Soil Washing Optimization, Recycling of the Solution, and Ecotoxicity Assessment for the Remediation of Pb-Contaminated Sites Using EDDS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Characterization
2.2. Soil Washing and Recycle of the SWS
2.3. Ecotoxicity Tests
3. Results and Discussion
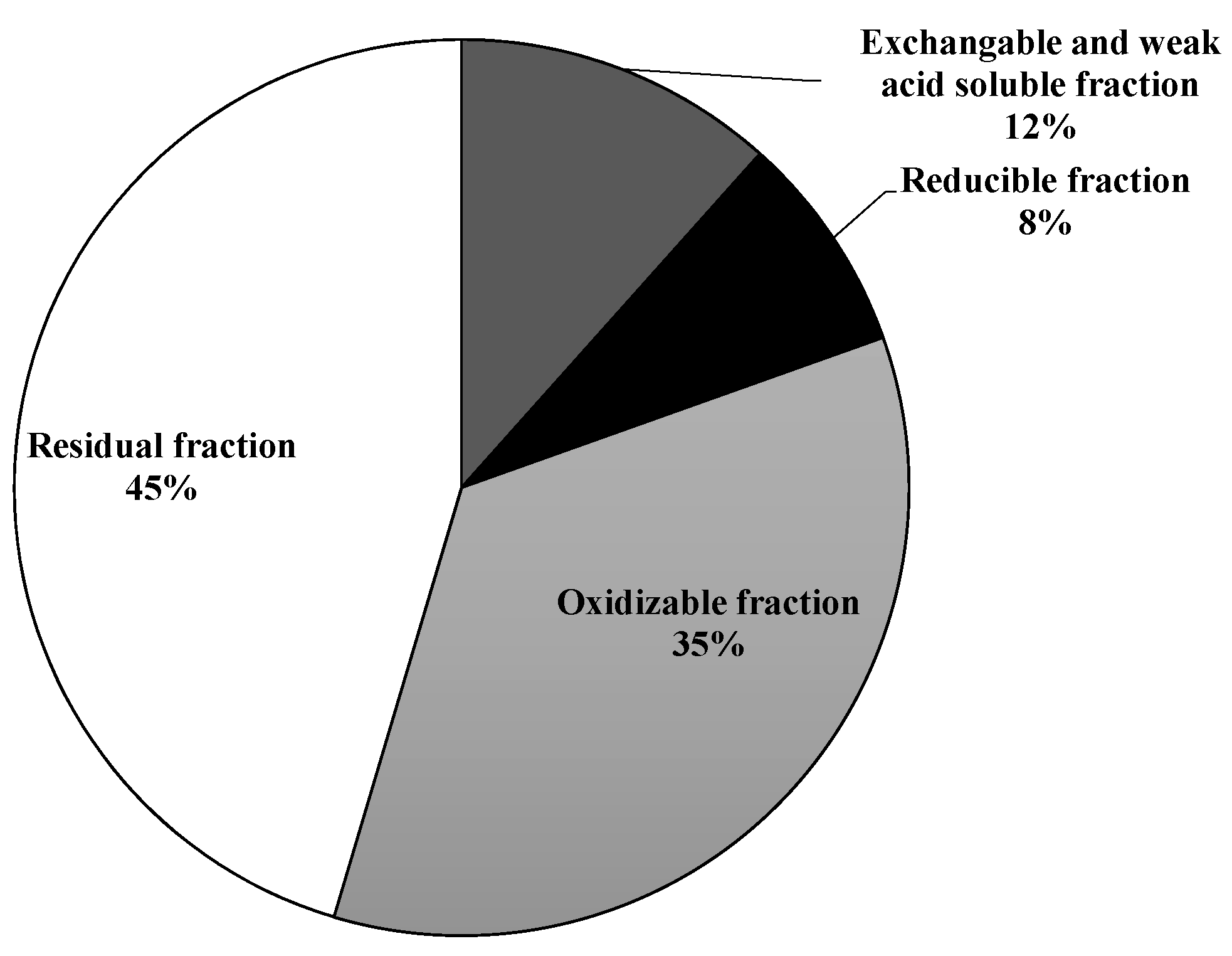
3.1. Soil Characterization
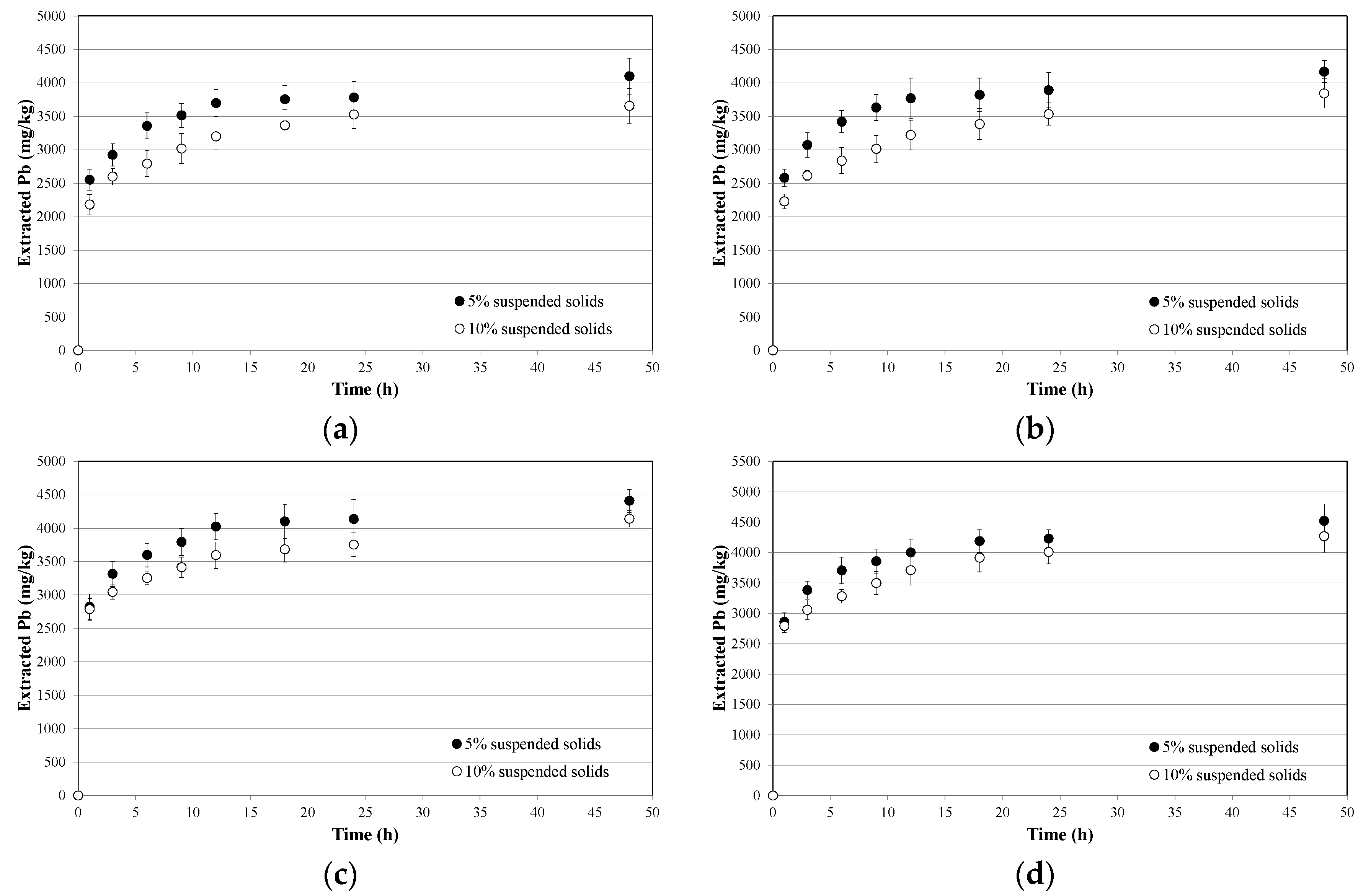
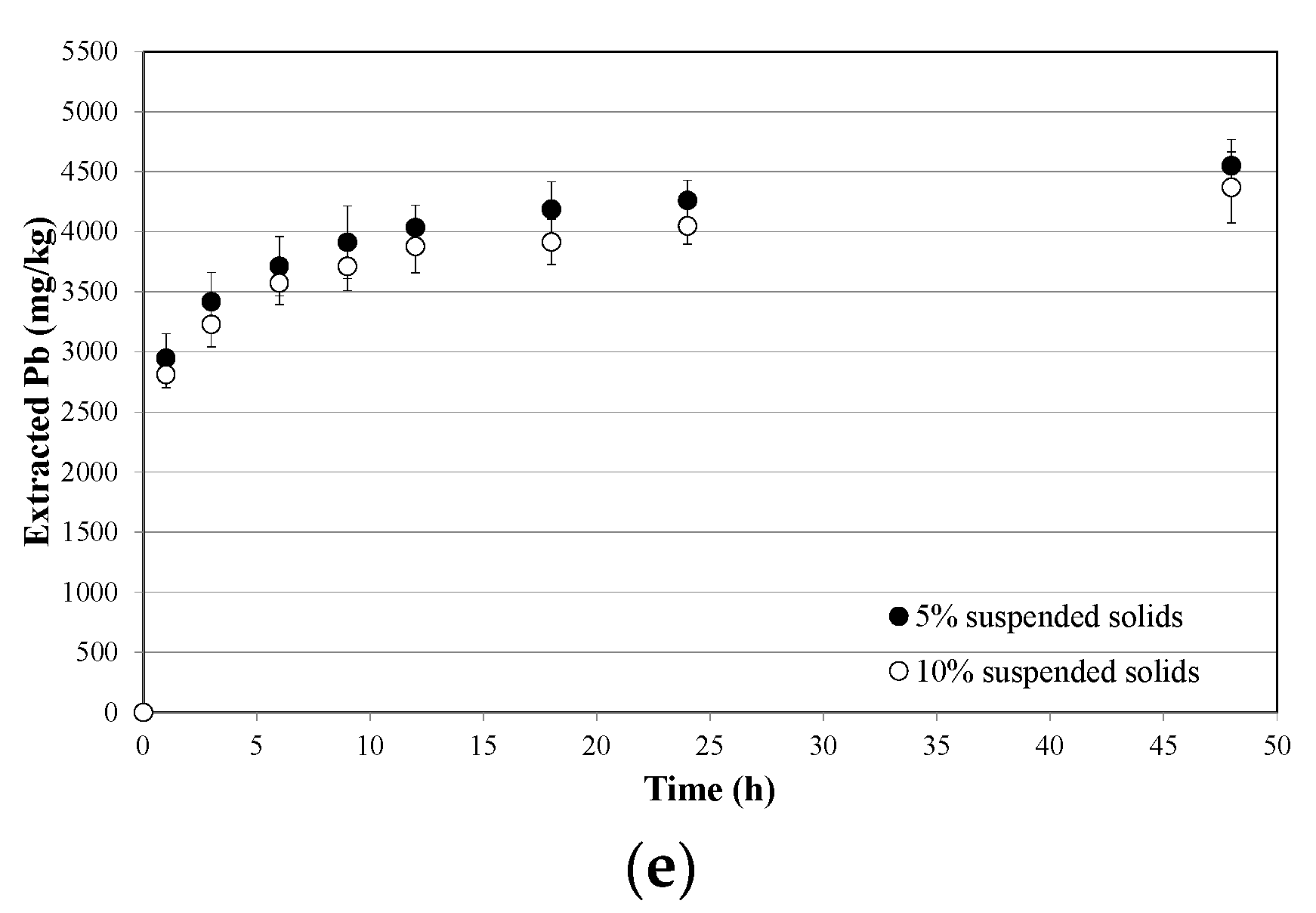
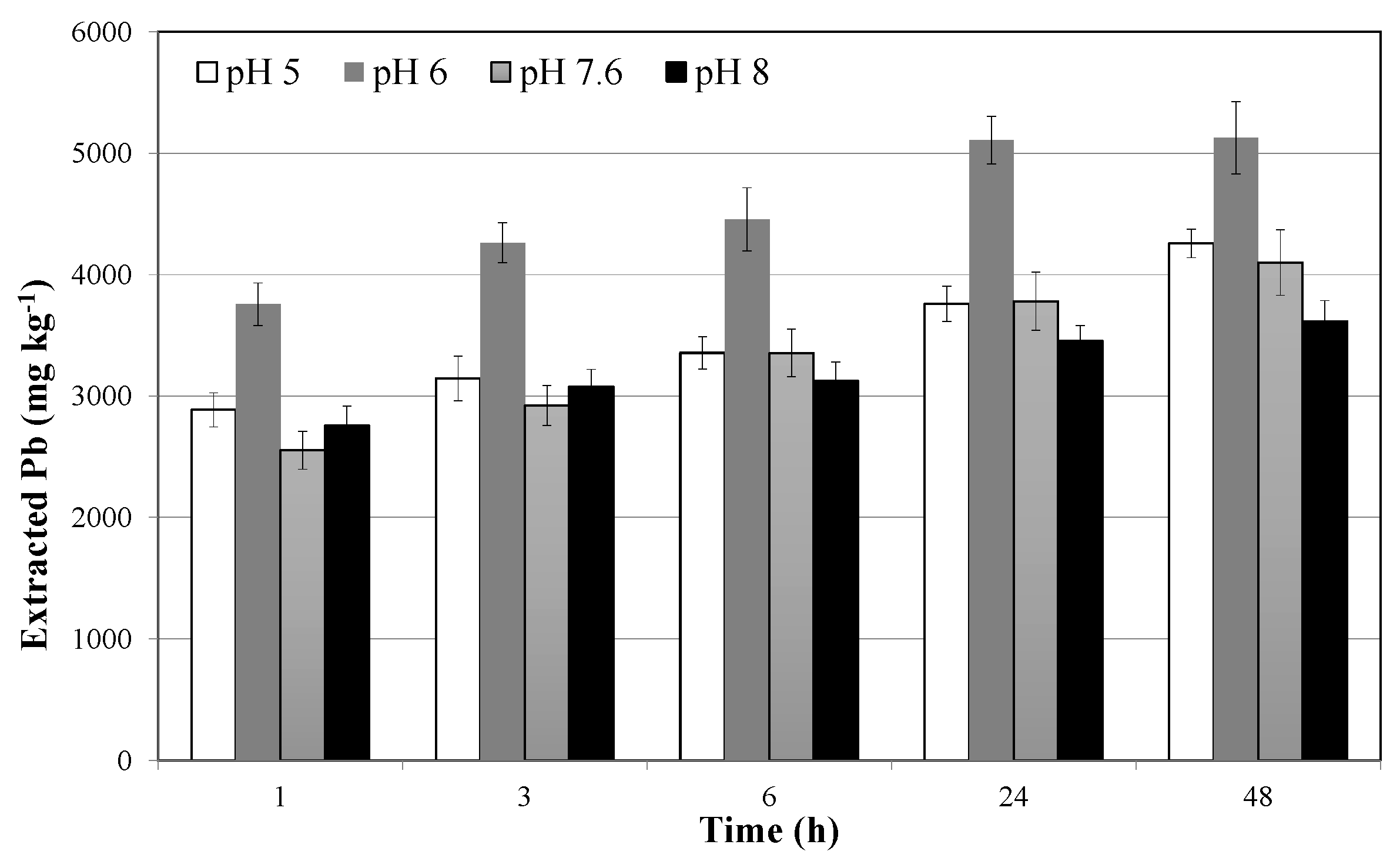
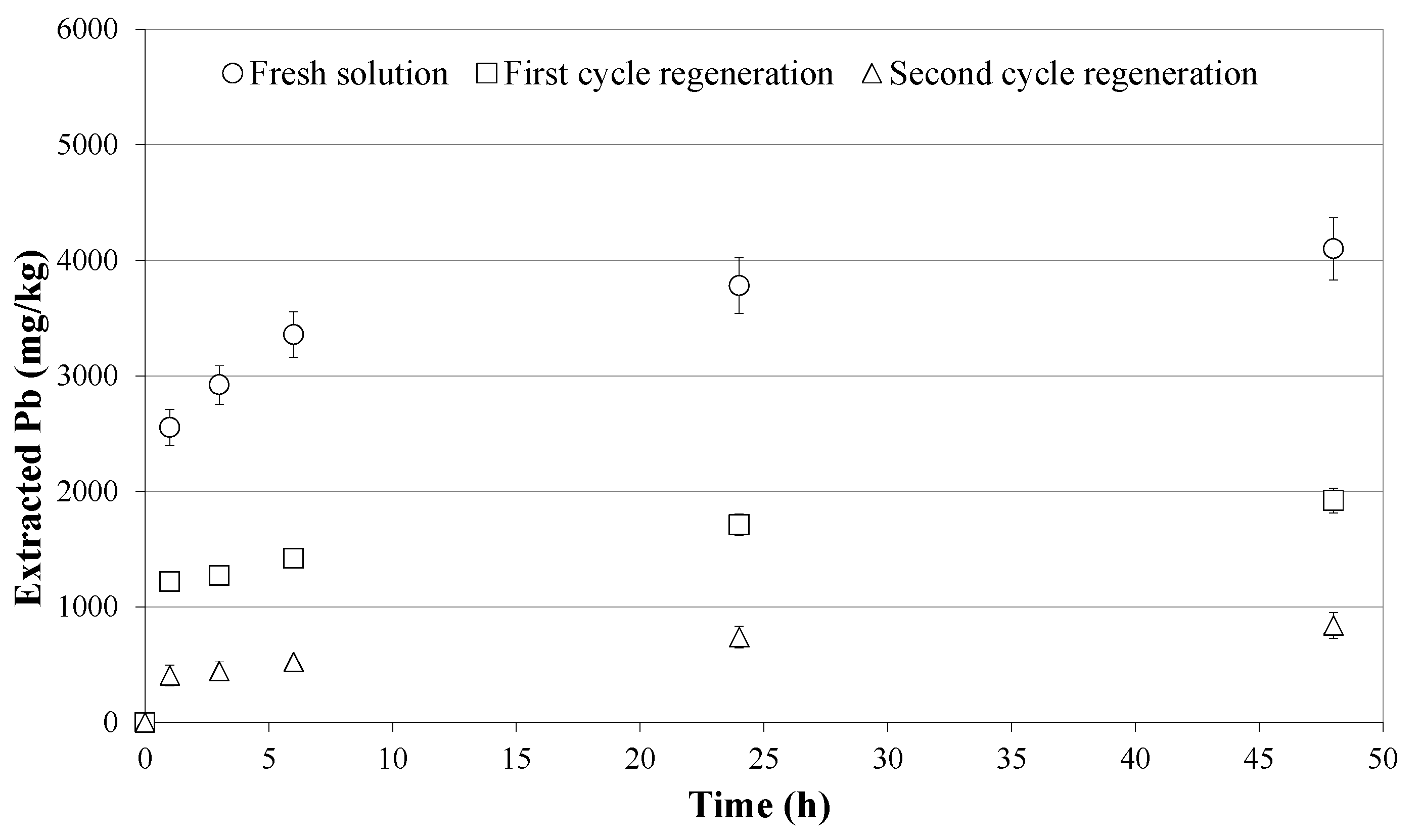
3.2. Soil Washing and Recycle of the SWS
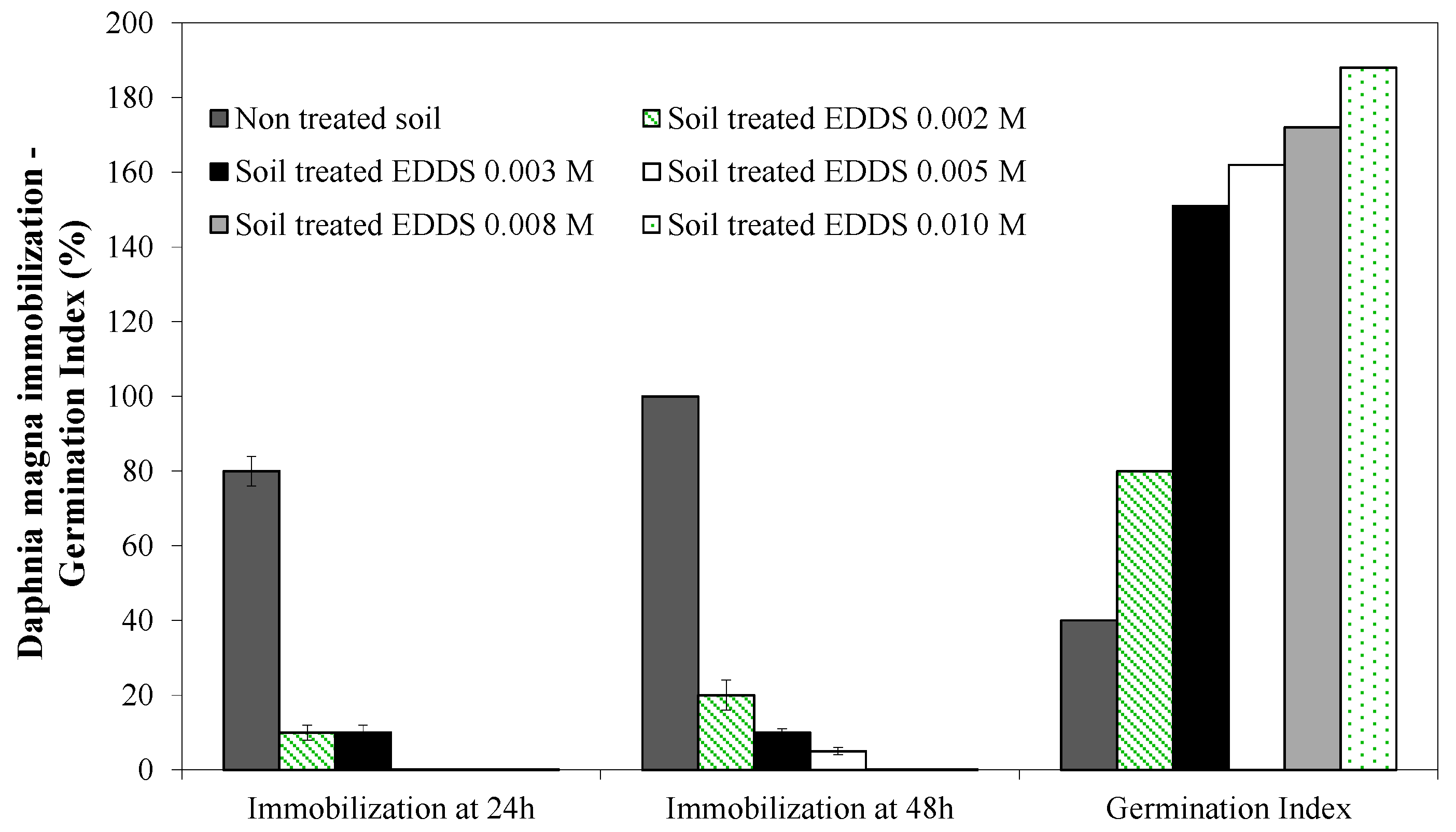
3.3. Ecotoxicity Tests
4. Conclusions
- use of EDDS for soil washing of Pb-contaminated soil is an available option to almost completely remove the mobile fraction of the metal bound to the soil particles;
- process kinetics are characterized by two distinctive steps, corresponding to an initial fast external exchange, followed by a slower internal exchange dominated by intraparticle diffusion, and can be modeled using a two-step exponential expression;
- kinetics parameters corresponding to the maximum extractive capacity linearly depend on washing solution molarity, while those corresponding to the extraction rates are almost independent of it;
- extraction efficiencies are optimal at pH close to the neutrality, because of the possible competition between hydrogen and Pb ions at lower values, and the possible formation of Pb hydroxide at higher values;
- the spent solutions can be regenerated and reused for more than one washing cycle, decreasing the overall cost of the treatment;
- spent solutions containing Pb–EDDS chelates do not have any toxic effect on the activated sludge of the wastewater treatment plant, but they are not biodegradable, therefore requiring the involvement of appropriate chemical processes before final disposal;
- the soil treated with EDDS has no toxic effect on living species, and can be returned to its original location after treatment.
Author Contributions
Conflicts of Interest
References
- Dermont, G.; Bergeron, M.; Mercier, G.; Richer-Laflèche, M. Soil washing for metal removal: A review of physical/chemical technologies and field applications. J. Hazard. Mater. 2008, 152, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Tandy, S.; Bossart, K.; Mueller, R.; Ritschel, J.; Hauser, L.; Schulin, R.; Nowack, B. Extraction of Heavy Metals from Soils Using Biodegradable Chelating Agents. Environ. Sci. Technol. 2004, 38, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Wuana, R.A.; Okieimen, F.E. Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecol. 2011, 2011, 402647. [Google Scholar] [CrossRef]
- Ferraro, A.; Fabbricino, M.; van Hullebusch, E.D.; Esposito, G.; Pirozzi, F. Effect of soil/contamination characteristics and process operational conditions on aminopolycarboxylates enhanced soil washing for heavy metals removal: A review. Rev. Environ. Sci. Bio/Technol. 2015, 1–35. [Google Scholar] [CrossRef]
- Satyro, S.; Race, M.; Marotta, R.; Dezotti, M.; Guida, M.; Clarizia, L. Photocatalytic processes assisted by artificial solar light for soil washing effluent treatment. Environ. Sci. Pollut. Res. 2017, 24. [Google Scholar] [CrossRef] [PubMed]
- Ng, Y.S.; Sen Gupta, B.; Hashim, M.A. Performance evaluation of two-stage electrokinetic washing as soil remediation method for lead removal using different wash solutions. Electrochim. Acta 2014, 147, 9–18. [Google Scholar] [CrossRef]
- Dos Santos, G.C.G.; Valladares, G.S.; Abreu, C.A.; de Camargo, O.A.; Grego, C.R. Assessment of Copper and Zinc in Soils of a Vineyard Region in the State of São Paulo, Brazil. Appl. Environ. Soil Sci. 2013, 2013, 790795. [Google Scholar] [CrossRef]
- Li, Y.; Hu, P.; Zhao, J.; Dong, C. Remediation of cadmium-and lead-contaminated agricultural soil by composite washing with chlorides and citric acid. Environ. Sci. Pollut. Res. 2015, 22, 5563–5571. [Google Scholar] [CrossRef] [PubMed]
- Gitipour, S.; Ahmadi, S.; Madadian, E.; Ardestani, M. Soil washing of chromium-and cadmium-contaminated sludge using acids and ethylenediaminetetra acetic acid chelating agent. Environ. Technol. 2016, 37, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Lestan, D.; Luo, C.; Li, X. The use of chelating agents in the remediation of metal-contaminated soils: A review. Environ. Pollut. 2008, 153, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Vandevivere, P.; Hammes, F.; Verstraete, W.; Feijtel, T.; Schowanek, D. Metal Decontamination of Soil, Sediment, and Sewage Sludge by Means of Transition Metal Chelant [S,S]-EDDS. J. Environ. Eng. 2001, 127, 802–811. [Google Scholar] [CrossRef]
- Ferraro, A.; Fabbricino, M.; van Hullebusch, E.D.; Esposito, G. Investigation of different ethylenediamine-N,N′-disuccinic acid-enhanced washing configurations for remediation of a Cu-contaminated soil: Process kinetics and efficiency comparison between single-stage and multi-stage configurations. Environ. Sci. Pollut. Res. 2017, 24, 21960–21972. [Google Scholar] [CrossRef] [PubMed]
- Jelusic, M.; Lestan, D. Effect of EDTA washing of metal polluted garden soils. Part I: Toxicity hazards and impact on soil properties. Sci. Total Environ. 2014, 475, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Fountain, J.S.; Reith, D.M. Dangers of “EDTA”. N. Z. Med. J. 2014, 127, 126–127. [Google Scholar] [PubMed]
- Shahid, M.; Austruy, A.; Echevarria, G.; Arshad, M.; Sanaullah, M.; Aslam, M.; Nadeem, M.; Nasim, W.; Dumat, C. EDTA-enhanced phytoremediation of heavy metals: A review. Soil Sediment Contam. Int. J. 2014, 23, 389–416. [Google Scholar] [CrossRef]
- Meers, E.; Tack, F.M.G.; Verloo, M.G. Degradability of ethylenediaminedisuccinic acid (EDDS) in metal contaminated soils: Implications for its use soil remediation. Chemosphere 2008, 70, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Epelde, L.; Hernández-Allica, J.; Becerril, J.M.; Blanco, F.; Garbisu, C. Effects of chelates on plants and soil microbial community: Comparison of EDTA and EDDS for lead phytoextraction. Sci. Total Environ. 2008, 401, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Attinti, R.; Barrett, K.R.; Datta, R.; Sarkar, D. Ethylenediaminedisuccinic acid (EDDS) enhances phytoextraction of lead by vetiver grass from contaminated residential soils in a panel study in the field. Environ. Pollut. 2017, 225, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, Q.; Shan, Y.; Wang, Y.; Song, X.; Lei, X. A comparative study on the efficiency of biodegradable EDDS and micro-electric field on the promotion of the phytoextraction by Commelina communis L. in Cu-contaminated soils. Geoderma 2018, 314, 1–7. [Google Scholar] [CrossRef]
- Komínková, D.; Fabbricino, M.; Gurung, B.; Race, M.; Tritto, C.; Ponzo, A. Sequential application of soil washing and phytoremediation in the land of fires. J. Environ. Manag. 2018, 206. [Google Scholar] [CrossRef]
- Race, M. Applicability of alkaline precipitation for the recovery of EDDS spent solution. J. Environ. Manag. 2017, 203. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zeng, Y.; Xie, Y.; He, Q.; Zhao, F.; Wang, Y.; Zeng, G. Single and combined removal of Cr (VI) and Cd (II) by nanoscale zero-valent iron in the absence and presence of EDDS. Water Sci. Technol. 2017. [Google Scholar] [CrossRef] [PubMed]
- McCann, C.M.; Gray, N.D.; Tourney, J.; Davenport, R.J.; Wade, M.; Finlay, N.; Hudson-Edwards, K.A.; Johnson, K.L. Remediation of a historically Pb contaminated soil using a model natural Mn oxide waste. Chemosphere 2015, 138, 211–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henry, H.; Naujokas, M.F.; Attanayake, C.; Basta, N.T.; Cheng, Z.; Hettiarachchi, G.M.; Maddaloni, M.; Schadt, C.; Scheckel, K.G. Bioavailability-based in situ remediation to meet future lead (Pb) standards in urban soils and gardens. Environ. Sci. Technol. 2015, 49, 8948–8958. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.G.; Spliethoff, H.M.; Ribaudo, L.N.; Lopp, D.M.; Shayler, H.A.; Marquez-Bravo, L.G.; Lambert, V.T.; Ferenz, G.S.; Russell-Anelli, J.M.; Stone, E.B. Lead (Pb) and other metals in New York City community garden soils: Factors influencing contaminant distributions. Environ. Pollut. 2014, 187, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Gurung, B.; Race, M.; Fabbricino, M.; Komínková, D.; Libralato, G.; Siciliano, A.; Guida, M. Assessment of metal pollution in the Lambro Creek (Italy). Ecotoxicol. Environ. Saf. 2018, 148. [Google Scholar] [CrossRef] [PubMed]
- Komínková, D.; Nabelková, J. The risk assessment of heavy metals in the ecosystem of urban creeks. Water Sci. Technol. 2006, 53, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Meers, E.; Qadir, M.; De Caritat, P.; Tack, F.M.G.; Du Laing, G.; Zia, M.H. EDTA-assisted Pb phytoextraction. Chemosphere 2009, 74, 1279–1291. [Google Scholar]
- Shen, Z.-G.; Li, X.-D.; Wang, C.-C.; Chen, H.-M.; Chua, H. Lead phytoextraction from contaminated soil with high-biomass plant species. J. Environ. Qual. 2002, 31, 1893–1900. [Google Scholar] [CrossRef] [PubMed]
- Mason, L.H.; Harp, J.P.; Han, D.Y. Pb neurotoxicity: Neuropsychological effects of lead toxicity. Biomed. Res. Int. 2014, 2014, 840547. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. USEPA Method 3051, Microwave Assisted Digestion of Sediments, Sludges, Soils and Oils; Official Methods/US EPA Methods; United States Environmental Protection Agency: Washington, DC, USA, 2001.
- Pueyo, M.; Mateu, J.; Rigol, A.; Vidal, M.; López-Sánchez, J.F.; Rauret, G. Use of the modified BCR three-step sequential extraction procedure for the study of trace element dynamics in contaminated soils. Environ. Pollut. 2008, 152, 330–341. [Google Scholar] [CrossRef] [PubMed]
- Race, M.; Nabelkova, J.; Fabbricino, M.; Pirozzi, F.; Raia, P. Analysis of Heavy Metal Sources for Urban Creeks in the Czech Republic. Water Air Soil Pollut. 2015, 226. [Google Scholar] [CrossRef]
- Alvarenga, P.; Palma, P.; Gonçalves, A.P.; Fernandes, R.M.; De Varennes, A.; Vallini, G.; Duarte, E.; Cunha-Queda, A.C. Evaluation of tests to assess the quality of mine-contaminated soils. Environ. Geochem. Health 2008, 30, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Spasiano, D.; Siciliano, A.; Race, M.; Marotta, R.; Guida, M.; Andreozzi, R.; Pirozzi, F. Biodegradation, ecotoxicity and UV254/H2O2 treatment of imidazole, 1-methyl-imidazole and N,N′-alkyl-imidazolium chlorides in water. Water Res. 2016, 106. [Google Scholar] [CrossRef] [PubMed]
- Polettini, A.; Pomi, R.; Calcagnoli, G. Assisted washing for heavy metal and metalloid removal from contaminated dredged materials. Water Air Soil Pollut. 2009, 196, 183–198. [Google Scholar] [CrossRef]
- Polettini, A.; Pomi, R.; Rolle, E. The effect of operating variables on chelant-assisted remediation of contaminated dredged sediment. Chemosphere 2007, 66, 866–877. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, A.; Fabbricino, M.; van Hullebusch, E.D.; Esposito, G. Calibration and validation of a two-step kinetic mathematical model for predicting Cu extraction efficiency in an EDDS-enhanced soil washing. Water Air Soil Pollut. 2016, 227, 71. [Google Scholar] [CrossRef]
- Janssen, P.H.M.; Heuberger, P.S.C. Calibration of process-oriented models. Ecol. Model. 1995, 83, 55–66. [Google Scholar] [CrossRef]
- Race, M.; Marotta, R.; Fabbricino, M.; Pirozzi, F.; Andreozzi, R.; Cortese, L.; Giudicianni, P. Copper and zinc removal from contaminated soils through soil washing process using ethylenediaminedisuccinic acid as a chelating agent: A modeling investigation. J. Environ. Chem. Eng. 2016, 4, 2878–2891. [Google Scholar] [CrossRef]
- Fabbricino, M.; Ferraro, A.; Del Giudice, G.; D’Antonio, L. Current views on EDDS use for ex situ washing of potentially toxic metal contaminated soils. Rev. Environ. Sci. Bio/Technol. 2013, 12, 391–398. [Google Scholar] [CrossRef]
- Neale, C.N.; Bricka, R.Y.; Chao, A.C. Evaluating acids and chelating agents for removing heavy metals from contaminated soils. Environ. Prog. 1997, 16, 274–280. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of Adsorption on Carbon from Solution. J. Sanit. Eng. Div. 1963, 89, 31–60. [Google Scholar]
- Yan, D.Y.S.; Yip, T.C.M.; Yui, M.M.T.; Tsang, D.C.W.; Lo, I.M.C. Influence of EDDS-to-metal molar ratio, solution pH, and soil-to-solution ratio on metal extraction under EDDS deficiency. J. Hazard. Mater. 2010, 178, 890–894. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Luo, C.; Liu, Y.; Quan, L.; Chen, Y.; Shen, Z. Residual effects of EDDS leachates on plants during EDDS-assisted phytoremediation of copper contaminated soil. Sci. Total Environ. 2013, 444, 263–270. [Google Scholar] [CrossRef] [PubMed]

| Metal | Concentration [mg/kg] | LD a—F-AAS [mg/L] | LD a—GF-AAS [mg/L] |
|---|---|---|---|
| Cd | 1 ± 0.2 | 5.0 × 10−2 | 5.0 × 10−4 |
| Zn | 50 ± 3.2 | 5.0 × 10−2 | 2.0 × 10−4 |
| Pb | 8600 ± 300 | 1.0 | 5.0 × 10−3 |
| Cu | 25 ± 1.7 | 0.2 | 5.0 × 10−3 |
| Cr | 2 ± 0.7 | 0.5 | 5.0 × 10−3 |
| Ni | 40 ± 2.5 | 0.3 | 5.0 × 10−3 |
| Ca | 35,411 ± 634 | 1.0 × 10−2 | - |
| Fe | 8974 ± 289 | 0.25 | 5.0 × 10−3 |
| Mn | 300 ± 18 | 0.1 | 1.0 × 10−3 |
| k1 [1/h] | k2 [1/h] | b1 [mg/kg] | b2 [mg/kg] | Meff | |
|---|---|---|---|---|---|
| EDDS 0.002 M | 0.2 | 6 | 1500 | 2280 | 0.939 |
| EDDS 0.003 M | 0.2 | 6 | 1560 | 2330 | 0.957 |
| EDDS 0.005 M | 0.2 | 6 | 1690 | 2420 | 0.952 |
| EDDS 0.008 M | 0.2 | 6 | 1800 | 2520 | 0.964 |
| EDDS 0.010 M | 0.2 | 6 | 1890 | 2580 | 0.963 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fabbricino, M.; Ferraro, A.; Luongo, V.; Pontoni, L.; Race, M. Soil Washing Optimization, Recycling of the Solution, and Ecotoxicity Assessment for the Remediation of Pb-Contaminated Sites Using EDDS. Sustainability 2018, 10, 636. https://doi.org/10.3390/su10030636
Fabbricino M, Ferraro A, Luongo V, Pontoni L, Race M. Soil Washing Optimization, Recycling of the Solution, and Ecotoxicity Assessment for the Remediation of Pb-Contaminated Sites Using EDDS. Sustainability. 2018; 10(3):636. https://doi.org/10.3390/su10030636
Chicago/Turabian StyleFabbricino, Massimiliano, Alberto Ferraro, Vincenzo Luongo, Ludovico Pontoni, and Marco Race. 2018. "Soil Washing Optimization, Recycling of the Solution, and Ecotoxicity Assessment for the Remediation of Pb-Contaminated Sites Using EDDS" Sustainability 10, no. 3: 636. https://doi.org/10.3390/su10030636





