Gypsum and Legume Residue as a Strategy to Improve Soil Conditions in Sustainability of Agrosystems of the Humid Tropics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Soil Sampling and Analyses
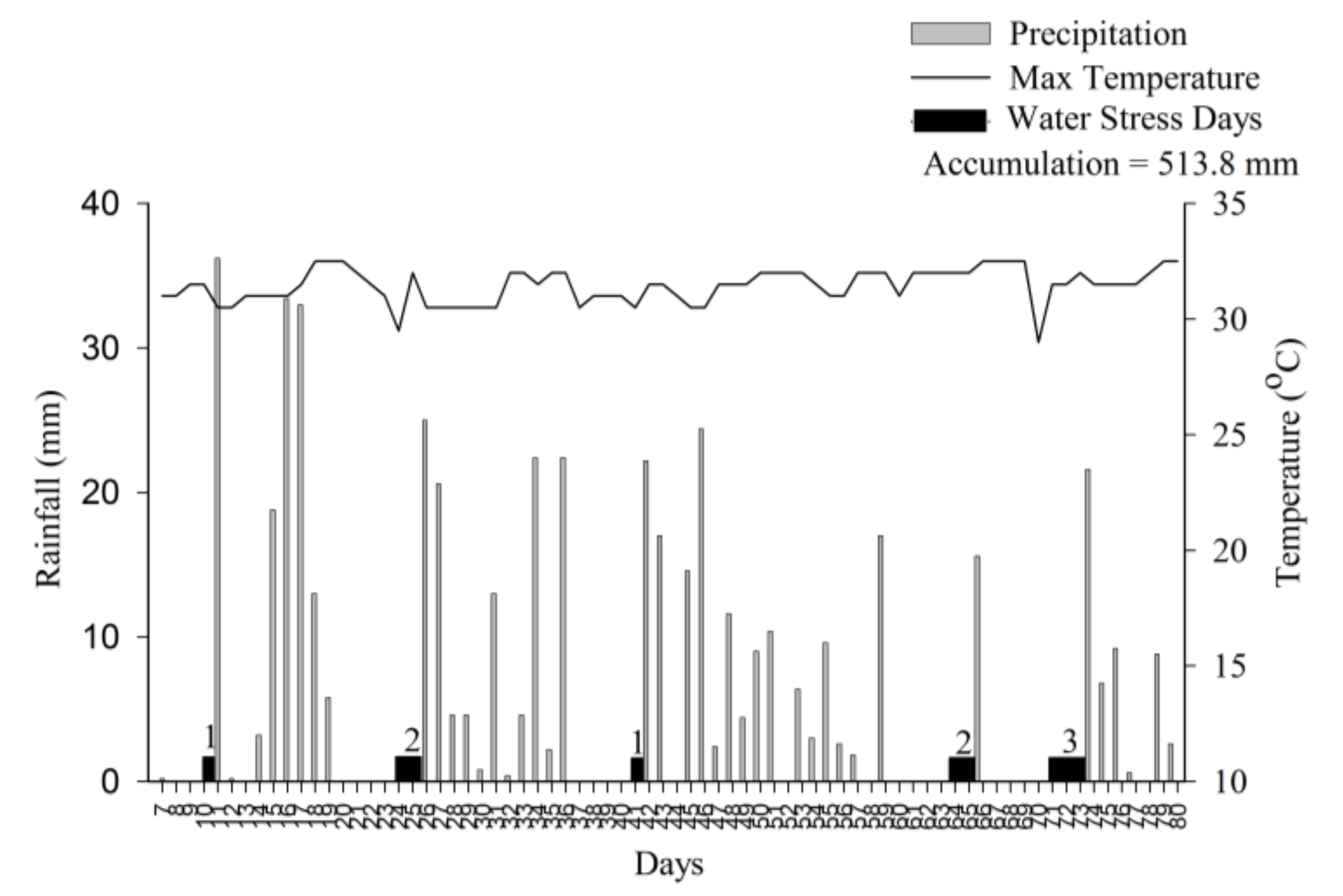
2.3. Plant Analysis, Yield Components and Water Stress Days
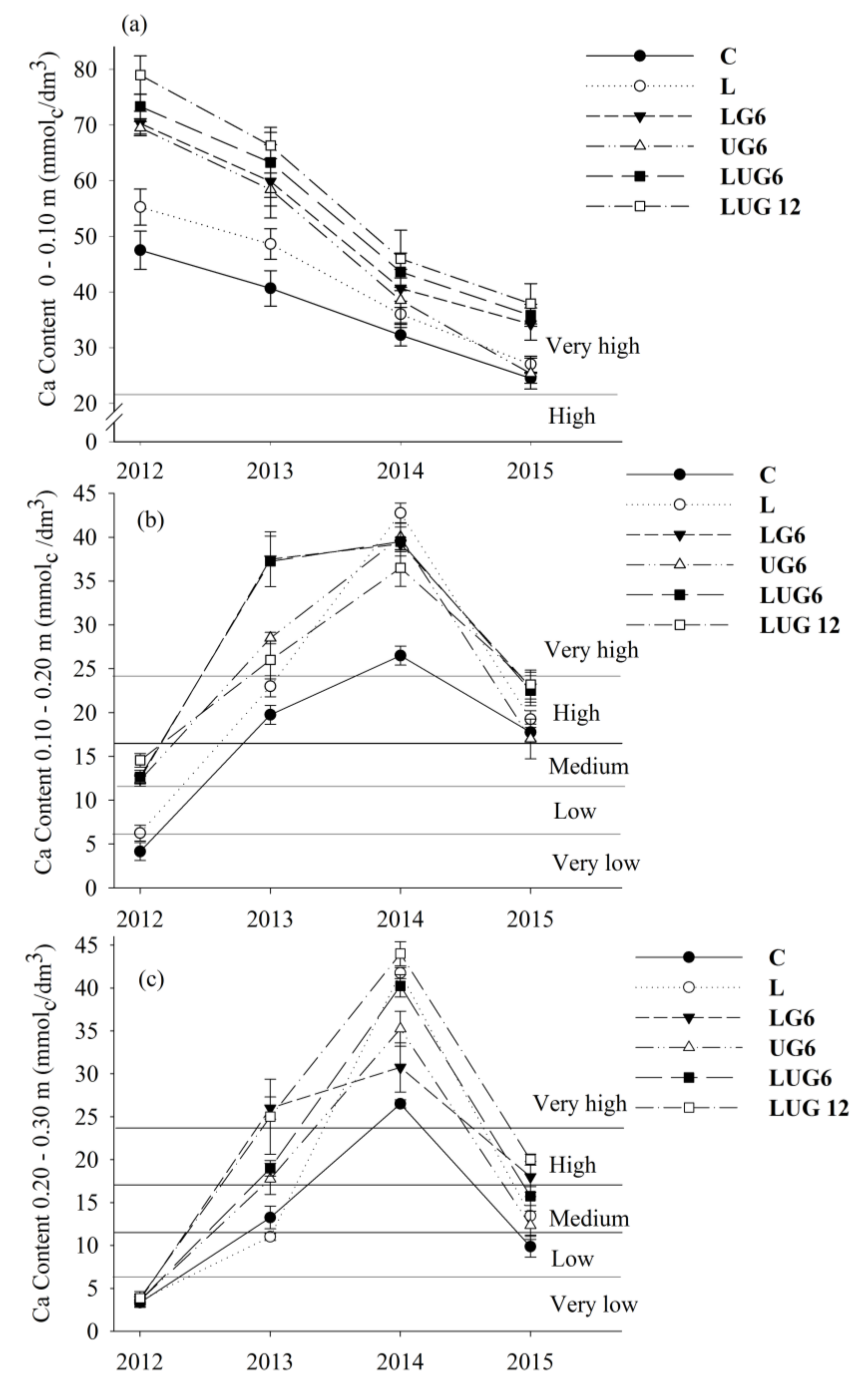
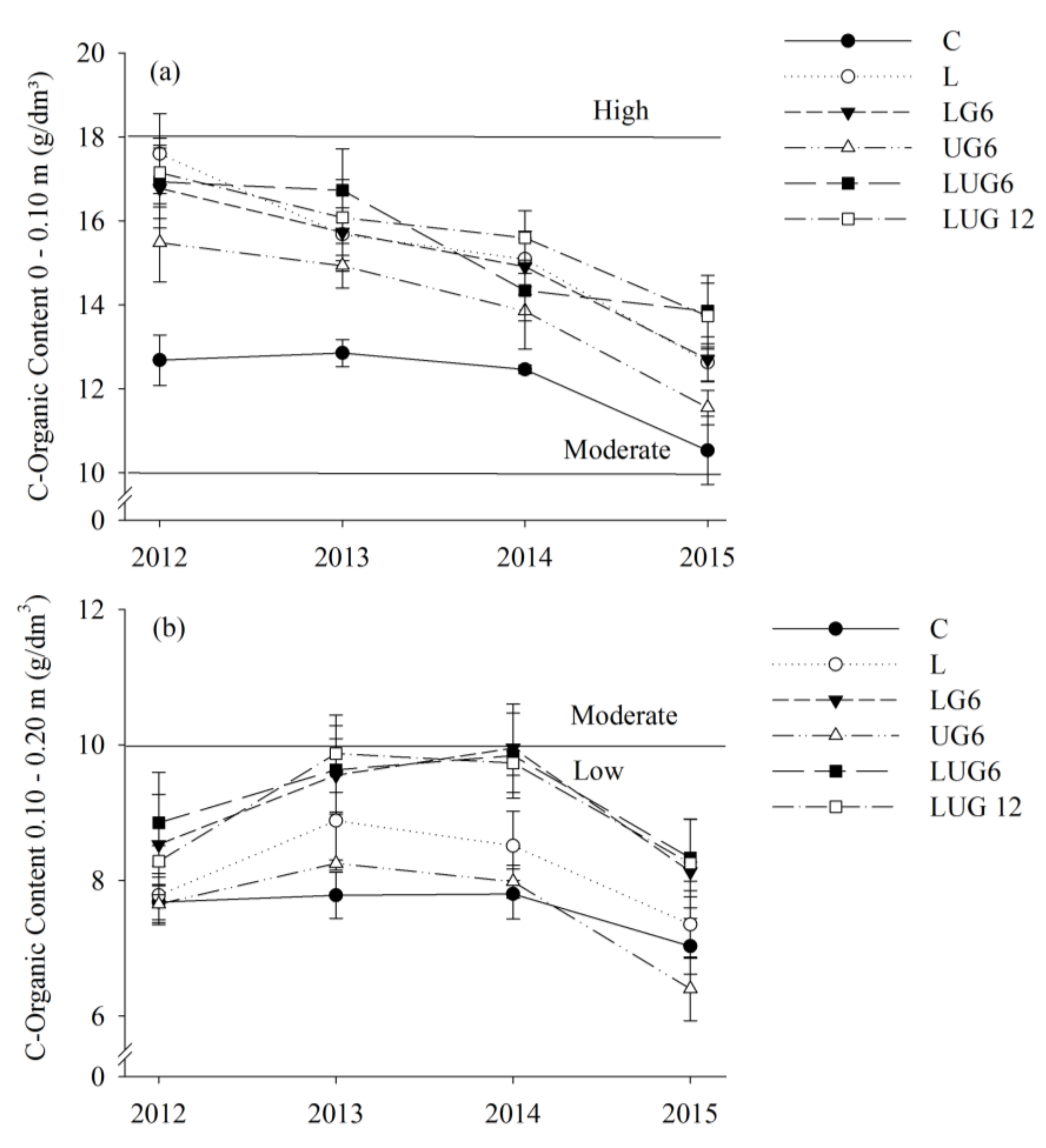
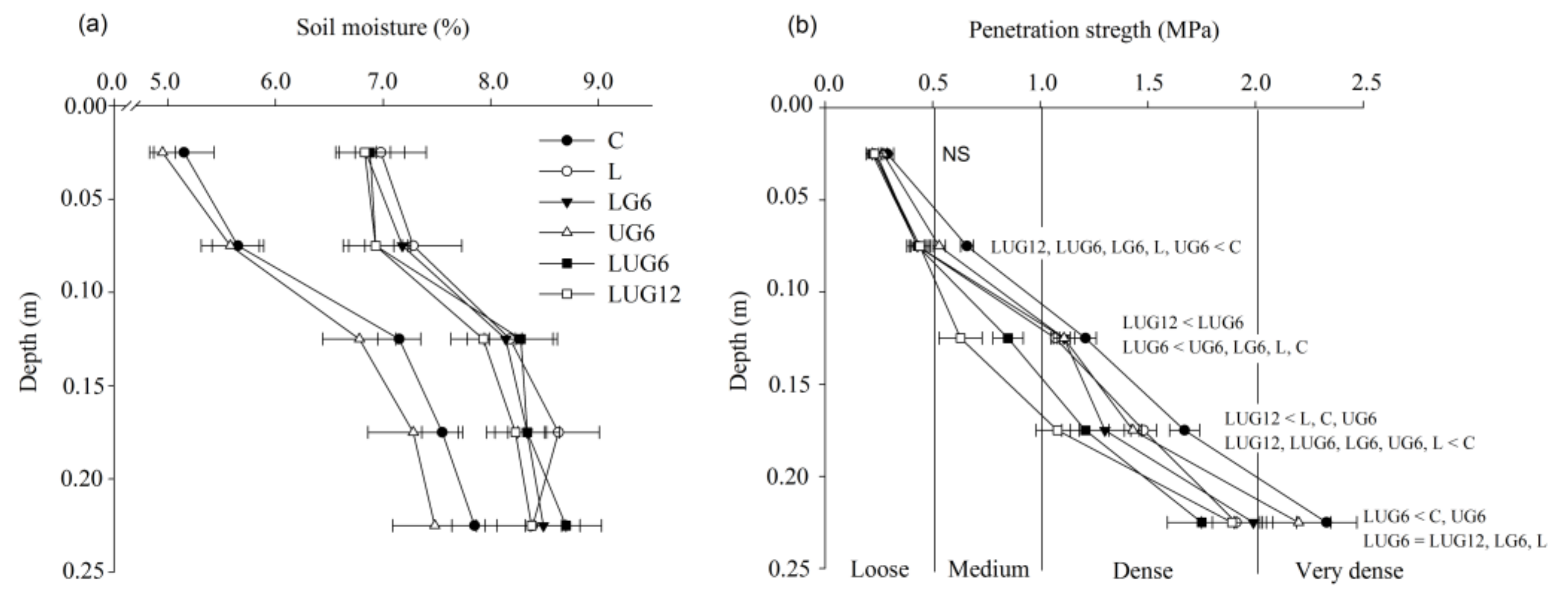
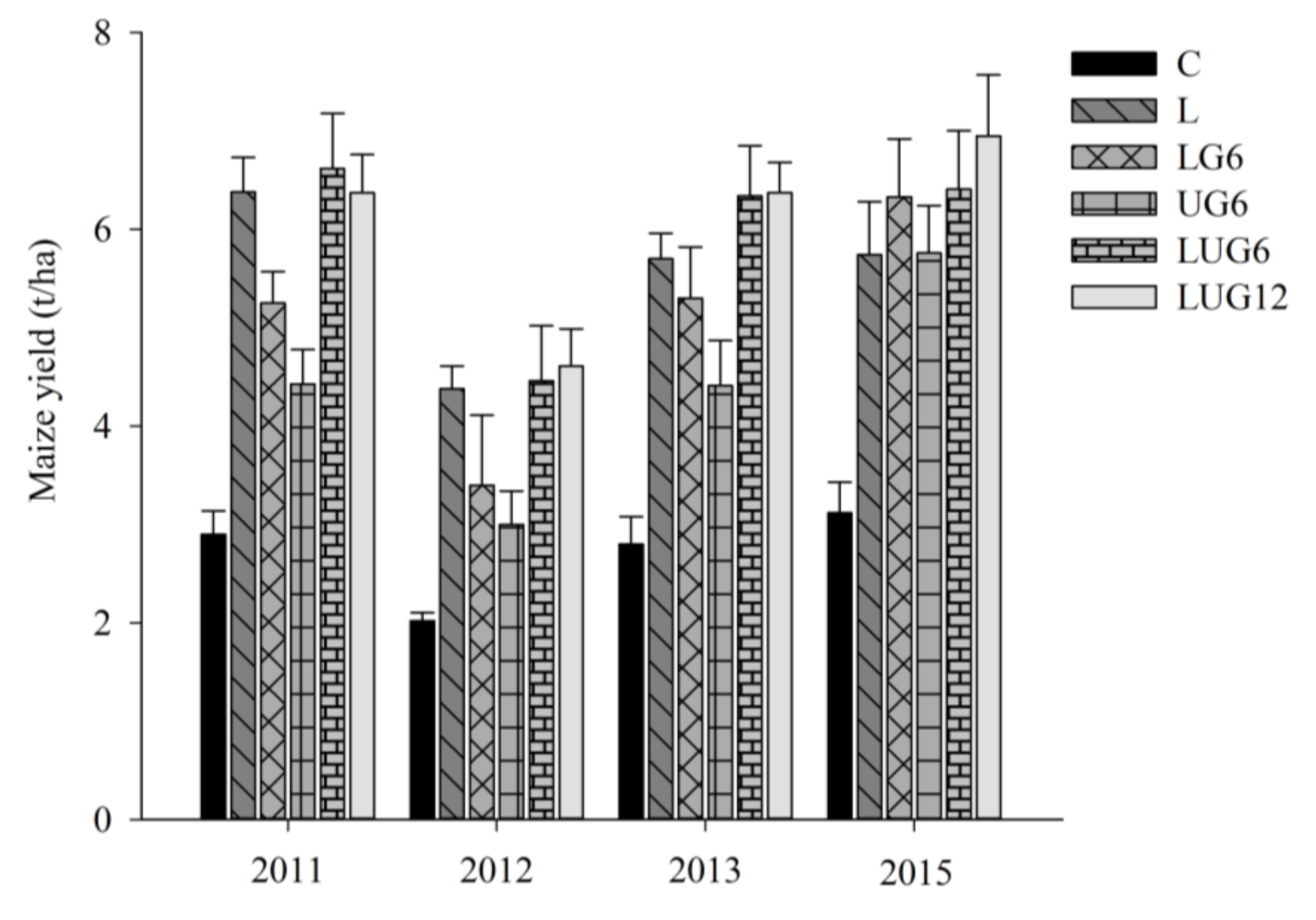
3. Results
3.1. Enhancement of Root Environment and Responses in Maize Grain Yield
3.2. The Leaf Area Index, Nutrient Uptake and Nitrogen Remobilization
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Daniells, I.G. Hardsetting soils: A review. Soil Res. 2012, 50, 349–359. [Google Scholar] [CrossRef]
- Giarola, N.F.B.; Silva, A.P.; Tormena, C.; Souza, L.S.; Ribeiro, L.P. Similaridades entre o caráter coeso dos solos e o comportamento hardsetting: Estudo de caso. Rev. Bras. Cienc. Solo 2011, 25, 239–247. [Google Scholar] [CrossRef]
- Wong, M.T.F.; Asseng, S. Yield and environmental benefits of ameliorating subsoil constraints under variable rainfall in a Mediterranean environment. Plant Soil 2007, 297, 29–42. [Google Scholar] [CrossRef]
- Denmead, O.T.; Shaw, R.H. The effects of soil moisture stress at different stages of growth on the development and yield of corn. Agron. J. 1960, 52, 272–274. [Google Scholar] [CrossRef]
- Benjamin, J.G.; Nielsen, D.C.; Vigil, M.F. Quantifying effects of soil conditions on plant growth and crop production. Geoderma 2003, 116, 137–148. [Google Scholar] [CrossRef]
- Moura, E.G.; Oliveira, A.K.; Coutinho, C.G.; Pinheiro, K.M.; Aguiar, A.C.F. Management of a cohesive tropical soil to enhance rootability and increase the efficiency of nitrogen and potassium use. Soil Use Manag. 2012, 28, 368–375. [Google Scholar] [CrossRef]
- Dechert, G.; Veldkamp, E.; Brumme, R. Are partial nutrient balances suitable to evaluate nutrient sustainability of land use systems? Results from a case study in Central Sulawesi, Indonesia. Nutr. Cycl. Agroecosyst. 2005, 72, 201–212. [Google Scholar] [CrossRef]
- Mulumba, L.N.; Lal, R. Mulching effects on selected soil physical properties. Soil Tillage Res. 2008, 98, 106–111. [Google Scholar] [CrossRef]
- Sumner, M.E. Gypsum improves subsoil root growth. In Proceedings of the International symposium “Root Reseacher and Aplications, BOKU, Viena, Austria, 2–4 September 2009. [Google Scholar]
- Badorreck, A.; Krümmelbein, J.; Raab, T. Long-term effects of deep soil loosening on root distribution and soil physical parameters in compacted lignite mine soils. Geophys. Res. Abstr. 2015, 17, EGU2015-14990. Available online: http://meetingorganizer.copernicus.org/EGU2015/EGU2015-14990.pdf (accessed on 24 March 2018).
- Carrizo, M.E.; Alesso, C.A.; Cosentino, D.; Imhoff, S. Aggregation agents and structural stability in soils with different texture and organic carbon contents. Sci. Agric. 2015, 72, 75–82. [Google Scholar] [CrossRef]
- Moussadek, R.; Mrabet, R.; Dahan, R.; Zouahri, A.; El Mourid, M.; Van Ranst, E. Tillage System Affects Soil Organic Carbon Storage and Quality in Central Morocco. Appl. Environ. Soil Sci. 2014, 2014, 654796. [Google Scholar] [CrossRef]
- Christensen, B.T. Organic Matter in Soil-Structure, Function and Turnover; DIAS Report. Plant Production, 30; Danish Institute of Agricultural Sciences, Research Center Foulum: Tjele, Denmark, 2000. [Google Scholar]
- Moura, E.G.; Marques, E.S.; Silva, T.M.B.; Piedade, A.; Aguiar, A.C.F. Interactions among leguminous trees, crops and weeds in a no-till alley cropping system. Int. J. Plant Prod. 2014, 8, 441–456. Available online: http://ijpp.gau.ac.ir/article_1719_3de34834108cef844800c6fc10334a2d.pdf (accessed on 24 March 2018).
- Wieder, R.W.; Cleveland, C.C.; Townsend, A.R. Controls over leaf litter decomposition in wet tropical forests. Ecology 2009, 90, 3333–3341. Available online: https://scholarworks.umt.edu/cgi/viewcontent.cgi?referer=https://www.google.com.br/&httpsredir=1&article=1009&context=decs_pubs (accessed on 24 March 2018). [CrossRef] [PubMed]
- Blanco-Canqui, H.; Lal, R. Soil and crop response to harvesting corn residues for biofuel production. Geoderma 2007, 141, 355–362. [Google Scholar] [CrossRef]
- Anikwe, M.A.N.; Eze, J.C.; Ibudialo, A.N. Influence of lime and gypsum application on soil properties and yield of cassava (Manihot esculenta Crantz.) in a degraded Ultisol in Agbani, Enugu Southeastern Nigeria. Soil Tillage Res. 2016, 158, 32–38. [Google Scholar] [CrossRef]
- Moura, E.G.; Sena, V.G.L.; Sousa, C.C.M.; Silva, F.R.; Coelho, M.J.A.; Macedo, V.R.A.; Aguiar, A.C.F. Enhancement of the rootability of a structurally fragile tropical soil using gypsum and leguminous residues to increase the maize yield. Soil Use Manag. 2016, 32, 118–126. [Google Scholar] [CrossRef]
- Wuddivira, M.N.; Camps-Roach, G. Effects of organic matter and calcium on soil structural stability. Eur. J. Soil Sci. 2007, 58, 722–727. [Google Scholar] [CrossRef]
- Whittinghill, K.; Hobbie, A.; Sarah, E. Effects of pH and calcium on soil organic matter dynamics in Alaskan tundra. Biogeochemistry 2012, 111, 569–581. [Google Scholar] [CrossRef]
- CPTEC/INPE. Center for Weather Forecasting and Climate Research. 2016. Available online: http://www.cptec.inpe.br/home/in (accessed on 13 May 2016).
- Moura, E.G.; Moura, N.G.; Marques, E.S.; Pinheiro, K.M.; Costa Sobrinho, J.R.S.; Aguiar, A.C.F. Evaluating chemical and physical quality indicators for a structurally fragile tropical soil. Soil Use Manag. 2009, 25, 368–375. [Google Scholar] [CrossRef]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; USDA-Natural Resources Conservation Service: Washington, DC, USA, 2012. [Google Scholar]
- Aguiar, A.C.F.; Bicudo, S.J.; Costa Sobrinho, J.R.S.; Martins, A.L.S.; Coelho, K.P.; Moura, E.G. Nutrient recycling and physical indicators of an alley cropping system in a sandy loam soil in the Pre-Amazon region of Brazil. Nutr. Cycl. Agroecosyst. 2010, 86, 189–198. [Google Scholar] [CrossRef]
- Raij, B.; Quaggio, J.A.; Silva, N.M. Extraction of phosphorus, potassium, calcium, and magnesium from soils by an ion exchange resin procedure. Commun. Soil Sci. Plant Anal. 1986, 17, 547–566. [Google Scholar] [CrossRef]
- Heckman, J.R. Soil fertility test interpretation: Phosphorus, potassium, magnesium and calcium. In Fact Sheet FS719, Rutgers Cooperative Extension, New Jersey Agricultural Experimental Extension; 2006; Available online: file:///C:/Users/User/Downloads/fs719.pdf (accessed on 24 March 2018).
- Tiessen, H.; Moir, J.O. Total and organic carbon. In Soil Sampling and Methods of Analysis; Carter, M.E., Ed.; Lewis Publishers: Ann Arbor, MI, USA, 1993; pp. 187–200. [Google Scholar]
- Hazelton, P.; Murphy, B. Interpreting Soil Test Results. What Do All the Numbers Mean? CSIRO Publishing: Clayton, Australia, 2007. [Google Scholar]
- Montgomery, E.G. Correlation studies of corn. Annu. Rep. 1911, 24, 108–159. [Google Scholar]
- Cottenie, A. Soil and Plant Testing as a Basis of Fertilizer Recommendations; FAO Soil Bulletin: Rome, Italy, 1980. [Google Scholar]
- Kolahchi, Z.; Jalali, M. Effect of water quality on the leaching of potassium from sandy soil. J. Arid. Environ. 2007, 68, 624–639. [Google Scholar] [CrossRef]
- Moore, T.R.; Turunen, J. Carbon accumulation and storage in mineral subsoil beneath peat. Soil Sci. Soc. Am. J. 2004, 68, 690–696. [Google Scholar] [CrossRef]
- Oste, L.A.; Temminghoff, E.J.M.; Van Riemsdijk, W.H. Solid solution partitioning of organic matter in soils as influenced by an increase in pH or Ca concentration. Environ. Sci. Technol. 2002, 36, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, M.A.; Harrison, R.; Webb, J. Managing soil organic matter-implications for structure on organic farms. Soil Use Manag. 2002, 18, 284–292. [Google Scholar] [CrossRef]
- Mbah, E.U.; Eke-Okoro, O. Relationship between some growth parameters, dry matter content and yield of some sweet potato genotypes grown under rainfed weathered ultisols in the humid tropics. J. Agron. 2015, 14, 121–129. [Google Scholar] [CrossRef]
- Sadras, V.O.; Milroy, S.P. Soil—Water thresholds for the responses of leaf expansion and gas exchange: A review. Field Crop. Res. 1996, 47, 253–266. [Google Scholar] [CrossRef]
- Sivasankar, S.; Rothstein, S.; Oaks, A. Regulation of the accumulation and reduction of nitrate by nitrogen and carbon metabolites in maize seedlings. Plant Physiol. 1997, 114, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Garnett, T.; Conn, V.; Kaiser, B.N. Root based approaches to improving nitrogen use efficiency in plants. Plant Cell Environ. 2009, 32, 1272–1283. [Google Scholar] [CrossRef] [PubMed]
- Masclaux-Daubresse, C.; Daniel-Vedel, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L. Nitrogen uptake, assimilation and remobilization in plants: Challenges for sustainable and productive agriculture. Ann. Bot. 2010, 105, 1141–1157. [Google Scholar] [CrossRef] [PubMed]
- Borrel, A.K.; Hammer, G.L.; Henzell, R.G. Does maintaining green leaf area in sorghum improve yield under drought? II. Dry matter production and yield. Crop Sci. 2000, 40, 1037–1048. [Google Scholar] [CrossRef]
| C | U | L | LG6 | UG6 | LUG6 | LUG12 | |
|---|---|---|---|---|---|---|---|
| LAI (m2 m−2) | 2.45 b | 2.58 b | 3.06 a | 3.03 a | 3.04 a | 3.08 a | 3.29 a |
| NT (Kg ha−1) | 34.69 c | 57.98 c | 76.38 b | 69.43 b | 75.45 b | 103.16 a | 94.28 a |
| NR (Kg ha−1) | 8.38 c | 13.68 c | 38.67 b | 37.65 b | 27.89 b | 56.41 a | 52.35 a |
| NPT (Kg ha−1) | 27.24 c | 28.00 c | 41.19 ab | 50.46 a | 37.19 b | 42.20 a | 45.16 a |
| NG (Kg ha−1) | 39.43 d | 52.18 d | 72.28 bc | 88.35 ab | 70.14 c | 88.61 ab | 94.50 a |
| TN (Kg ha−1) | 61.93 c | 85.98 bc | 117.57 ab | 119.89 a | 112.64 ab | 145.36 a | 139.44 a |
| DM (Mg ha−1) | 6.72 e | 9.14 d | 11.21 c | 12.95 ab | 11.37 bc | 13.46 a | 13.23 ab |
| C | U | L | LG6 | UG6 | LUG6 | LUG12 | |
|---|---|---|---|---|---|---|---|
| Weight of ear (g) | 118.48 c | 162.10 b | 188.71 ab | 183.85 ab | 190.61 ab | 198.26 a | 199.40 a |
| Number of grains ear −1 | 447 c | 555 b | 619 ab | 619 ab | 618 ab | 624 a | 628 a |
| Weight of 100 grains (g) | 26.24 b | 28.99 ab | 30.50 a | 29.65 a | 30.84 a | 31.96 a | 31.52 a |
| Weight of grains (t ha−1) | 3.12 c | 4.33 bc | 5.74 ab | 6.33 a | 5.76 ab | 6.41 a | 6.95 a |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Moura, E.G.; Portela, S.B.; Macedo, V.R.A.; Sena, V.G.L.; Sousa, C.C.M.; Aguiar, A.D.C.F. Gypsum and Legume Residue as a Strategy to Improve Soil Conditions in Sustainability of Agrosystems of the Humid Tropics. Sustainability 2018, 10, 1006. https://doi.org/10.3390/su10041006
De Moura EG, Portela SB, Macedo VRA, Sena VGL, Sousa CCM, Aguiar ADCF. Gypsum and Legume Residue as a Strategy to Improve Soil Conditions in Sustainability of Agrosystems of the Humid Tropics. Sustainability. 2018; 10(4):1006. https://doi.org/10.3390/su10041006
Chicago/Turabian StyleDe Moura, Emanoel Gomes, Stefanny Barros Portela, Vinicius Ribamar Alencar Macedo, Virley Gardeny Lima Sena, Carlos Cesar Martin Sousa, and Alana Das Chagas Ferreira Aguiar. 2018. "Gypsum and Legume Residue as a Strategy to Improve Soil Conditions in Sustainability of Agrosystems of the Humid Tropics" Sustainability 10, no. 4: 1006. https://doi.org/10.3390/su10041006




