Macro Level Modeling of a Tubular Solid Oxide Fuel Cell
Abstract
:1. Introduction
2. SOFC Model Description
2.1. Methodology
- The fuel is a mixture of gases, which consists of any combination of CH4, H2, H2O, CO, CO2, O2, and N2.
- The air supplied to the fuel cell consists of any combination of O2, N2, CO2, and H2O.
- Chemical components behave as ideal gases at the operating temperature and pressure of the SOFC.
- Every SOFC within a stack operates at uniform temperature and pressure.
2.2. Chemical and Electrochemical Reactions
2.3. Electrochemical and Thermodynamic Calculations
3. Model Inputs and Outputs
| Parameter | Value | Units |
|---|---|---|
| Fuel utilization factor, Uf | 0.85 | - |
| Actual current density, iact | 3200 | A/m2 |
| γanode (Equation 13) | 2.0 × 108 | A/m2 |
| γcathode (Equation 14) | 1.5 × 108 | A/m2 |
| Transfer coefficient (anode), βanode | 0.5 | - |
| Transfer coefficient (cathode), βcathode | 0.5 | - |
| Activation energy (anode), Eactiv,anode | 105 | kJ/mol |
| Activation energy (cathode), Eactiv,cathode | 110 | kJ/mol |
| Constant A (Equation 16) | 2.0 × 108 | Ω-m2 |
| Constant B (Equation 16) | 9000 | K |
| Limiting current density, iL | 6500 | A/m2 |
| Limiting current density correction factor a (Equation 18), a | 0.05 | - |
| Parameter | Units |
|---|---|
| Nernst voltage, Vrev | V |
| Actual operating voltage, Vact | V |
| Overall activation loss, Vactiv | V |
| Ohmic loss, VOhm | V |
| Exchange current density (anode), i0,anode | A/m2 |
| Exchange current density (cathode), i0,cathode | A/m2 |
| Activation loss (anode and cathode) | V |
| Pressure adjusted limiting current density, iL,adj | A/m2 |
| Power produced, W | W |
| Heat produced, Q | W |
4. Results and Discussion
4.1. Activation Loss Sensitivity Analysis
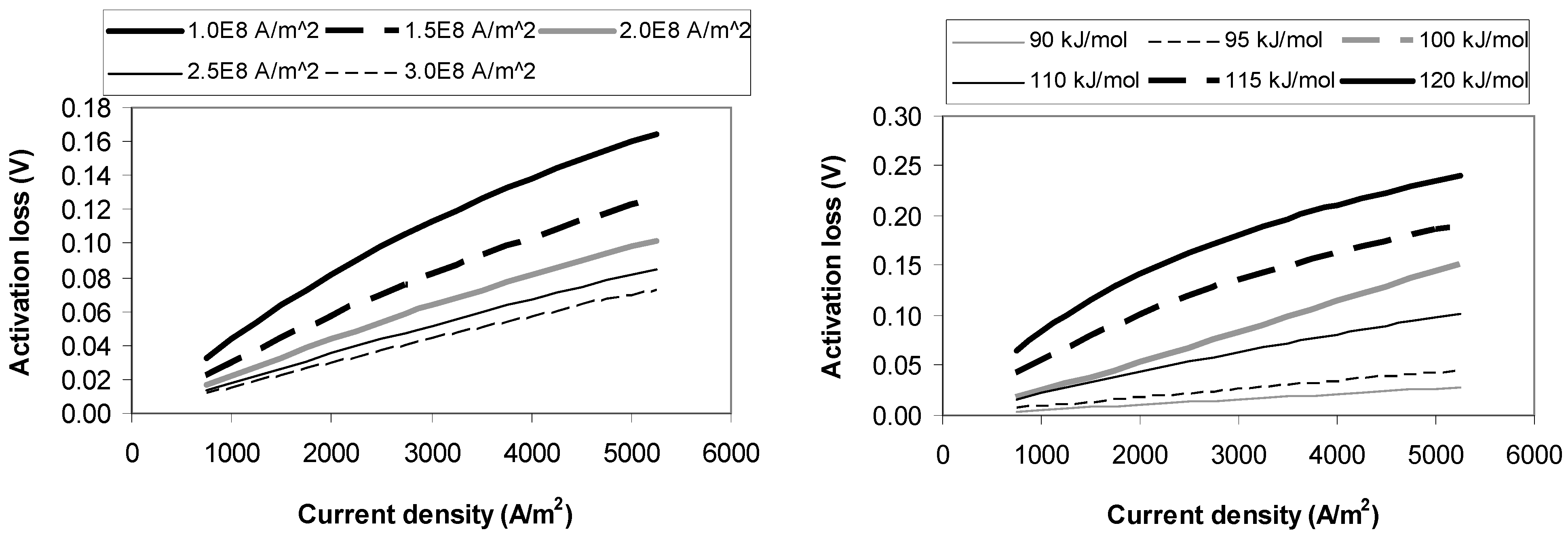
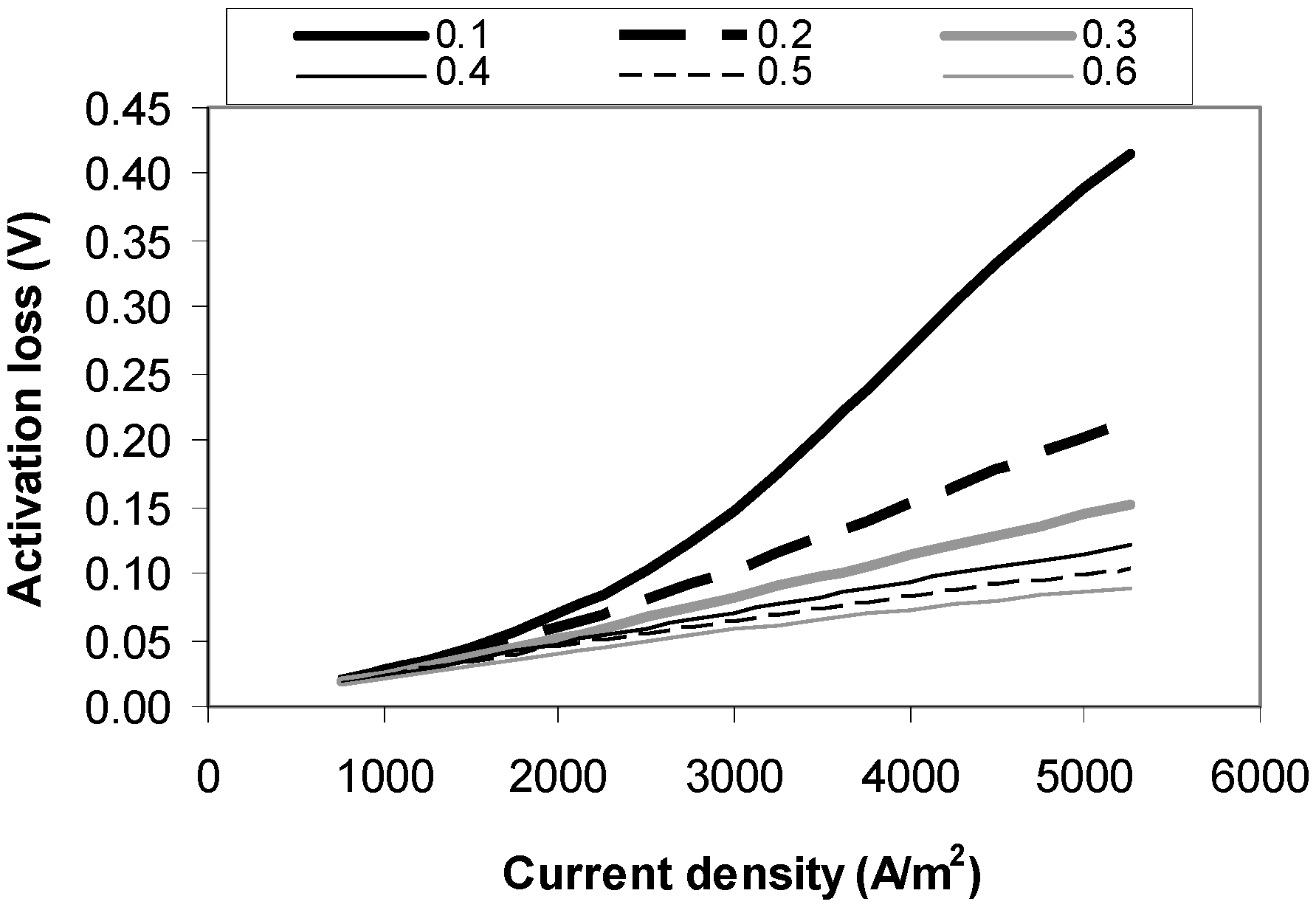
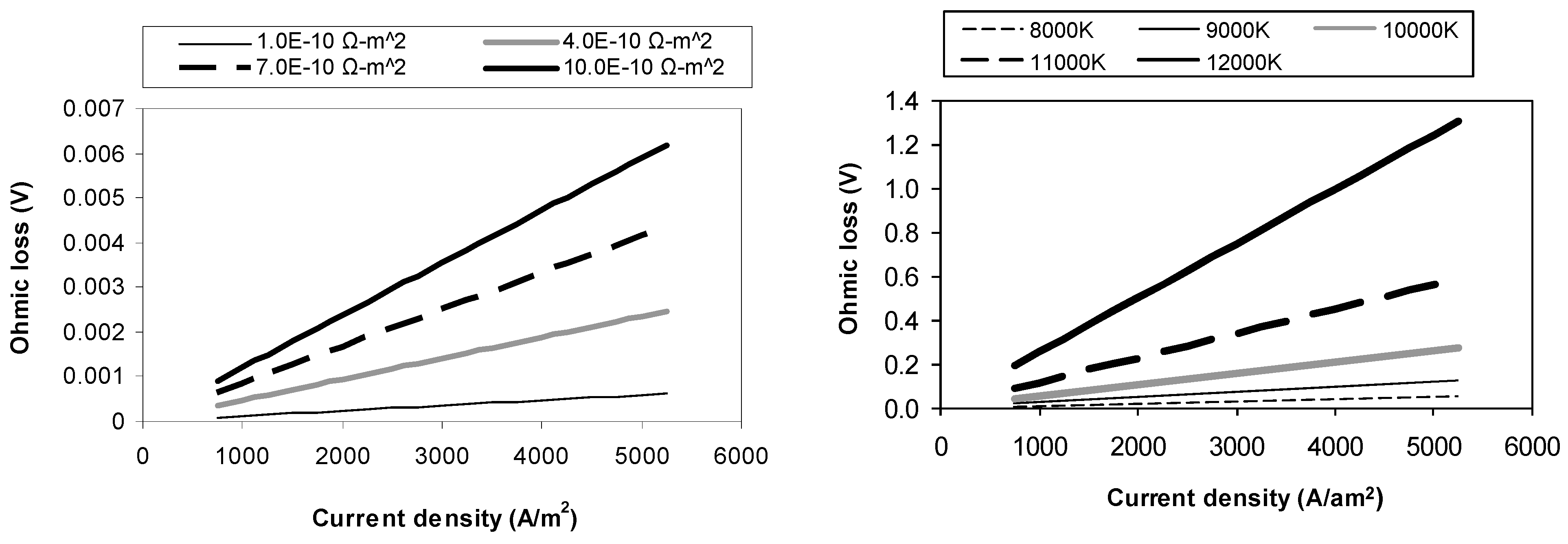
4.2. Ohmic Loss Sensitivity Analysis
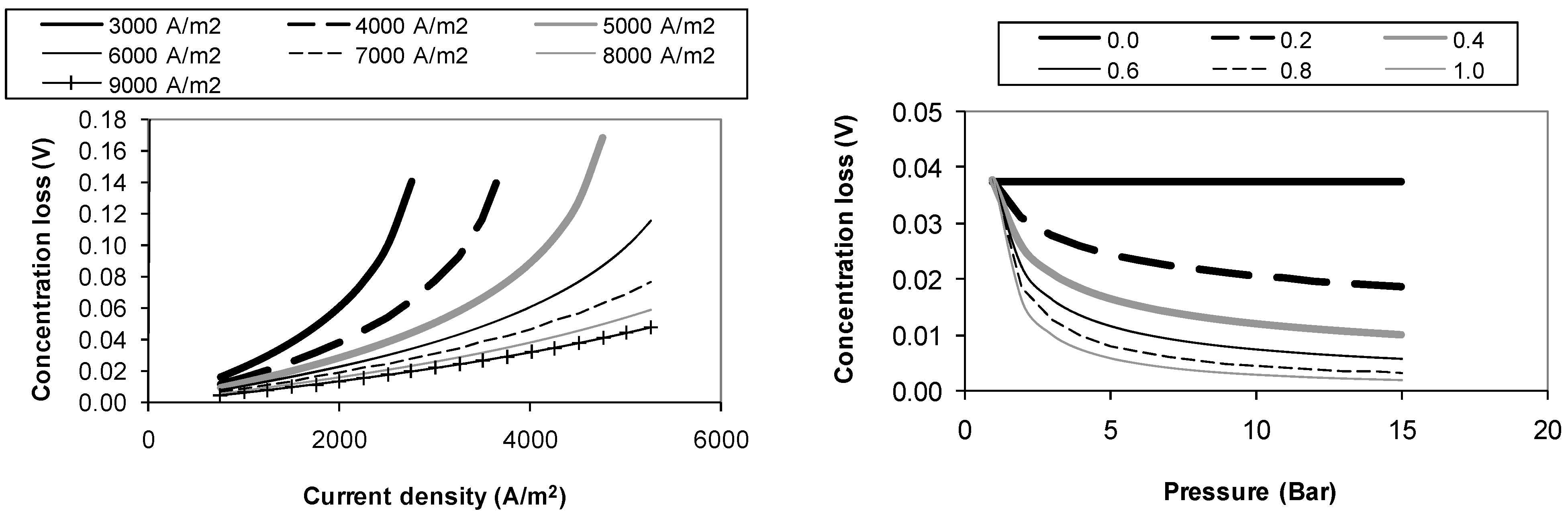
4.3. Concentration Loss Sensitivity Analysis
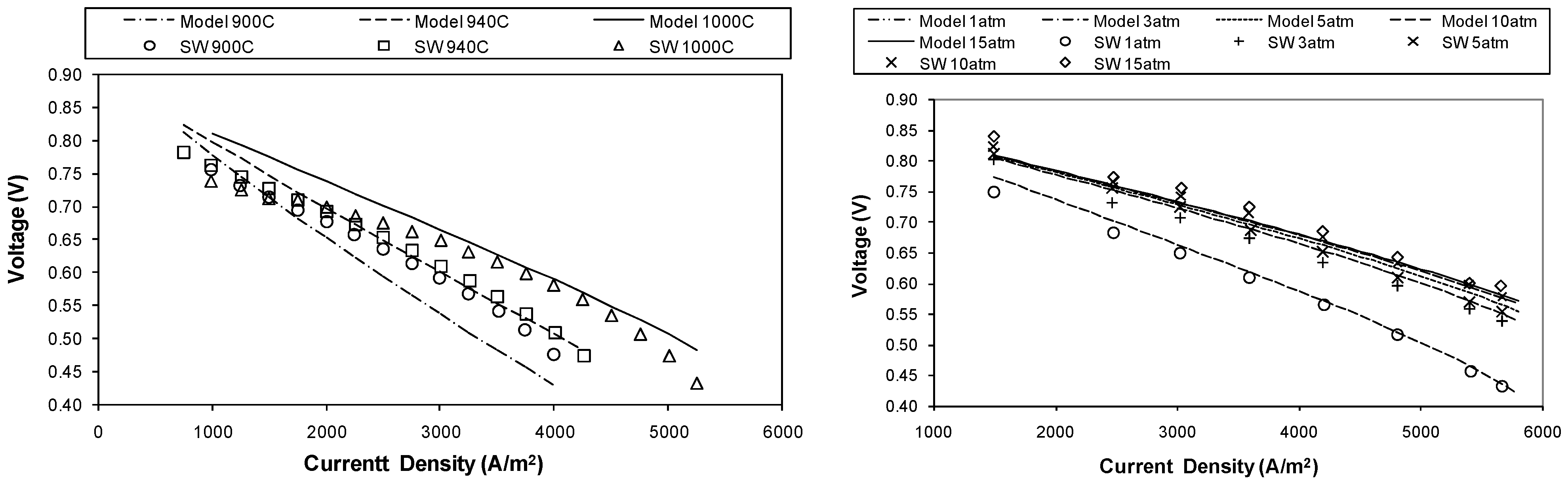
5. Model Validation
6. Conclusions
Nomenclature
| E | activation energy, J/mol |
| F | Faraday’s constant, A/mol |
| g | Gibbs free energy, J/mol |
| h | enthalpy, kJ/kg |
| i | current density, A/m2 |
| i0 | exchange current density, A/m2 |
| iL | limiting current density, A/m2 |
| k | equilibrium constant |
mass flow rate, kg/s | |
| NT | total number of moles, mol |
| P | pressure, Pa |
| Q | heat, W |
| R | resistance, Ωm2 |
| Ru | universal gas constant, J* mol−1 K−1 |
| T | temperature, °C |
| Uf | fuel utilization factor |
| V | voltage, V |
power, W | |
heat rate, W |
Greek Letters
| Λ | active cell area, m2 |
| β | transfer coefficient |
| ν | stoichiometric coefficient |
| γ | pre-exponential factor, A/m2 |
Subscripts
| act | actual |
| activ | activation |
| adj | adjusted |
| conc | concentration |
| I | internal |
| Ohm | Ohmic |
| op | operating |
| ref | reference |
| rev | reversible |
Acknowledgements
References
- Singhal, S.C.; Kendall, K. High Temperature Solid Oxide Fuel Cells; Elsevier: Oxford, UK, 2003. [Google Scholar]
- Power Generation. Available online: http://www.powergeneration.siemens.com (accessed on 15 November 2010).
- Larminie, J; Dicks, A. Fuel Cell Systems Explained; Wiley: New York, NY, USA, 2004. [Google Scholar]
- Omosun, A.O.; Bauen, A.; Brandon, N.P.; Adjiman, C.S.; Hart, D. Modeling system efficiencies and costs of two biomass-fuelled SOFC systems. J. Power Sources 2004, 131, 96–106. [Google Scholar] [CrossRef]
- Pfafferodt, M.; Heidebrecht, P.; Stelter, M.; Sundmacher, K. Model-based prediction of suitable operating range of a SOFC for an Auxiliary Power Unit. J. Power Sources 2005, 149, 53–62. [Google Scholar] [CrossRef]
- Palsson, J.; Selimovic, A.; Sjunnesson, L. Combined solid oxide fuel cell and gas turbine systems for efficient power and heat generation. J. Power Sources 2000, 86, 442–448. [Google Scholar] [CrossRef]
- Song, T.W.; Sohn, J.L.; Kim, J.H.; Kim, T.S.; Ro, S.T.; Suzuki, K. Performance analysis of a tubular solid oxide fuel cell/micro gas turbine hybrid power system based on a quasi-two dimensional model. J. Power Sources 2005, 142, 30–42. [Google Scholar] [CrossRef]
- Yakabe, H.; Ogiwara, T.; Hishinuma, M.; Yasuda, I. 3D model calculation for planar SOFC. J. Power Sources 2001, 102, 144–154. [Google Scholar] [CrossRef]
- Roos, M.; Batawi, E.; Harnisch, U.; Hocker, T. Efficient simulation of fuel cell stacks with the volume averaging method. J. Power Sources 2003, 118, 86–95. [Google Scholar] [CrossRef]
- Lazzaretto, A.; Toffolo, A.; Zanon, F. Parameter setting for a tubular SOFC simulation model. J. Energ. Resour. Technol. 2004, 126, 40–46. [Google Scholar] [CrossRef]
- Costamagna, P.; Selimovic, A.; Del Borghi, M.; Agnew, G. Electrochemical model of the integrated planar solid oxide fuel cell. Chem. Eng. J. 2003, 102, 61–69. [Google Scholar] [CrossRef]
- Noren, D.A.; Hoffman, M.A. Clarifying the Butler-Volmer equation and related approximations for calculating activation losses in solid oxide fuel cell models. J. Power Sources 2005, 152, 175–181. [Google Scholar] [CrossRef]
- Calise, F.; Palombo, A.; Vanoli, L. Design and partial load exergy analysis of hybrid SOFC-GT power plant. J. Power Sources 2006, 158, 225–244. [Google Scholar] [CrossRef]
- Freeh, J.E.; Pratt, J.W.; Brouwer, J. Development of A Solid-oxide Fuel Cell/gas Turbine Hybrid System Model for Aerospace Applications. In Proceedings of the ASME Turbo Expo 2004, Vienna, Austria, 14–17 June 2004.
- Kuchonthara, P.; Bhattacharya, S.; Tsutsumi, A. Energy recuperation in solid oxide fuel cell (SOFC) and gas turbine (GT) combined system. J. Power Sources 2003, 117, 7–13. [Google Scholar] [CrossRef]
- Hoogers, G. Fuel Cell Technology Handbook; CRC Press: New York, NY, USA, 2003. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Suther, T.; Fung, A.; Koksal, M.; Zabihian, F. Macro Level Modeling of a Tubular Solid Oxide Fuel Cell. Sustainability 2010, 2, 3549-3560. https://doi.org/10.3390/su2113549
Suther T, Fung A, Koksal M, Zabihian F. Macro Level Modeling of a Tubular Solid Oxide Fuel Cell. Sustainability. 2010; 2(11):3549-3560. https://doi.org/10.3390/su2113549
Chicago/Turabian StyleSuther, Torgeir, Alan Fung, Murat Koksal, and Farshid Zabihian. 2010. "Macro Level Modeling of a Tubular Solid Oxide Fuel Cell" Sustainability 2, no. 11: 3549-3560. https://doi.org/10.3390/su2113549




