Hexachlorocyclohexanes, Cyclodiene, Methoxychlor, and Heptachlor in Sediment of the Alvarado Lagoon System in Veracruz, Mexico
Abstract
:1. Introduction
2. Materials and Methods
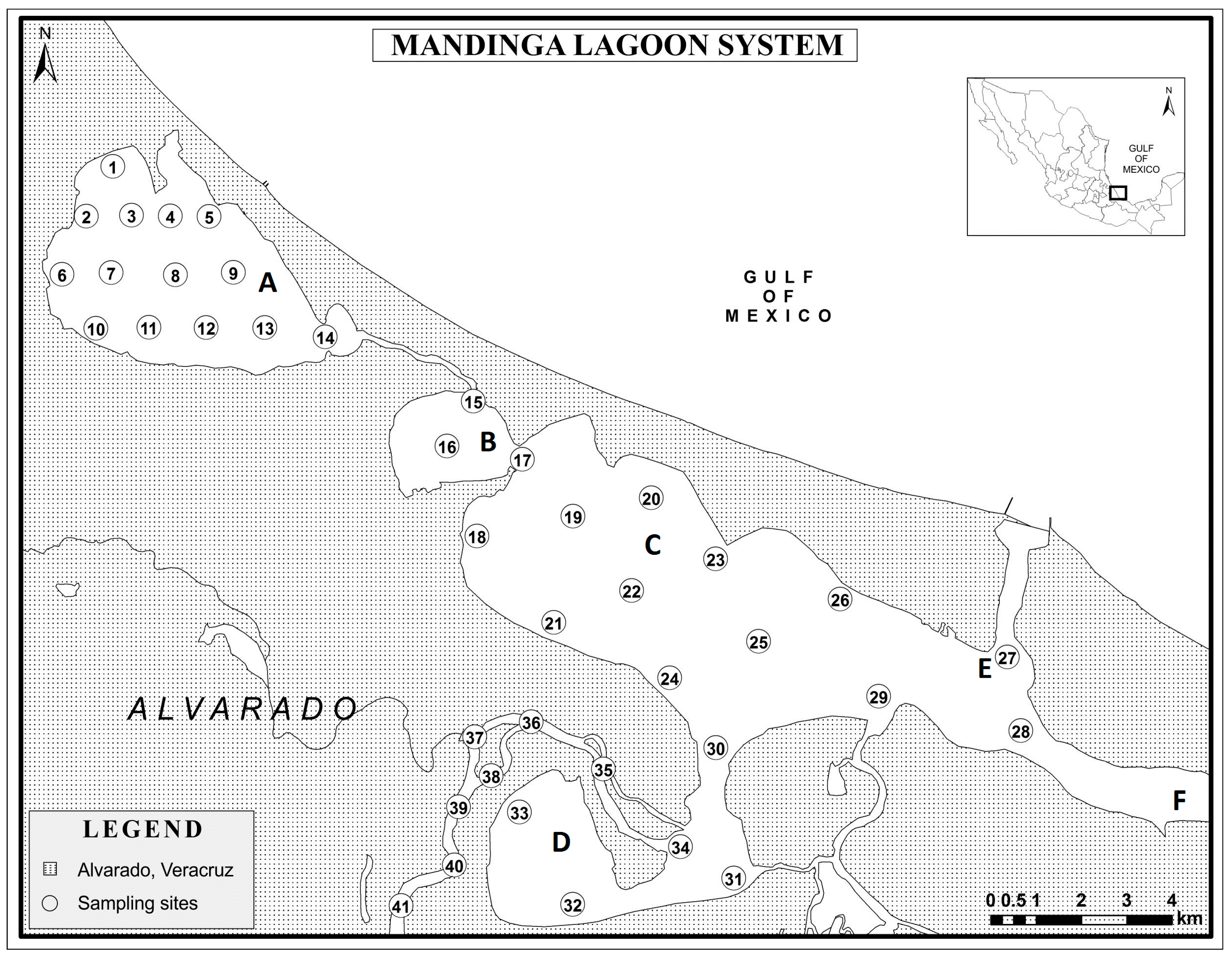
2.1. Study Area
2.2. Collection and Treatment of Sediment Samples for Analysis
2.3. Analytical Procedures
2.4. Microwave Extraction
2.5. Separation and Cleaning of Organochlorine Pesticides in Samples.
2.6. Preparation of the Calibration Curve
2.7. Quantification of Organochlorine Pesticides
3. Results and Discussion
3.1. Sediments as a Source of Pollutant Distribution
3.2. Hexachlorocyclohexane (HCH)
3.3. Cyclodienes (Endrin and Endrin Aldehyde, Aldrin and Dieldrin)
3.4. Methoxichlor and Heptachlor
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- She, J.; Holden, A.; Sharp, M.; Tañer, M.; Williams-Derry, C.; Hooper, K. Polybrominated Diphenyl Ethers (PBDEs) and Polychlorinated Biphenyls (PCBs) in Breast Milk from the Pacific Northwest. Chemosphere 2007, 67, S307–S317. [Google Scholar] [CrossRef] [PubMed]
- Grimalt, J.; Fernández, P.; Berdie, L.; Vilanova, R.M.; Catalán, J.; Psenner, R.; Hofer, R.; Appleby, P.G.; Rosseland, B.O.; Lien, L.; et al. Selective trapping of organochlorine compounds in mountain lakes of temperate areas. Environ. Sci. Technol. 2001, 35, 2690–2697. [Google Scholar] [CrossRef] [PubMed]
- Arbeli, Z. Biodegradación de compuestos orgánicos persistentes (COP): I. El Caso de los Bifenilos Policlorados (PCB). Acta Biol. Colomb. 2009, 14, 57–88. [Google Scholar]
- Lupi, L.; Bedmar, F.; Wunderlin, D.A.; Miglioranza, K.S.B. Organochlorine pesticides in agricultural soils and associated biota. Environ. Earth Sci. 2016, 75, 519. [Google Scholar] [CrossRef]
- García-Gutiérrez, C.; Rodríguez-Meza, G.D. Problemática y riesgo ambiental por el uso de plaguicidas en Sinaloa. Ra Ximhai 2012, 8, 1–10. [Google Scholar]
- Waliszewski, S.M.; Caba, M.; Goméz-Arroyo, S.; Villalobos-Pietrini, R.; Mártinez, A.; Valencia-Quintana, R.; Lozano-Flores, M.E.; Regalado-Torres, M.A. Niveles de plaguicidas organoclorados en habitantes de México. Rev. Int. Contam. Ambient. 2013, 29, 121–131. [Google Scholar]
- Arellano-Aguilar, O.; Betancourt-Lozano, M.; Aguilar-Zárate, G.; Ponce de León-Hill, C. Agrochemical loading in drains and rivers and its connection with pollution in coastal lagoons of the Mexican Pacific. Environ. Monit. Assess. 2017, 189, 270. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Morales, J.B.; Valdez-Torres, J.B.; Bastidas-Bastidas, P.J.; Angulo-Escalante, M.A.; Sarmiento-Sánchez, J.I.; Barraza-Lobo, A.L.; Olmeda-Rubio, C.; Chaidez-Quiroz, C. Monitoring of pesticides residues in northwestern Mexico rivers. Acta Univ. 2017, 27, 45–54. [Google Scholar] [CrossRef]
- Castañeda-Chávez, M.R.; Lango-Reynoso, F.; Landeros-Sánchez, C. DDT in Crassostrea virginica (Gmelin, 1791) of Coastal Lagoons in the Gulf of Mexico. J. Agric. Sci. 2011, 3, 183–193. [Google Scholar] [CrossRef]
- Lango-Reynoso, F.; Castañeda-Chávez, M.R.; Landeros-Sánchez, C.; Galavíz-Villa, I.; Navarrete-Rodríguez, G.; Soto-Estrada, A. Cd, Cu, Hg and Pb, and organochlorine pesticides in commercially important benthic organisms from coastal lagoons along the SW Gulf of Mexico. Agric. Sci. 2013, 1, 63–80. [Google Scholar] [CrossRef]
- Palmerín, R.C.; Ponce, V.G.; Botello, A.V. Evaluación de plaguicidas organoclorados en sedimentos y organismos filtradores de la laguna de Alvarado, Veracruz, México. In Golfo de México. Contaminación e Impacto Ambiental: Diagnóstico y Tendencias, 3rd ed.; Botello, A.V., Rendón von Osten, J., Benítez, J., Gold-Bouchot, G., Eds.; La Universidad Nacional Autónoma de México (UNAM): de la Ciudad de México, Mexico, 2014; pp. 285–308. ISBN 978-607-7887-71-3. [Google Scholar]
- Navarrete-Rodríguez, G.; Landeros-Sánchez, C.; Soto-Estrada, A.; Castañeda-Chávez, M.R.; Lango-Reynoso, F.; Pérez-Vázquez, A.; Nikolskii-Gavrilov, I. Endosulfan: Its Isomers and Metabolites in Commercially Aquatic Organisms from the Gulf of Mexico and the Caribbean. J. Agric. Sci. 2016, 8, 8–24. [Google Scholar] [CrossRef]
- Ramírez-Elías, M.A.; Córdova-Quiroz, A.V.; Cerón-Bretón, J.G.; Cerón-Bretón, R.M.; Rendón-von Osten, J.; Hipólito-Cortés, S.J. Dichloro-Diphenyl-Trichloroethane (DDT) and Endosulfan in Sediments of Sabancuy Lagoon, Campeche, Mexico. Open J. Ecol. 2016, 6, 22–31. [Google Scholar] [CrossRef]
- Albert, L.A.; Benítez, J.A. Impacto ambiental de los plaguicidas en los ecosistemas costeros. In Golfo de México Contaminación e Impacto Ambiental: Diagnóstico y Tendencias, 2nd ed.; Botello, A.V., Rendón von Osten, J., Gold Bouchot, G., Hernández, C.A., Eds.; Univ. Nal. Autón. de México, Instituto Nacional de Ecología: de Campeche, Mexico, 2005; pp. 157–176. ISBN 968-5722-37-4. [Google Scholar]
- Albert, L.A. Los plaguicidas y sus riesgos para el ambiente. In Golfo de México Contaminación e Impacto Ambiental: Diagnóstico y Tendencias, 3rd ed.; Botello, A.V., Rendón von Osten, J., Benítez, J., Gold-Bouchot, G., Eds.; La Universidad Nacional Autónoma de México (UNAM): de la Ciudad de México, Mexico, 2014; pp. 183–212. ISBN 978-607-7887-71-3. [Google Scholar]
- Leyva-Cardoso, D.O.; Ponce-Vélez, G.; Botello, A.V.; Díaz-González, G. Persistent organochlorine pesticides in coastal sediments from Petacalco Bay, Guerrero, México. Bull. Environ. Contam. Toxicol. 2003, 71, 1244–1251. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, C.A.; Montalvo, C.; Rodríguez, L.; Cerón, J.G.; Cerón, R.M. American oyster (Crassostrea virginica) and sediments as a coastal zone pollution monitor by heavy metals. Int. J. Environ. Sci. Technol. 2012, 9, 579–586. [Google Scholar] [CrossRef]
- Villanueva, F.S.; Páez-Osuna, F. Contaminantes críticos: Metales. In Golfo de México, Contaminación e Impacto Ambiental: Diagnostico y Tendencias; Botello, A.V., Rojas-Galaviz, J.L., Benítez, J., Zárate-Lomelí, D., Eds.; EPOMEX Serie Científica 5; Universidad Autónoma de Campeche: México D.F., Mexico, 1995; pp. 681–710. [Google Scholar]
- Carvalho, F.P.; Gonzalez-Farias, F.; Villeneuve, J.P.; Cattini, C.; Hernandez-Garza, M.; Mee, L.D. Distribution, fate and effects of pesticide residues in tropical coastal lagoons of Northwestern Mexico. Environ. Toxicol. 2002, 23, 1257–1270. [Google Scholar]
- Gonzalez-Farías, F.; Hernández-Garza, M.; Díaz-González, G. Organic carbon and pesticide pollution in a tropical coastal lagoon-estuarine system in Northwest Mexico. Int. J. Environ. Pollut. 2006, 26, 234–253. [Google Scholar] [CrossRef]
- USGS (U.S. Geological Survey). Pesticides in Stream Sediment and Aquatic Biota. Current Understanding of Distribution and Major Influences. Pesticide National Synthesis Project. 2017. Available online: https://water.usgs.gov/nawqa/pnsp/pubs/fs09200/fs09200.pdf (accessed on 28 May 2017).
- Portilla-Ochoa, E. Ficha Informativa de los Humedales de Ramsar (FIR). Nombre del Sitio Ramsar: Sistema Lagunar Alvarado. 2003. Available online: http://ramsar.conanp.gob.mx/docs/sitios/FIR_RAMSAR/Veracruz/Sistema_Lagunar_Alvarado/Sistema%20Lagunar%20Alvarado.pdf (accessed on 30 July 2017).
- Carta Nacional Pesquera. Ecosistemas Lagunares Costeros. Diario Oficial de la Federación. Available online: http://www.conapesca.sagarpa.gob.mx/wb/cona/cona_parte_4_ (accessed on 29 July 2017).
- Castañeda-Chávez, M.R.; Sedas, V.P.; Orrantia-Borunda, E. Influence of water temperature and salinity on seasonal occurrences of Vibrio cholerae and enteric bacteria in oyster-producing areas of Veracruz, México. Mar. Pollut. Bull. 2005, 50, 1641–1648. [Google Scholar] [CrossRef] [PubMed]
- Bello, P.J.; Cervantes, A.M.; Gómez, L.; Magaña, V.; Graizbord, B.; Hipólito, R.V. Sitio piloto Río Papaloapan-Laguna de Alvarado. In Adaptabilidad a los Impactos del Cambio Climático en los Humedales Costeros del Golfo de Mexico; Buenfil-Friedman, J., Ed.; Secretaría de Medio Ambiente y Recursos Naturales (Semarnat): Ciudad de México, Mexico, 2009; Volume 2, pp. 435–456. [Google Scholar]
- Portilla-Ochoa, E.; Sánchez-Hernández, A.I.; Ortega-Argueta, A.; Juárez-Eusebio, A.; Escobar-López, H.E.; Gutiérrez-García, R.; Montejo-Díaz, J.E.; Cortina-Julio, B.E.; Garza-Garza, S.; García-Hernández, C. Establecimiento de Unidades de Gestión Ambiental en el Humedal de Alvarado, Veracruz, México: Bases para su Ordenamiento Ecológico y Social; Informe Técnico Semestral; Universidad Veracruzana: Jalapa, Mexico, 2003; p. 45. [Google Scholar]
- Vázquez-Lule, A.D.; Rodríguez-Zúñiga, M.T.; Ramírez-García, P. Caracterización del Sitio de Manglar Sistema Lagunar de Alvarado Veracruz, en Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (CONABIO). Sitios de Manglar con Relevancia Biológica y con Necesidades de Rehabilitación Ecológica; CONABIO. Available online: http://www.biodiversidad.gob.mx/ecosistemas/manglares/pdf/caracterizacion/GM53_Sistema_Lagunar_de_Alvarado_veracruz_caracterizacion.pdf (accessed on 13 August 2017).
- CONABIO (Comisión Nacional para el Conocimiento y Uso de la Biodiversidad). Ficha Técnica para la Evaluación de los Sitios Prioritarios para la Conservación de los Ambientes Costeros y Oceánicos de México: Sistema Lagunar de Alvarado. Available online: http://www.conabio.gob.mx/gap/images/1/1b/60_Sistema_Lagunar_Alvarado.pdf (accessed on 13 August 2017).
- Waliszewski, S.M.; Mójica, G.X.; Infanzón, R.M.; Barradas, D.C.M.; Carvajal, Z.O. Uso del ácido sulfúrico en las determinaciones de plaguicidas organoclorados. I. Calidad químico-analítica de la preparación de grasas por el ácido sulfúrico Concentrado. Rev. Int. Contam. Ambient. 2008, 24, 33–38. [Google Scholar]
- Murphy, P.G. Sulfuric acid for the cleanup of animal tissues for analysis of acid-stable chlorinated hydrocarbon residues. J. Assoc. Off. Anal. Chem. 1972, 55, 1360–1362. [Google Scholar] [PubMed]
- Mamta; Rao, R.J.; Wani, K.A. Concentration of organochlorine and organophosphorus pesticides in different molluscs from Tighra Reservoir, Gwalior, India. Bull. Environ. Contam. Toxicol. 2015, 95, 332–339. [Google Scholar]
- Okoya, A.A.; Ogunfowokan, A.O.; Asubiojo, O.I.; Torto, N. Organochlorine Pesticide Residues in Sediments and Waters from Cocoa Producing Areas of Ondo State, Southwestern Nigeria. ISRN Soil Sci. 2013, 2013, 131647. [Google Scholar] [CrossRef]
- CICOPLAFEST. Catálogo Oficial de Plaguicidas. Mexico: Secretaría de Medio Ambiente y Recursos Naturales (Semarnap)/Secretaría de Comercio y Fomento Industrial (Secofi)/SAGAR/Secretaría de Salud (SSA); COFEPRIS: Ciudad de México, Mexico, 2004; p. 483. [Google Scholar]
- Rosales-Hoz, M.T.L.; Alvarez-León, R. Niveles actuales de hidrocarburos organoclorados en sedimentos de lagunas costeras del golfo de México. Anales del Centro de Ciencias del Mar y Limnología. In Proceedings of the VI Congreso Nacional de Oceanografía, Ensenada, Mexico, 10–13 April 1978. [Google Scholar]
- Botello, A.V.; Díaz, G.; Rueda, L.; Villanueva, S.F. Organochlorine compounds in oysters and sediments from coastal lagoons of the Gulf of Mexico. Bull. Environ. Contam. Toxicol. 1994, 53, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Díaz-González, G.; Rueda Quintana, L. Niveles de concentraciones de plaguicidas organoclorados en las lagunas del Carmen, Machona y Alvarado. In Golfo de México, Contaminación e Impacto Ambiental: Diagnóstico y Tendencias; Botello, A.V., Rojas-Galavíz, J.L., Benítez, J.A., Zárate-Lomelí, Y.D., Eds.; EPOMEX Serie Científica, 5; Universidad Autónoma de Campeche: Campeche, Mexico, 1996; pp. 177–185. [Google Scholar]
- Betancourt, J.M.; Ramírez-Triana, G. Estudio de los procesos relacionados con la presencia de plaguicidas organoclorados en la ciénaga grande de Santa Marta. Bol. Invest. Mar. Cost. 2005, 34, 121–139. [Google Scholar] [CrossRef]
- Calva Benítez, L.G.; Torres-Alvarado, M.R. Textura de sedimentos y carbono orgánico en el sistema costero lagunar Alvarado, Veracruz. Contactos 2011, 81, 11–16. [Google Scholar]
- ATSDR (Agency for Toxic Substances and Disease Registry). Resumen de Salud Pública. Hexacloro-ciclohexano. Agencia para Sustancias Tóxicas y el Registro de Enfermedades. Atlanta, GA: Departamento de Salud y Servicios Humanos de los EE.UU., Servicio de Salud Pública. Available online: https://www.atsdr.cdc.gov/es/phs/es_phs43.pdf (accessed on 29 August 2017).
- Kuranchie-Mensahm, H.; Manukure-Atiemo, S.; Naa-Dedei-Palm, L.M.; Blankson-Arthur, S.; Osei-Tutu, A.; Fosu, P. Determination of organochlorine pesticide residue in sediment and water from the Densu river basin, Ghana. Chemosphere 2012, 86, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Loomis, D.; Mattpck, H.; Straif, K.; Zavadil, A. Carcinogenicity of lindane, DDT, and 2, 4-dichlorophenoxyacetic acid. Lancet Oncol. 2015, 16, 891–892. [Google Scholar] [CrossRef]
- Castañeda, L.O.y.F.C.E. Los ecosistemas costeros del estado de Veracruz. Gobierno del Estado de Veracruz; Secretaría de Desarrollo Agropecuario, Forestal y Pesquero: Tabasco, Mexico, 1995; p. 144.
- Montes, A.M.; Gonzalez-Farias, F.A.; Botello, A.V. Pollution by organochlorine pesticides in Navachiste-Macapule, Sinaloa, Mexico. Environ. Monit. Assess. 2012, 184, 1359–1369. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.B. Residue analysis of organochlorine pesticides in water and sediments from Agboyi Creek, Lagos. Afr. J. Environ. Sci. Technol. 2013, 7, 267–273. [Google Scholar] [CrossRef]
- ATSDR. Resumen de Salud Pública. Endrina. Agencia para Sustancias Tóxicas y el Registro de Enfermedades. Atlanta, GA: Departamento de Salud y Servicios Humanos de los EE.UU, Servicio de Salud Pública. 2016. Available online: https://www.atsdr.cdc.gov/es/toxfaqs/es_tfacts89.html (accessed on 29 August 2017).
- ATSDR. Resumen de Salud Pública. Heptacloro. Agencia para Sustancias Tóxicas y el Registro de Enfermedades. Atlanta, GA: Departamento de Salud y Servicios Humanos de los EE.UU., Servicio de Salud Pública. 2016. Available online: https://www.atsdr.cdc.gov/es/phs/es_phs12.html (accessed on 27 August 2017).
- ATSDR. Resumen de Salud Pública. Metoxicloro. Agencia para Sustancias Tóxicas y el Registro de Enfermedades. Atlanta, GA: Departamento de Salud y Servicios Humanos de los EE.UU., Servicio de Salud Pública. 2016. Available online: https://www.atsdr.cdc.gov/es/toxfaqs/es_tfacts47.html (accessed on 27 August 2017).
- ATSDR. Toxicological Profile for Heptachlor and Heptachlor Epoxide; U.S. Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2007; p. 158.
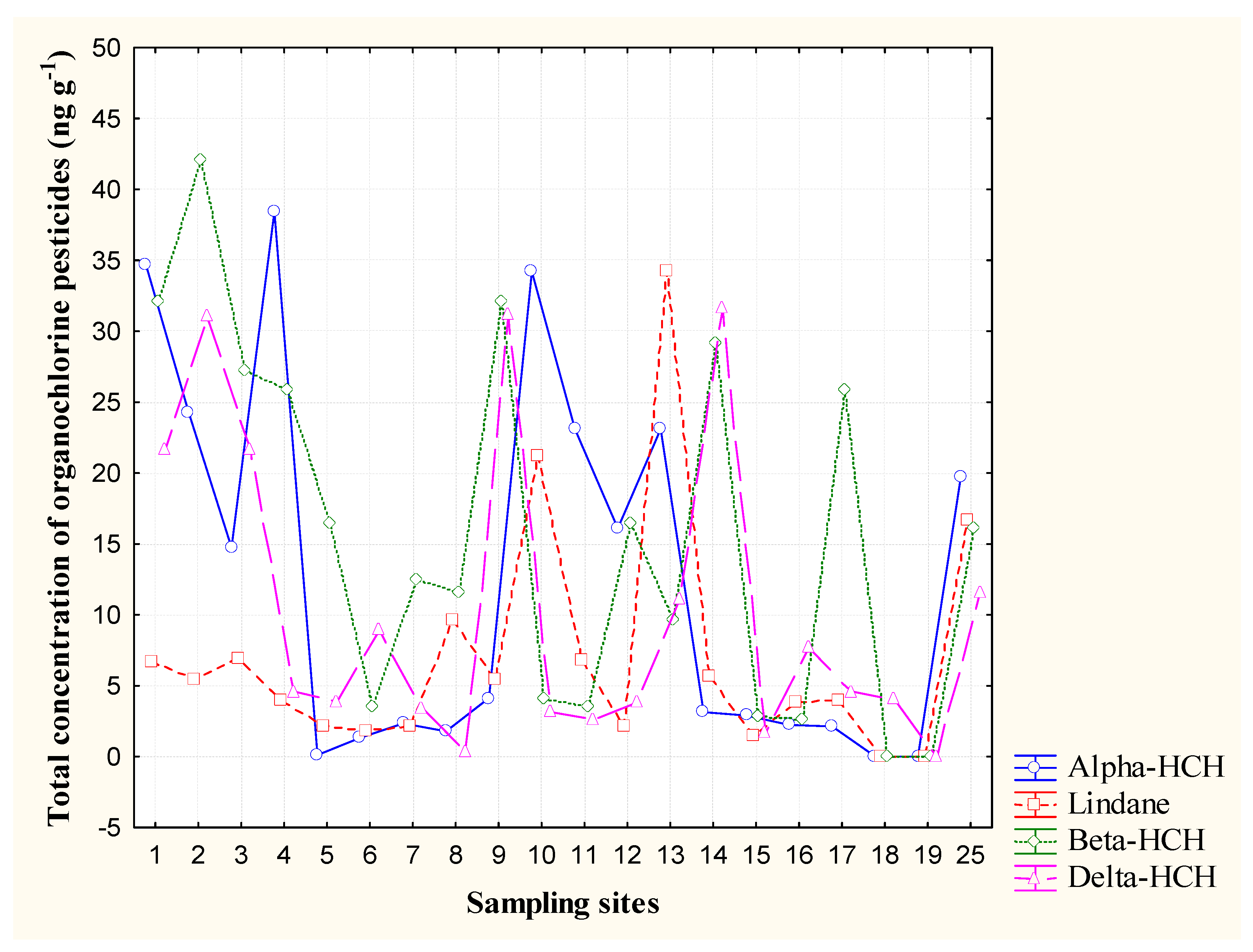
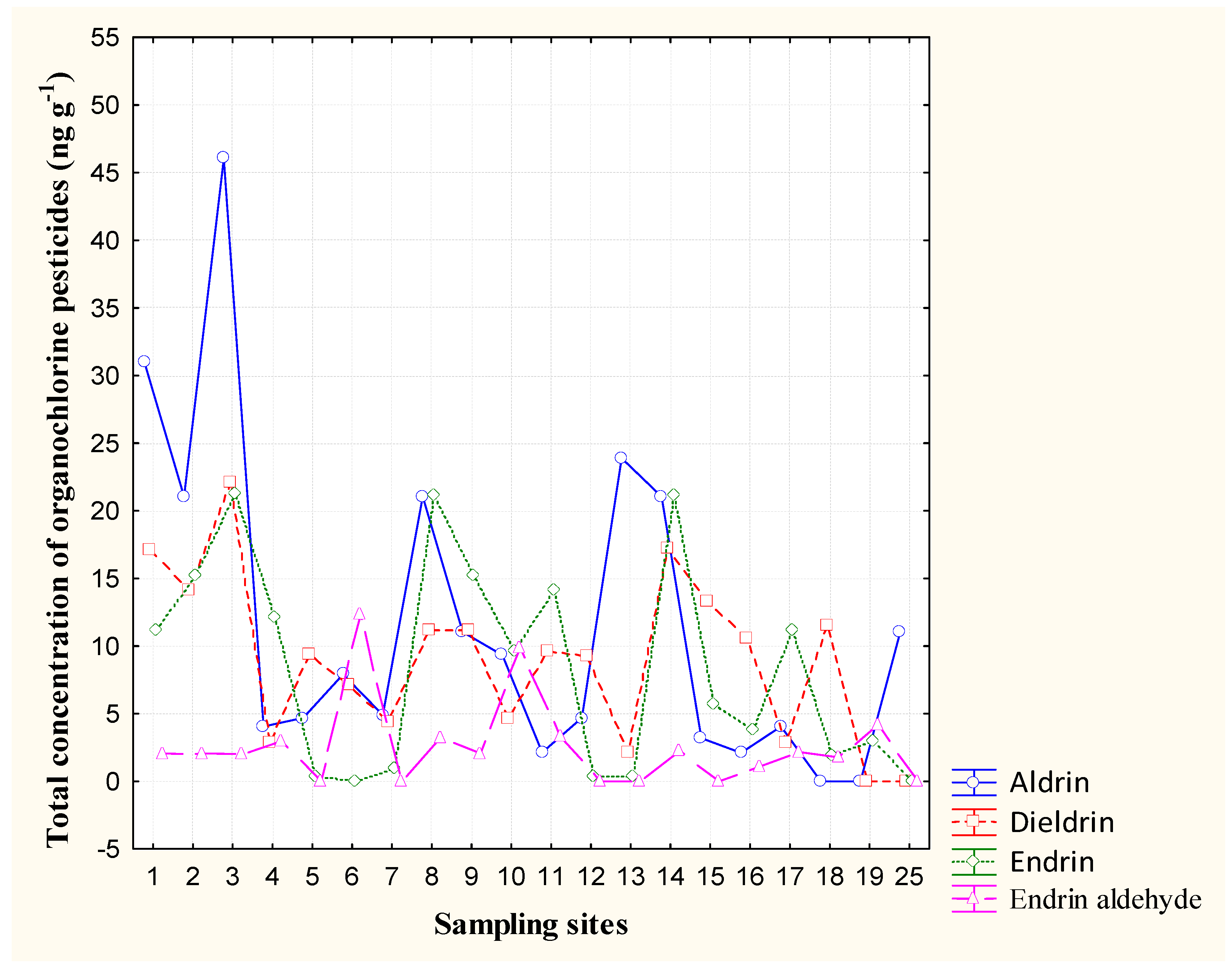
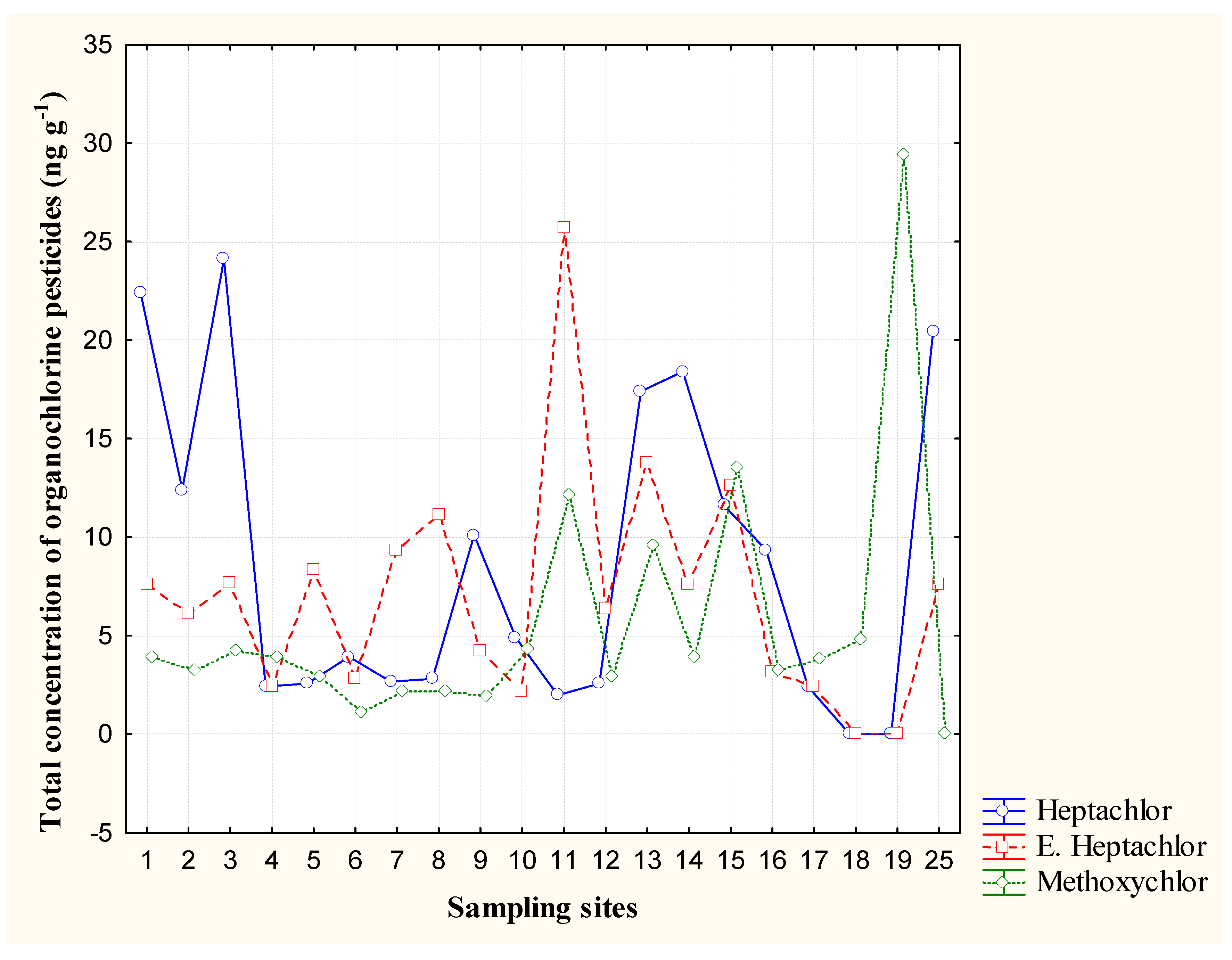
| Compound | Minimum | Maximum | Mean | Standard Deviation |
|---|---|---|---|---|
| alpha-HCH | 0.121 | 38.44 | 12.428 | 13.264 |
| gamma-HCH (Lindane) | 1.45 | 34.20 | 7.010 | 8.287 |
| beta-HCH | 2.56 | 42.11 | 15.683 | 12.737 |
| delta-HCH | 0.34 | 31.61 | 10.424 | 10.801 |
| Heptachlor | 1.98 | 24.11 | 8.621 | 8.000 |
| Aldrin | 2.11 | 46.05 | 11.667 | 12.088 |
| Heptachlor epoxide | 2.13 | 25.70 | 7.052 | 5.884 |
| Dieldrin | 2.18 | 22.13 | 9.041 | 6.051 |
| Endrin | 0.34 | 21.23 | 8.420 | 7.724 |
| Endrin aldehyde | 0.01 * | 12.40 | 2.581 | 3.224 |
| Methoxichlor | 1.13 | 29.40 | 5.650 | 6.561 |
| Compound | Díaz-González and Rueda-Quintana (ng g−1) [34] | Botello et al. (ng g−1) [35] | Palmerín et al. (ng g−1) [11] * |
|---|---|---|---|
| alpha-HCH | 2.61 | 0.47 ± 0.29 | 3.49 |
| γ-HCH (Lindane) | 2.73 | 0.85 ± 0.63 | 4.784 |
| beta-HCH | 2.08 | 1.86 ± 0.60 | 28.49 |
| δ-HCH | NA | NA | 17.29 |
| Heptachlor | 2.91 | 3.91 ± 2.21 | 46 |
| Aldrin | 6.61 | 2.11 ± 1.66 | 82.36 |
| Heptachlor epoxide | 2.17 | 0.86 ± 0.45 | 52.32 |
| Dieldrin | ND | 2.05 ± 1.08 | ND |
| Endrin | 7.95 | 7.82 ± 4.77 | 4.42 |
| Endrin aldehyde | 1.92 | 1.77 | 18.51 |
| Methoxichlor | NA | NA | NA |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castañeda-Chávez, M.D.R.; Lango-Reynoso, F.; Navarrete-Rodríguez, G. Hexachlorocyclohexanes, Cyclodiene, Methoxychlor, and Heptachlor in Sediment of the Alvarado Lagoon System in Veracruz, Mexico. Sustainability 2018, 10, 76. https://doi.org/10.3390/su10010076
Castañeda-Chávez MDR, Lango-Reynoso F, Navarrete-Rodríguez G. Hexachlorocyclohexanes, Cyclodiene, Methoxychlor, and Heptachlor in Sediment of the Alvarado Lagoon System in Veracruz, Mexico. Sustainability. 2018; 10(1):76. https://doi.org/10.3390/su10010076
Chicago/Turabian StyleCastañeda-Chávez, María Del Refugio, Fabiola Lango-Reynoso, and Gabycarmen Navarrete-Rodríguez. 2018. "Hexachlorocyclohexanes, Cyclodiene, Methoxychlor, and Heptachlor in Sediment of the Alvarado Lagoon System in Veracruz, Mexico" Sustainability 10, no. 1: 76. https://doi.org/10.3390/su10010076




