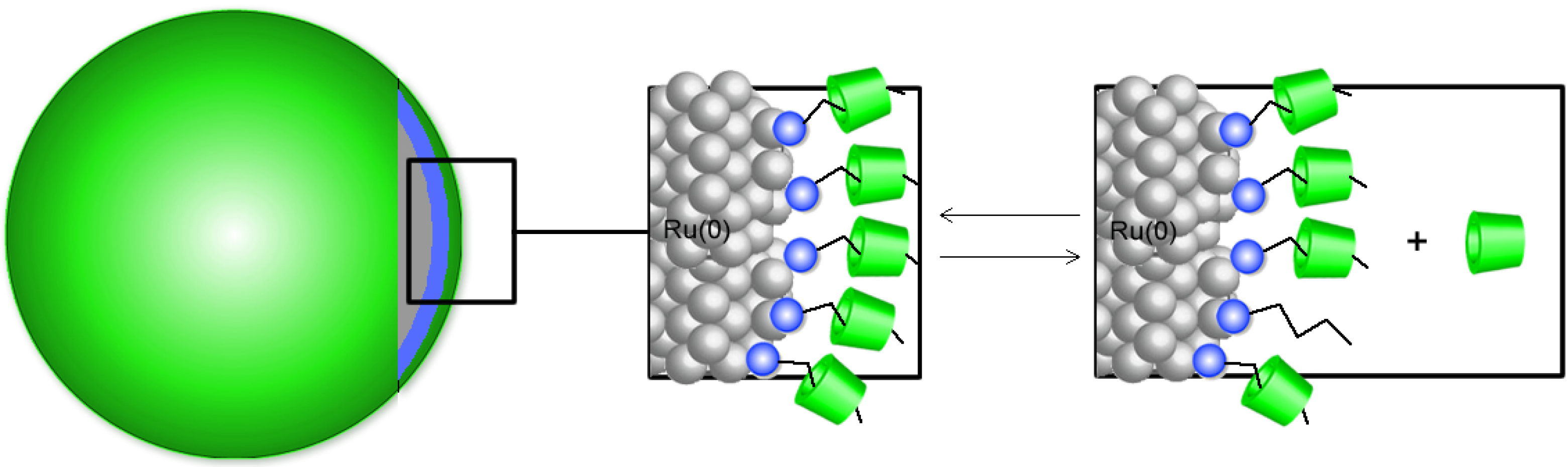
2.3. Water-Soluble Catalytically Active Metallic Nanoparticles Stabilized by Cyclodextrins
2.4. Dispersion and Activation of Palladium Charcoal by Cyclodextrins
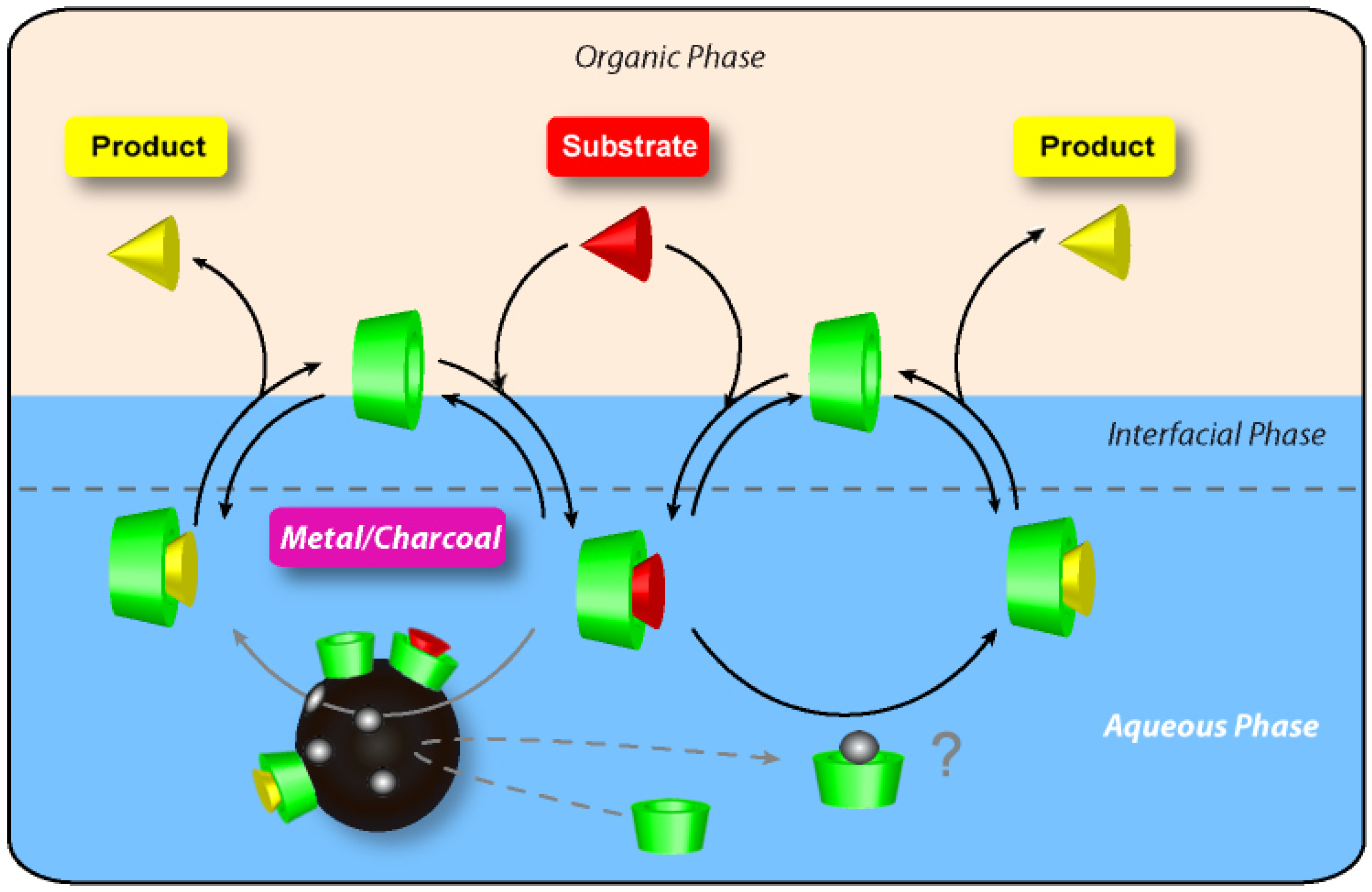
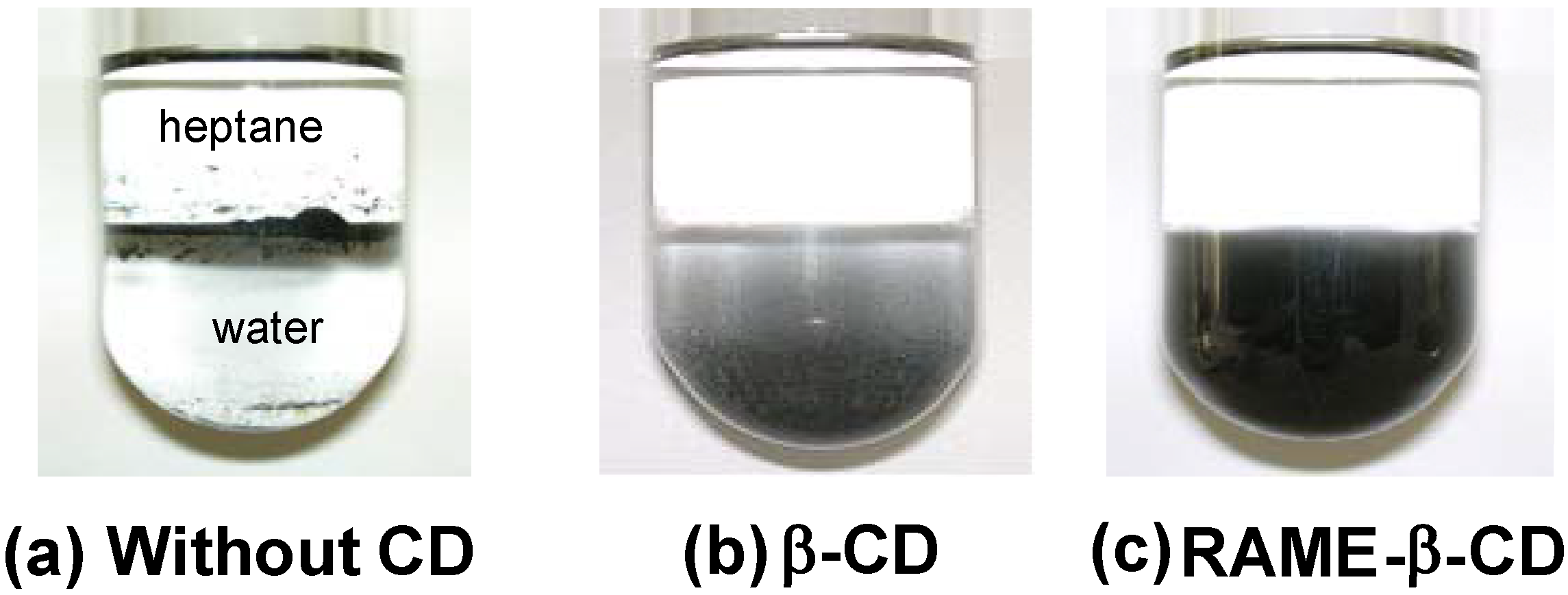
3. Conclusions
Acknowledgements
References and Notes
- Shaughnessy, K.H. Hydrophilic ligands and their application in aqueous-phase metal-catalyzed reactions. Chem. Rev. 2009, 109, 643–710. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xiao, J. Toward green catalytic synthesis—Transition metal-catalyzed reactions in non-conventional media. J. Mol. Catal. A: Chem. 2007, 270, 1–43. [Google Scholar] [CrossRef]
- Schulz, J.; Roucoux, A.; Patin, H. Unprecedented efficient hydrogenation of arenes in biphasic liquid–liquid catalysis by re-usable aqueous colloidal suspensions of rhodium. Chem. Commun. 1999, 535–536. [Google Scholar] [CrossRef]
- Hubert, C.; Denicourt-Nowicki, A.; Guégan, J.P.; Roucoux, A. Polyhydroxylated ammonium chloride salt: a new efficient surfactant for nanoparticles stabilisation in aqueous media. Characterization and application in catalysis. J. Chem Soc. Dalton Trans. 2009, 36, 7356–7358. [Google Scholar]
- Nowicki, A.; Le Boulaire, V.; Roucoux, A. Nanoheterogeneous catalytic hydrogenation of arenes: Evaluation of the surfactant-stabilized aqueous ruthenium(0) colloidal suspension. Adv. Synth. Catal. 2007, 349, 2326–2330. [Google Scholar] [CrossRef]
- Cornils, B. Industrial aqueous biphasic catalysis: Status and directions. Org. Process Res. Dev. 1998, 2, 121–127. [Google Scholar] [CrossRef]
- Cole-Hamilton, D.J.; Tooze, R.P. Catalyst Separation, Recovery and Recycling: Chemistry and Process Design; Series: Catalysis by Metal Complexes; Springer: Dordrecht, The Netherlands, 2006. [Google Scholar]
- Monflier, E.; Komiyama, M. Cyclodextrins and Catalysis. In Cyclodextrins and Their Complexes: Chemistry, Analytical Methods, Applications; Dodziuk, H., Ed.; Wiley-VCH: Weinheim, Germany, 2006; pp. 93–105. [Google Scholar]
- Szejtli, J. Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 1998, 98, 1743–1753. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.R.; Forgo, P.; Stine, K.J.; D’Souza, V.T. Methods for selective modifications of cyclodextrins. Chem. Rev. 1998, 98, 1977–1996. [Google Scholar] [CrossRef] [PubMed]
- Martin del Valle, E.M. Cyclodextrins and their uses: a review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Zahalka, H.A.; Januszkiewicz, K.; Alper, H. Olefin oxidation catalyzed by palladium chloride using cyclodextrins as phase transfer agents. J. Mol. Catal. 1986, 35, 249–253. [Google Scholar] [CrossRef]
- Harada, A.; Hu, Y.; Takahashi, S. Cyclodextrin-palladium choride. New catalytic system for selective oxidation of olefins to ketones. Chem. Lett. 1986, 15, 2083–2084. [Google Scholar] [CrossRef]
- Lee, J.T.; Alper, H. The hydridopentacyanocobaltate anion induced deoxygenation of allylic alcohols using β-cyclodextrin as a phase transfer agent. Tetrahedron Lett. 1990, 31, 4101–4104. [Google Scholar] [CrossRef]
- Lee, J.T.; Alper, H. β-cyclodextrin and hydridopentacyanocobaltate catalyzed selective hydrogenation of α,β-unsaturated acids and their derivatives. Tetrahedron Lett. 1990, 31, 1941–1942. [Google Scholar] [CrossRef]
- Pinel, C.; Gendreau-Diaz, N.; Bréhéret, A.; Lemaire, M. Asymmetric hydrogenation of α-keto ester with diamine-complex metal. J. Mol. Catal. A: Chem. 1996, 112, L157–L161. [Google Scholar] [CrossRef]
- Lee, J.T.; Alper, H. Regioselective hydrogenation of conjugated dienes catalyzed by hydridopentacyanocobaltate anion using β-cyclodextrin as the phase transfer agent and lanthanide halides as promoters. J. Org. Chem. 1990, 55, 1854–1856. [Google Scholar] [CrossRef]
- Zahalka, H.; Alper, H. β-cyclodextrin-promoted, rhodium(I)-catalyzed conversion of carbonyl compounds to hydrocarbons under remarkably mild conditions. Organometallics 1986, 5, 1909–1911. [Google Scholar] [CrossRef]
- Hirano, K.; Yorimitsu, H.; Oshima, K. Alkylation of aldehydes with trialkylboranes in water. Adv. Synth. Catal. 2006, 348, 1543–1546. [Google Scholar] [CrossRef]
- Hirano, K.; Yorimitsu, H.; Oshima, K. Nickel-catalysed reactions with trialkylboranes and silacyclobutanes. Chem. Commun. 2008, 28, 3234–3241. [Google Scholar] [CrossRef] [Green Version]
- Benyei, A.; Joo, F. Organometallic catalysis in aqueous solutions: The biphasic transfer hydrogenation of aldehydes catalyzed by water-soluble phosphine complexes of ruthenium, rhodium and iridium. J. Mol. Catal. 1990, 58, 151–163. [Google Scholar] [CrossRef]
- Anderson, J.R.; Campi, E.M.; Jackson, W.R. Hydroformylation of olefins with water-soluble rhodium catalysts in the presence of α-cyclodextrin. Catal. Lett. 1991, 9, 55–58. [Google Scholar] [CrossRef]
- Monflier, E.; Blouet, E.; Barbaux, Y.; Mortreux, A. Wacker oxidation of 1-decene to 2-decanone in the presence of a chemically modified cyclodextrin system: A happy union of host-guest chemistry and homogeneous catalysis. Angew. Chem., Int. Ed. Engl. 1994, 33, 2100–2102. [Google Scholar] [CrossRef]
- Monflier, E.; Tilloy, S.; Blouet, E.; Barbaux, Y.; Mortreux, A. Wacker oxidation of various olefins in the presence of per(2,6-di-O-methyl)-β-cyclodextrin: Mechanistic investigations of a multistep catalysis in a solvent free two phase system. J. Mol. Catal. A: Chem. 1996, 109, 27–35. [Google Scholar] [CrossRef]
- Monflier, E.; Tilloy, S.; Fremy, G.; Barbaux, Y.; Mortreux, A. A very useful and efficient Wacker oxidation of higher α-olefins in the presence of per(2,6-di-O-methyl)-β-cyclodextrin. Tetrahedron. Lett. 1995, 36, 387–388. [Google Scholar] [CrossRef]
- Maksimov, A.L.; Sakharov, D.A.; Filippova, T.Y.; Zhuchkova, A.Y.; Karakhanov, E.A. Supramolecular catalysts on the basis of molecules-receptors. Ind. Eng. Chem. Res. 2005, 44, 8644–8653. [Google Scholar] [CrossRef]
- Karakhanov, E.A.; Filippova, T.Y.; Martynova, S.A.; Maximov, A.L.; Predeina, V.V.; Topchieva, I.N. New catalytic systems for selective oxidation of aromatic compounds by hydrogen peroxide. Catal. Today 1998, 44, 189–198. [Google Scholar] [CrossRef]
- Karakhanov, E.A.; Kardasheva, Y.; Kirillov, A.; Maximov, A.; Predeina, V.; Runova, E. Surface active macromolecular and supramolecular complexes: Design and catalysis. Macromol. Symp. 2000, 156, 137–145. [Google Scholar] [CrossRef]
- Tilloy, S.; Bricout, H.; Monflier, E. Cyclodextrins as inverse phase transfer catalysts for the biphasic catalytic hydrogenation of aldehydes: A green and easy alternative to conventional mass transfer promoters. Green Chem. 2002, 4, 188–193. [Google Scholar] [CrossRef]
- Monflier, E.; Tilloy, S.; Castanet, Y.; Mortreux, A. Chemically modified β-cyclodextrins: efficient supramolecular carriers for the biphasic hydrogenation of water-insoluble aldehydes. Tetrahedron Lett. 1998, 39, 2959–2960. [Google Scholar] [CrossRef]
- Hapiot, F.; Lyskawa, J.; Tilloy, S.; Bricout, H.; Monflier, E. Cyclodextrins or calixarenes: What is the best mass transfer promoter for Suzuki cross-coupling reactions in water? Adv. Synth. Catal. 2004, 346, 83–89. [Google Scholar] [CrossRef]
- Lacroix, T.; Bricout, H.; Tilloy, S.; Monflier, E. Chemically modified β-cyclodextrin as supramolecular carriers in biphasic palladium catalyzed cleavage of allylic carbonates: activity enhancement and substrate selective catalysis. Eur. J. Org. Chem. 1999, 11, 3127–3129. [Google Scholar] [CrossRef]
- Monflier, E.; Fremy, G.; Castanet, Y.; Mortreux, A. Molecular recognition between chemically modified β-cyclodextrin and dec-1-ene: New prospects for biphasic hydroformylation of water-insoluble olefins. Angew. Chem. Int. Ed. Engl. 1995, 34, 2269–2271. [Google Scholar] [CrossRef]
- Monflier, E.; Tilloy, S.; Fremy, G.; Castanet, Y.; Mortreux, A. A further breakthrough in biphasic, rhodium catalyzed hydroformylation: the use of per(2,6-di-O-methyl)-β-cyclodextrin as inverse phase transfer catalyst. Tetrahedron Lett. 1995, 36, 9481–9484. [Google Scholar] [CrossRef]
- Kalck, P.; Miquel, L.; Dessoudeix, M. Various approaches to transfers improvement during biphasic catalytic hydroformylation of heavy alkenes. Catal. Today 1998, 42, 431–440. [Google Scholar] [CrossRef]
- Dessoudeix, M.; Urrutigoïty, M.; Kalck, P. Catalytic activity enhancement of a cyclodextrin/water-soluble rhodium complex system due to its gradual supramolecular organization in the interphase. Eur. J. Inorg. Chem. 2001, 7, 1797–1800. [Google Scholar] [CrossRef]
- Mathivet, T.; Méliet, C.; Castanet, Y.; Mortreux, A.; Caron, L.; Tilloy, S.; Monflier, E. Rhodium catalyzed hydroformylation of water-insoluble olefins in the presence of chemically modified β-cyclodextrins: Evidence for ligand-cyclodextrin interactions and effect of various parameters on the activity and the aldehydes selectivity. J. Mol. Catal. A.: Chem. 2001, 176, 105–116. [Google Scholar] [CrossRef]
- Dabbawala, A.A.; Parmar, J.N.; Jasra, R.V.; Bajaj, H.C.; Monflier, E. Cobalt catalyzed hydroformylation of higher olefins in the presence of chemically modified cyclodextrins. Catal. Commun. 2009, 10, 1808–1812. [Google Scholar] [CrossRef]
- Monflier, E.; Tilloy, S.; Bertoux, F.; Castanet, Y.; Mortreux, A. New prospects for the palladium catalyzed hydrocarboxylation of higher α-olefins in two-phase system: The use of chemically modified-β-cyclodextrin. New J. Chem. 1997, 21, 857–859. [Google Scholar]
- Tilloy, S.; Bertoux, F.; Mortreux, A.; Monflier, E. Chemically modified β-cyclodextrins in biphasic catalysis: a fruitful contribution of the host-guest chemistry to the transition-metal catalyzed reactions. Catal. Today 1999, 48, 245–253. [Google Scholar] [CrossRef]
- Behr, A.; Leschinski, J.; Awungacha, C.; Simic, S.; Knoth, T. Telomerization of butadiene with glycerol: Reaction control through process engineering, solvents, and additives. Chem. Sus. Chem. 2009, 2, 71–76. [Google Scholar] [CrossRef]
- Bricout, H.; Caron, L.; Bormann, D.; Monflier, E. Substrate selective catalysis in aqueous/organic biphasic system with per(2,6-di-O-methyl)-β-cyclodextrin. Catal. Today 2001, 66, 355–361. [Google Scholar] [CrossRef]
- Torque, C.; Bricout, H.; Hapiot, F.; Monflier, E. Substrate-selective aqueous organometallic catalysis. How size and chemical modification of cyclodextrin influence the substrate selectivity. Tetrahedron 2004, 60, 6487–6493. [Google Scholar] [CrossRef]
- Cabou, J.; Bricout, H.; Hapiot, F.; Monflier, E. Methylated-β-cyclodextrins: Useful discriminating tools for substrate-selective reactions in aqueous organometallic catalysis. Catal. Commun. 2004, 5, 265–270. [Google Scholar] [CrossRef]
- Torque, C.; Sueur, B.; Cabou, J.; Bricout, H.; Hapiot, F.; Monflier, E. Substrate-selective aqueous organometallic catalysis. How small water-soluble organic molecules enhance the supramolecular discrimination. Tetrahedron 2005, 61, 4811–4817. [Google Scholar] [CrossRef]
- Leclercq, L.; Bricout, H.; Tilloy, S.; Monflier, E. Aqueous organometallic catalysis promoted by cyclodextrins. Can surface tension measurements explain the efficiency of chemically modified cyclodextrins? J. Colloid Interf. Sci. 2007, 307, 481–487. [Google Scholar] [CrossRef]
- Legrand, F.X.; Sauthier, M.; Flahaut, C.; Hachani, J.; Elfakir, C.; Fourmentin, S.; Tilloy, S.; Monflier, E. Aqueous hydroformylation reaction mediated by randomly methylated β-cyclodextrin: How substitution degree influences catalytic activity and selectivity. J. Mol. Catal. A: Chem. 2009, 303, 72–77. [Google Scholar] [CrossRef]
- Sieffert, N.; Wipff, G. Adsorption at the liquid-liquid interface in the biphasic rhodium catalyzed hydroformylation of olefins promoted by cyclodextrins: A molecular dynamics study. J. Phys. Chem. B 2006, 110, 4125–4134. [Google Scholar] [CrossRef] [PubMed]
- Sieffert, N.; Wipff, G. Importance of interfacial adsorption in the biphasic hydroformylation of higher olefins promoted by cyclodextrins: A molecular dynamics study at the decene/water interface. Chem. Eur. J. 2007, 13, 1978–1990. [Google Scholar] [CrossRef]
- Ding, L.; Jiao, X.; Deng, J.; Zhao, W.; Yang, W. Catalytic polymerizations of hydrophobic, substituted acetylene monomers in an aqueous medium by using a monomer/hydroxypropyl-β-cyclodextrin inclusion complexation. Macromol. Rapid Commun. 2009, 30, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Monflier, E.; Tilloy, S.; Méliet, C.; Mortreux, A.; Fourmentin, S.; Landy, D.; Surpateanu, G. First evidence of molecular recognition between cyclodextrins and a water-soluble ligand used in aqueous phase organometallic catalysis. New J. Chem. 1999, 23, 469–472. [Google Scholar] [CrossRef]
- Caron, L.; Tilloy, S.; Monflier, E.; Wieruszeski, J.M.; Lippens, G.; Fourmentin, S.; Landy, D.; Surpateanu, G. Study of inclusion complexes of the β-cyclodextrin with the sodium salt of trisulfonated triphenylphosphine. J. Inclusion Phenom. 2000, 38, 661–679. [Google Scholar] [CrossRef]
- da Costa, A.; Monflier, E.; Landy, D.; Fourmentin, S.; Surpateanu, G. Scanning tunneling microscopy investigation of an inclusion complex between the β-cyclodextrin and the sodium salt of the trisulfonated triphenylphosphine. Surface Sci. 2001, 470, 275–283. [Google Scholar] [CrossRef]
- Caron, L.; Christine, C.; Tilloy, S.; Monflier, E.; Landy, D.; Fourmentin, S.; Surpateanu, G. One and two-dimensional NMR investigations of the inclusion of the monosulfonated triphenylphosphine in the β-cyclodextrin. Supramol. Chem. 2002, 14, 11–20. [Google Scholar] [CrossRef]
- Canipelle, M.; Caron, L.; Christine, C.; Tilloy, S.; Monflier, E. Thermodynamic insight into the origin of the inclusion of monosulfonated isomers of triphenylphosphine in the β-cyclodextrin. Carbohydr. Res. 2002, 337, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Canipelle, M.; Tilloy, S.; Bricout, H.; Monflier, E. Complexation of monosulfonated triphenylphosphine with chemically modified β-cyclodextrins: Effect of substituents on the stability of inclusion complexes. J. Inclusion Phenom. 2005, 51, 79–85. [Google Scholar] [CrossRef]
- Binkowski, C.; Cabou, J.; Bricout, H.; Hapiot, F.; Monflier, E. Cleavage of water-insoluble alkylallylcarbonates catalyzed by a palladium/TPPTS/cyclodextrin system: Effect of phosphine/cyclodextrin interactions on the reaction rate. J. Mol. Catal. A: Chem. 2004, 215, 23–32. [Google Scholar] [CrossRef]
- Monflier, E.; Bricout, H.; Hapiot, F.; Tilloy, S.; Aghmiz, A.; Masdeu-Bulto, A.M. High-Pressure 31P{1H} NMR studies of RhH(CO)(TPPTS)3 in the presence of methylated cyclodextrins: New light on rhodium catalyzed hydroformylation reaction assisted by cyclodextrins. Adv. Synth. Catal. 2004, 346, 425–431. [Google Scholar] [CrossRef]
- Blach, P.; Landy, D.; Fourmentin, S.; Surpateanu, G.; Bricout, H.; Ponchel, A.; Hapiot, F.; Monflier, E. Sulfobutyl ether-β-cyclodextrins: Promising supramolecular carriers for aqueous organometallic catalysis. Adv. Synth. Catal. 2005, 347, 1301–1307. [Google Scholar] [CrossRef]
- Kirschner, D.; Green, T.; Hapiot, F.; Tilloy, S.; Leclercq, L.; Bricout, H.; Monflier, E. Heptakis(2,3-di-O-methyl-6-O-sulfopropyl)-β-cyclodextrin: A genuine supramolecular carrier for the Aqueous Organometallic Catalysis. Adv. Synth. Catal. 2006, 348, 379–386. [Google Scholar] [CrossRef]
- Kirschner, D.; Jaramillo, M.; Green, T.; Hapiot, F.; Leclercq, L.; Bricout, H.; Monflier, E. Fine tuning of sulfoalkylated cyclodextrin structures to improve their mass-transfer properties in an aqueous biphasic hydroformylation reaction. J. Mol. Catal. A: Chem. 2008, 286, 11–20. [Google Scholar] [CrossRef]
- Ferreira, M.; Bricout, H.; Sayede, A.; Ponchel, A.; Fourmentin, S.; Tilloy, S.; Monflier, E. Biphasic aqueous organometallic catalysis promoted by cyclodextrins: How to design the water-soluble phenylphosphane to avoid interaction with cyclodextrin. Adv. Synth. Catal. 2008, 350, 609–618. [Google Scholar] [CrossRef]
- Legrand, F.X.; Hapiot, F.; Tilloy, S.; Guerriero, A.; Peruzzini, M.; Gonsalvi, L.; Monflier, E. Aqueous rhodium-catalyzed hydroformylation of 1-decene in the presence of randomly methylated β-cyclodextrin and 1,3,5-triaza-7-phosphaadamantane derivatives. Appl. Catal. A: General 2009, 362, 62–66. [Google Scholar] [CrossRef]
- Tilloy, S.; Crowyn, G.; Monflier, E.; van Leeuwen, P.W.N.M.; Reek, J.N.H. Hydroformylation of 1-decene in aqueous medium catalyzed by rhodium/alkyl sulfonated diphosphines system in the presence of methylated β-cyclodextrins. How the flexibility of the diphosphine backbone influences the regioselectivity. New J. Chem. 2006, 30, 377–383. [Google Scholar] [CrossRef]
- Leclercq, L.; Hapiot, F.; Tilloy, S.; Ramkisoensing, K.; Reek, J.N.H.; van Leeuwen, P.W.N.M.; Monflier, E. Sulfonated Xantphos ligand and methylated cyclodextrin: A winning combination for rhodium catalysed hydroformylation of higher olefins in aqueous medium. Organometallics 2005, 24, 2070–2075. [Google Scholar] [CrossRef]
- Tilloy, S.; Hapiot, F.; Landy, D.; Fourmentin, S.; Michelet, V.; Genêt, J.P.; Monflier, E. Water-soluble triphenylphosphane-3,3’,3’’-tricarboxylate (m-TPPTC) ligand and methylated cyclodextrins: a new combination for biphasic rhodium catalyzed hydroformylation of higher olefins. Adv. Synth. Catal. 2006, 348, 1547–1522. [Google Scholar] [CrossRef]
- Leclercq, L.; Sauthier, M.; Castanet, Y.; Mortreux, A.; Bricout, H.; Monflier, E. Two-phase hydroformylation of higher olefins using randomly methylated α-cyclodextrin as mass transfer promoter: A smart solution for preserving the catalytic properties of the rhodium/trisulfonated triphenylphoshine catalytic system. Adv. Synth. Catal. 2005, 347, 55–59. [Google Scholar] [CrossRef]
- Sueur, B.; Leclercq, L.; Sauthier, M.; Castanet, Y.; Mortreux, A.; Bricout, H.; Tilloy, S.; Monflier, E. Rhodium complexes non-covalently bound to cyclodextrins: Novel water-soluble supramolecular catalysts for the biphasic hydroformylation of higher olefins. Chem. Eur. J. 2005, 11, 6228–6236. [Google Scholar] [CrossRef] [PubMed]
- Caron, L.; Canipelle, M.; Tilloy, S.; Bricout, H.; Monflier, E. Unexpected effect of cyclodextrins on water-soluble phosphine modified rhodium hydroformylation catalysts. Eur. J. Inorg. Chem. 2003, 595–599. [Google Scholar] [CrossRef]
- Binkowski-Machut, C.; Canipelle, M.; Bricout, H.; Tilloy, S.; Hapiot, F.; Monflier, E. Supramolecular trapping of phosphanes by cyclodextrins: A general approach to generate phosphane coordinatively unsaturated organometallic complexes. Eur. J. Inorg. Chem. 2006, 8, 1611–1619. [Google Scholar] [CrossRef]
- Machut, C.; Patrigeon, J.; Tilloy, S.; Bricout, H.; Hapiot, F.; Monflier, E. First example of self-assembled supramolecular bidentate ligands for aqueous organometallic catalysis. Angew. Chem. Int. Ed. 2007, 46, 3040–3042. [Google Scholar] [CrossRef]
- Caron, L.; Bricout, H.; Tilloy, S.; Ponchel, A.; Landy, D.; Fourmentin, S.; Monflier, E. Molecular recognition between a water-soluble organometallic complex and a β-cyclodextrin: First example of second-sphere coordination adducts possessing a catalytic activity. Adv. Synth. Catal. 2004, 346, 1449–1456. [Google Scholar] [CrossRef]
- Breslow, R.; Dong, S.D. Biomimetic reactions catalyzed by cyclodextrins and their derivatives. Chem. Rev. 1998, 98, 1997–2011. [Google Scholar] [CrossRef] [PubMed]
- Breslow, R.; Overman, L.E. Artificial enzyme combining a metal catalytic group and a hydrophobic binding cavity. J. Am. Chem. Soc. 1970, 92, 1075–1075. [Google Scholar] [CrossRef] [PubMed]
- Steinborn, D.; Junicke, H. Carbohydrate complexes of Platinum-group metals. Chem. Rev. 2000, 100, 4283–4317. [Google Scholar] [CrossRef] [PubMed]
- McNaughton, M.; Engmann, L.; Birmingham, A.; Powis, G.; Cotgreave, I.A. Cyclodextrin-derived diorganyl tellurides as glutathione peroxidase mimics and inhibitors of thioredoxin reductase and cancer cell growth. J. Med. Chem. 2004, 47, 233–239. [Google Scholar] [CrossRef] [PubMed]
- French, R.R.; Holzer, P.; Leuenberger, M.G.; Woggon, W.D. A supramolecular enzyme mimic that catalyzes the 15,15' double bond scission of β,β-carotene. Angew. Chem. Int. Ed. 2000, 39, 1267–1269. [Google Scholar] [CrossRef]
- Zhoua, Y.H.; Zhao, M.; Sun, H.; Mao, Z.W.; Ji, L.N. Effect of cyclodextrin dimers with bipyridyl and biphenyl linking groups on carboxyl ester hydrolysis catalyzed by zinc complex. J. Mol. Catal. A: Chem. 2009, 308, 61–67. [Google Scholar] [CrossRef]
- Tastan, P.; Akkaya, E.U. A novel cyclodextrin homodimer with dual-mode substrate binding and esterase activity. J. Mol. Catal. A: Chem. 2000, 157, 261–263. [Google Scholar] [CrossRef]
- Reetz, M.T.; Waldvogel, S.R. β-cyclodextrin-modified diphosphanes as ligands for supramolecular rhodium catalysts. Angew. Chem., Int. Ed. Engl. 1997, 36, 865–867. [Google Scholar] [CrossRef]
- Reetz, M.T. New approaches to supramolecular transition metal complexes. Top. Catal. 1997, 4, 187–200. [Google Scholar] [CrossRef]
- Reetz, M.T. New supramolecular transition metal catalysis. J. Heterocyclic Chem. 1998, 35, 1065–1073. [Google Scholar] [CrossRef]
- Reetz, M.T.; Frömbgen, C. Chemoselective reduction of halo-nitro aromatic compounds by β-cyclodextrin-modified transition metal catalysts in a biphasic system. Synthesis 1999, 9, 1555–1557. [Google Scholar] [CrossRef]
- Armspach, D.; Matt, D. Metal-capped α-cyclodextrins: The crowing of the oligosaccharide torus with precious metals. Chem. Commun. 1999, 12, 1073–1074. [Google Scholar] [CrossRef]
- Deshpande, R.M.; Fukuoka, A.; Ichikawa, M. Novel phosphinite capped cyclodextrin rhodium catalyst in substrate selective hydroformylation. Chem. Lett. 1999, 28, 13–14. [Google Scholar] [CrossRef]
- Karakhanov, E.A.; Maksimov, A.L.; Runova, E.A.; Kardasheva, Y.S.; Terenina, M.V.; Buchneva, T.S.; Guchkova, A.Y. Supramolecular catalytic system based on calixarenes and cyclodextrins. Macromol. Symp. 2003, 204, 159–173. [Google Scholar] [CrossRef]
- Karakhanov, E.; Maximov, A.; Kirillov, A. Biphasic Wacker-oxidation of 1-octene catalyzed by palladium complexes with modified β-cyclodextrins. J. Mol. Catal. A: Chem. 2000, 157, 25–30. [Google Scholar] [CrossRef]
- Karakhanov, E.A.; Karapetyan, L.M.; Kardasheva, Y.S.; Maksimov, A.L.; Runova, E.A.; Skorkin, V.A.; Terenina, M.V. Molecular recognition and catalysis: from macrocyclic receptors to molecularly imprinted metal complexes. Macromol. Symp. 2006, 235, 39–51. [Google Scholar] [CrossRef]
- Schlatter, A.; Kundu, M.K.; Woggon, W.D. Enantioselective reduction of aromatic and aliphatic ketones catalyzed by ruthenium complexes attached to β-Cyclodextrin. Angew. Chem. Int. Ed. 2004, 43, 6731–6734. [Google Scholar] [CrossRef]
- Schlatter, A.; Woggon, W.D. Enantioselective transfer hydrogenation of aliphatic ketones catalyzed by ruthenium complexes linked to the secondary face of β-Cyclodextrin. Adv. Synth. Catal. 2008, 350, 995–1000. [Google Scholar] [CrossRef]
- Komiyama, M.; Hirai, H. Colloidal rhodium dispersions protected by cyclodextrins. Bull. Chem. Soc. Jpn. 1983, 56, 2833–2834. [Google Scholar] [CrossRef]
- Mandler, D.; Willner, I. Effective photoreduction of CO2/HCO3– to formate using visible light. J. Am. Chem. Soc. 1987, 109, 7884–7885. [Google Scholar] [CrossRef]
- Willner, I.; Mandler, D. Characterization of Pd-β-cyclodextrin colloids as catalysts in the photosensitized reduction of bicarbonate to formate. J. Am. Chem. Soc. 1989, 111, 1330–1336. [Google Scholar] [CrossRef]
- Huang, T.; Meng, F.; Qi, L. Facile synthesis and one-dimensional assembly of cyclodextrin-capped gold nanoparticles and their applications in catalysis and surface-enhanced Raman scattering. J. Phys. Chem. C 2009, 113, 13636–13642. [Google Scholar] [CrossRef]
- Alvarez, J.; Liu, J.; Roman, E.; Kaifer, A.E. Water-soluble platinum and palladium nanoparticles modified with thiolated β-cyclodextrin. Chem. Commun. 2000, 1151–1152. [Google Scholar] [CrossRef]
- Liu, J.; Alvarez, J.; Ong, W.; Roman, E.; Kaifer, A.E. Tuning the catalytic activity of cyclodextrin modified palladium nanoparticles though host-guest binding interactions. Langmuir 2001, 17, 6762–6764. [Google Scholar] [CrossRef]
- Mhadgut, S.C.; Palaniappan, K.; Thimmaiah, M.; Hackney, S.A.; Török, B.; Liu, J. A metal nanoparticle-based supramolecular approach for aqueous biphasic reactions. Chem. Commun. 2005, 3207–3209. [Google Scholar] [CrossRef]
- Strimbu, L.; Liu, J.; Kaifer, A.E. Cyclodextrin-capped palladium nanoparticles as catalysts for the Suzuki reaction. Langmuir 2003, 19, 483–485. [Google Scholar] [CrossRef]
- Xue, C.; Palaniappan, K.; Arumugam, G.; Hackney, S.A.; Liu, J.; Liu, H. Sonogashira reactions catalyzed by water-soluble, β-cyclodextrin-capped palladium nanoparticles. Catal. Lett. 2007, 116, 94–100. [Google Scholar] [CrossRef]
- Nowicki, A.; Zhang, Y.; Léger, B.; Rolland, J.P.; Bricout, H.; Monflier, E.; Roucoux, A. Supramolecular shuttle and protective agent: A multiple role of methylated cyclodextrins in the chemoselective hydrogenation of benzene derivatives with ruthenium particles. Chem. Commun. 2006, 296–298. [Google Scholar] [CrossRef]
- Nowicki-Denicourt, A.; Ponchel, A.; Monflier, E.; Roucoux, A. Methylated Cyclodextrins: An efficient protective agent in water for zerovalent ruthenium nanoparticles and a supramolecular shuttle in alkene and arene hydrogenation reactions. J. Chem. Soc. Dalton Trans. 2007, 48, 5714–5719. [Google Scholar] [CrossRef]
- Hubert, C.; Denicourt-Nowicki, A.; Roucoux, A.; Landy, D.; Léger, B.; Crowyn, G.; Monflier, E. Catalytically active nanoparticles stabilized by host–guest inclusion complexes in water. Chem. Commun. 2009, 1228–1230. [Google Scholar] [CrossRef]
- Fornasier, R.; Marcuzzi, F.; Zorzi, D. High selectivity in the catalytic hydrogenation of acyl substituted pyridines in alkaline solution: Effect of complexation by β-cyclodextrin. J. Mol. Catal. 1987, 43, 21–26. [Google Scholar] [CrossRef]
- Shimizu, S.; Sasaki, Y.; Hirai, C. Inverse phase transfer catalysis by cyclodextrins. Palladium catalyzed reduction of bromoanisoles with sodium formate. Bull. Chem. Soc. Jpn. 1990, 63, 176–178. [Google Scholar] [CrossRef]
- Cassez, A.; Ponchel, A.; Bricout, H.; Fourmentin, S.; Landy, D.; Monflier, E. Eco-efficient catalytic hydrodechloration of carbon tetrachloride in aqueous cyclodextrin solutions. Catal. Lett. 2006, 108, 209–214. [Google Scholar] [CrossRef]
- Cassez, A.; Ponchel, A.; Hapiot, F.; Monflier, E. Unexpected multi-functional effects of methylated cyclodextrins in palladium charcoal-catalyzed Suzuki-Miyaura reaction. Org. Lett. 2006, 8, 4823–4826. [Google Scholar] [CrossRef] [PubMed]
- Cassez, A.; Kania, N.; Hapiot, F.; Fourmentin, S.; Monflier, E.; Ponchel, A. Chemically modified cyclodextrins adsorbed on Pd/C particles: New opportunities to generate highly chemo- and stereoselective catalysts for Heck reaction. Catal. Commun. 2008, 9, 1346–1351. [Google Scholar] [CrossRef]
- Ponchel, A.; Hapiot, F.; Tilloy, S.; Roucoux, A.; Monflier, E. Methylated cyclodextrins: New opportunities for aqueous catalysis. In Proceedings of the 14th International Cyclodextrins Symposium, Kyoto, Japan, May 8–11, 2008; pp. 60–66.
- Senra, J.D.; Malta, L.F.B.; de Souza, A.L.F.; Medeiros, M.E.; Aguiar, L.C.S.; Antunes, O.A.C. Phosphine-free Heck reactions in aqueous medium using hydroxypropylated cyclodextrins as supramolecular hosts. Tetrahedron Lett. 2007, 48, 8153–8156. [Google Scholar] [CrossRef]
- Senra, J.D.; Malta, L.F.B.; Souza, A.L.F.; Aguiar, L.C.S.; Antunes, O.A.C. Palladium on calcium carbonate combined to 2-Hydroxypropyl-α/β-cyclodextrins: A selective catalytic system for aqueous Heck coupling and hydroarylation. Adv. Synth. Catal. 2008, 350, 2551–2558. [Google Scholar] [CrossRef]
- Andreeva, L.V.; Maksimov, A.L.; Zhuchkova, A.Y.; Predeina, V.V.; Filippova, T.Y.; Karakhanov, E.A. Oxidation of unsaturated compounds in ionic liquids with the use of cyclodextrin-containing catalytic systems. Petrol. Chem. 2007, 47, 331–336. [Google Scholar] [CrossRef]
- Filardo, G.; di Blasi, M.; Galia, A.; Ponchel, A.H.; Bricout, H.; Sayede, A.D.; Monflier, E. Peracetylated β-cyclodextrin as solubilizer of arylphosphines in supercritical carbon dioxide. J. Supercrit. Fluids 2006, 36, 173–181. [Google Scholar] [CrossRef]
- Galia, A.; Navarre, E.C.; Scialdone, O.; Ferreira, M.; Filardo, G.; Tilloy, S.; Monflier, E. Complexation of phosphine ligands with peracetylated-β-cyclodextrin in supercritical carbon dioxide: Spectroscopic determination of equilibrium constants. J. Phys. Chem. B 2007, 111, 2573–2578. [Google Scholar] [CrossRef]
- Tortosa-Estorach, C.; Giménez-Pedrós, M.; Masdeu-Bultó, A.M.; Sayede, A.D.; Monflier, E. Hydroformylation of 1-octene in supercritical carbon dioxide with rhodium alkyl P-donor ligands using a peracetylated β-cyclodextrin as solubiliser. Eur. J. Inorg. Chem. 2008, 46, 2659–2663. [Google Scholar] [CrossRef]
- Galia, A.; Navarre, E.C.; Scialdone, O.; Filardo, G.; Monflier, E. Complexation of phosphine ligands with peracetylated β-cyclodextrin in supercritical carbon dioxide: Effect of temperature and cosolvent on the equilibrium constant. J. Supercrit. Fluids 2009, 49, 154–160. [Google Scholar] [CrossRef]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bricout, H.; Hapiot, F.; Ponchel, A.; Tilloy, S.; Monflier, E. Chemically Modified Cyclodextrins: An Attractive Class of Supramolecular Hosts for the Development of Aqueous Biphasic Catalytic Processes. Sustainability 2009, 1, 924-945. https://doi.org/10.3390/su1040924
Bricout H, Hapiot F, Ponchel A, Tilloy S, Monflier E. Chemically Modified Cyclodextrins: An Attractive Class of Supramolecular Hosts for the Development of Aqueous Biphasic Catalytic Processes. Sustainability. 2009; 1(4):924-945. https://doi.org/10.3390/su1040924
Chicago/Turabian StyleBricout, Hervé, Frédéric Hapiot, Anne Ponchel, Sébastien Tilloy, and Eric Monflier. 2009. "Chemically Modified Cyclodextrins: An Attractive Class of Supramolecular Hosts for the Development of Aqueous Biphasic Catalytic Processes" Sustainability 1, no. 4: 924-945. https://doi.org/10.3390/su1040924