Construction of an Environmentally Sustainable Development on a Modified Coastal Sand Mined and Landfill Site—Part 2. Re-Establishing the Natural Ecosystems on the Reconstructed Beach Dunes
Abstract
:1. Introduction
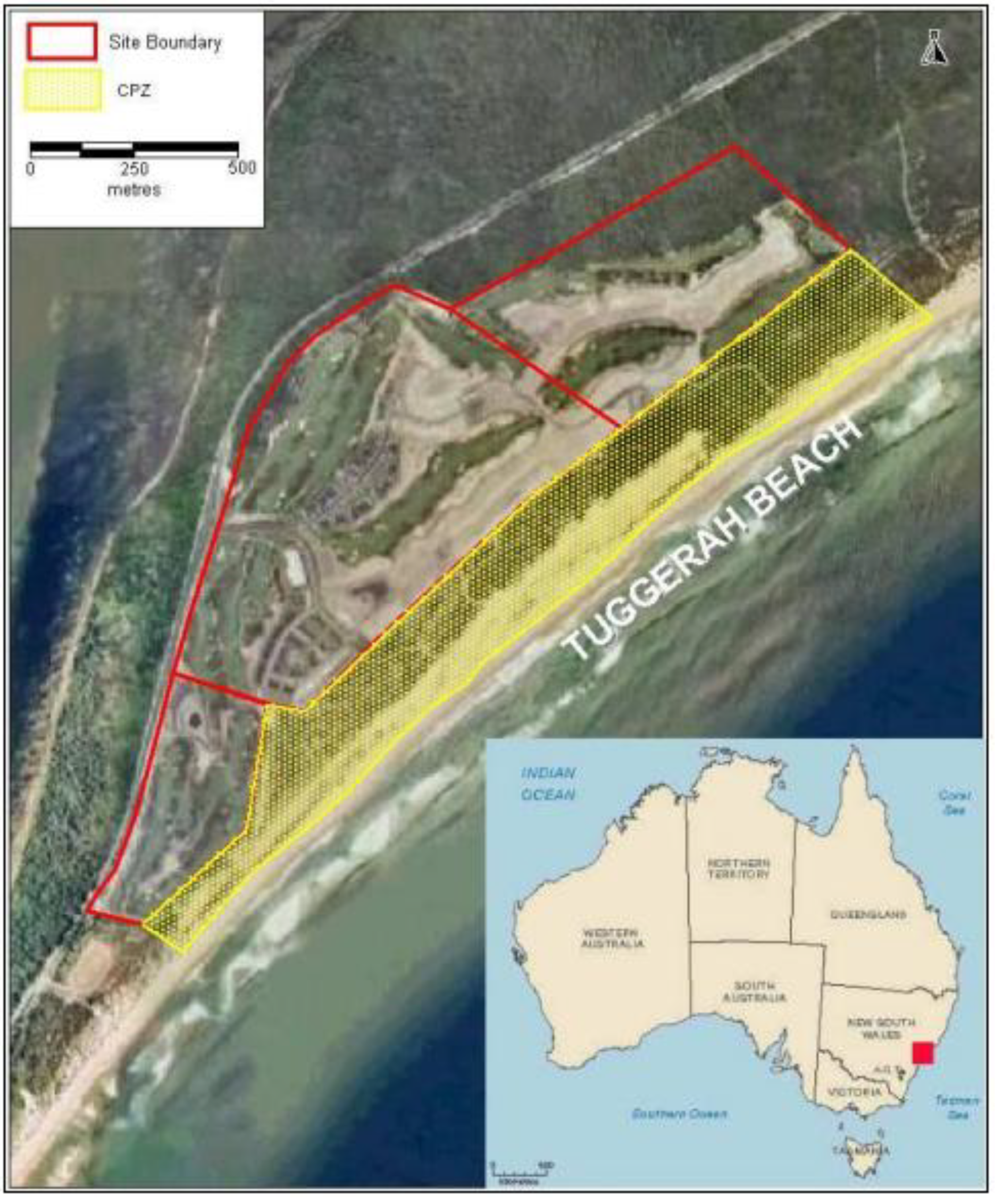
2. Vegetation and Sand Stabilityin 2004

3. Relationship between Vegetation, Mycorrhiza and Landform

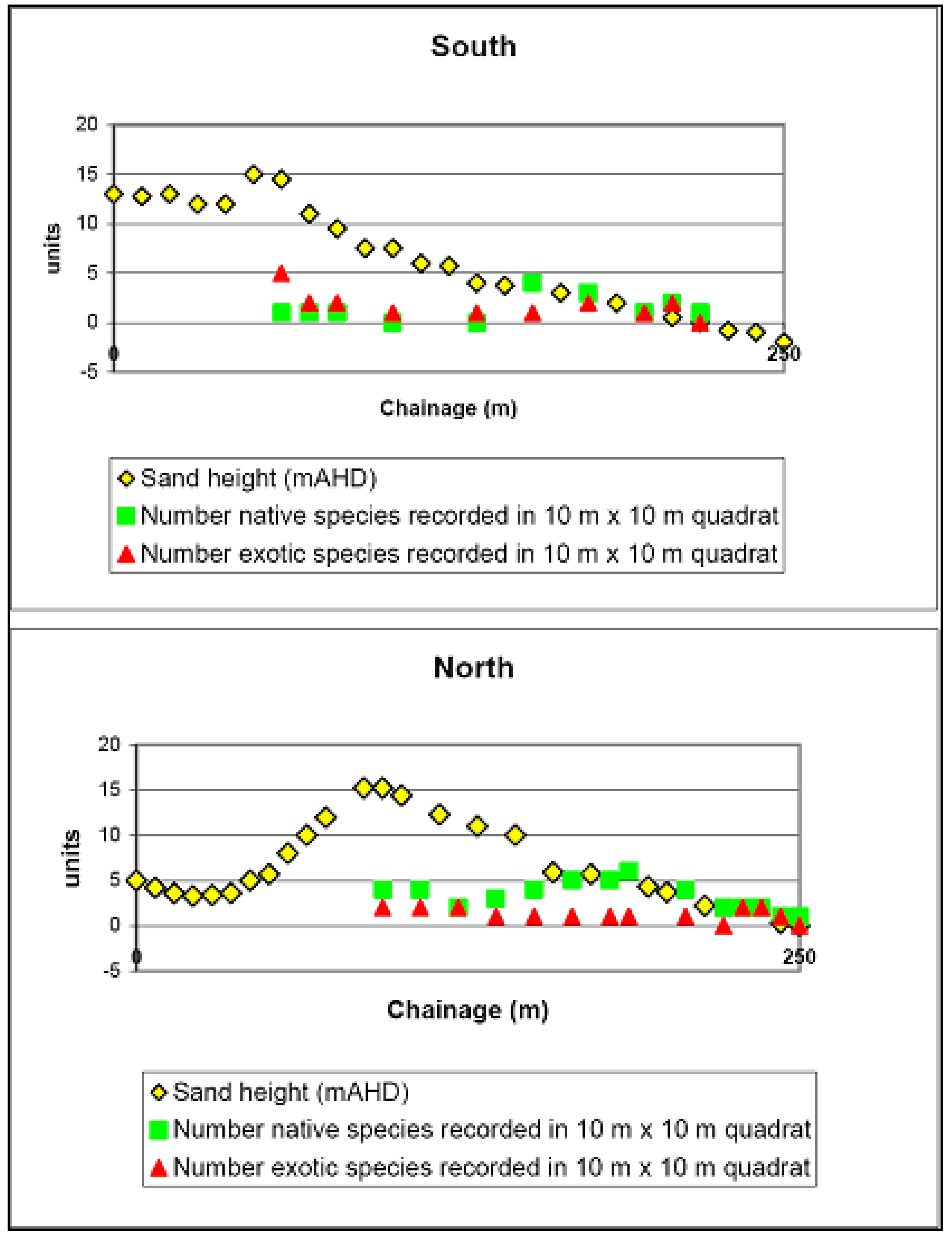

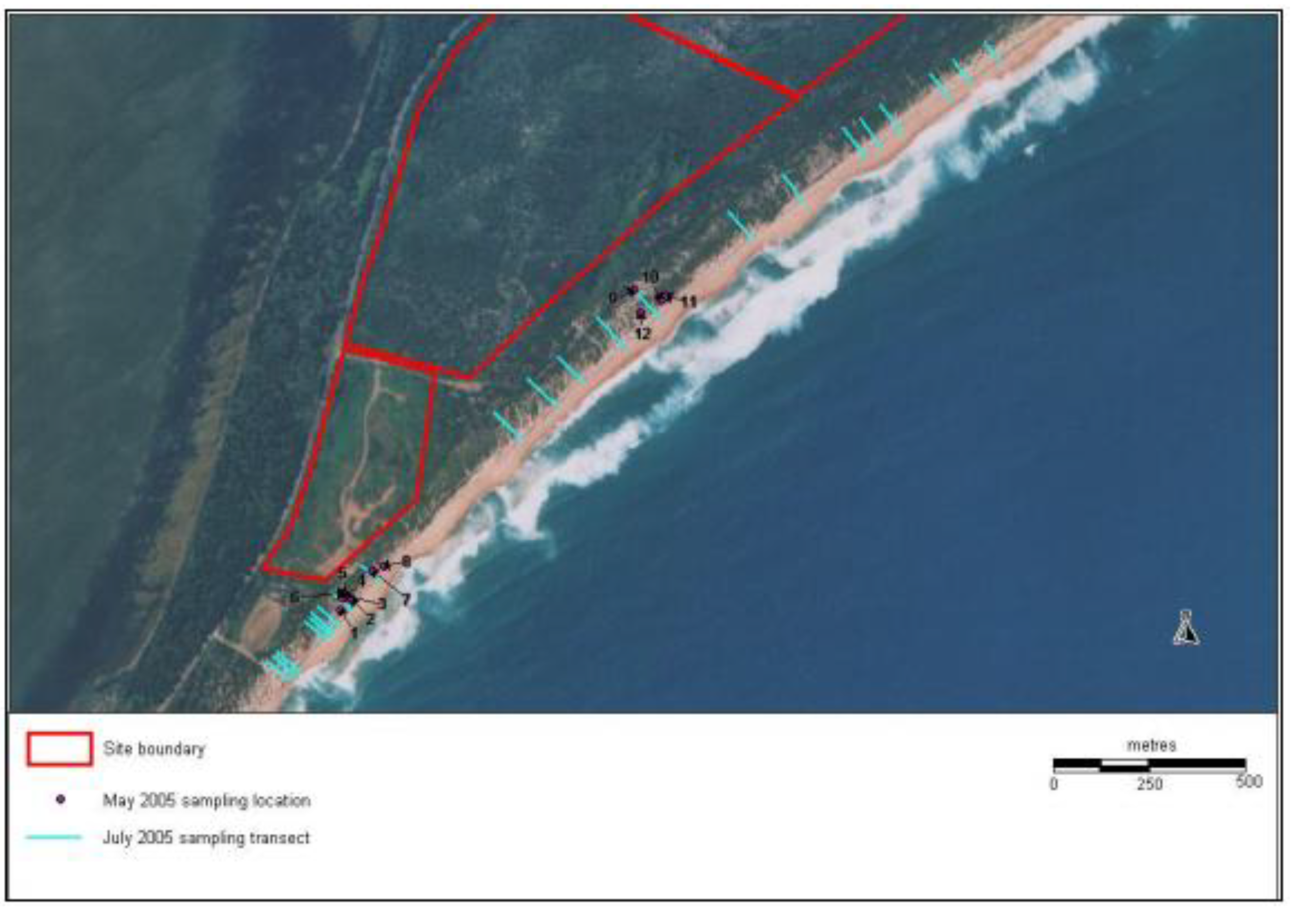
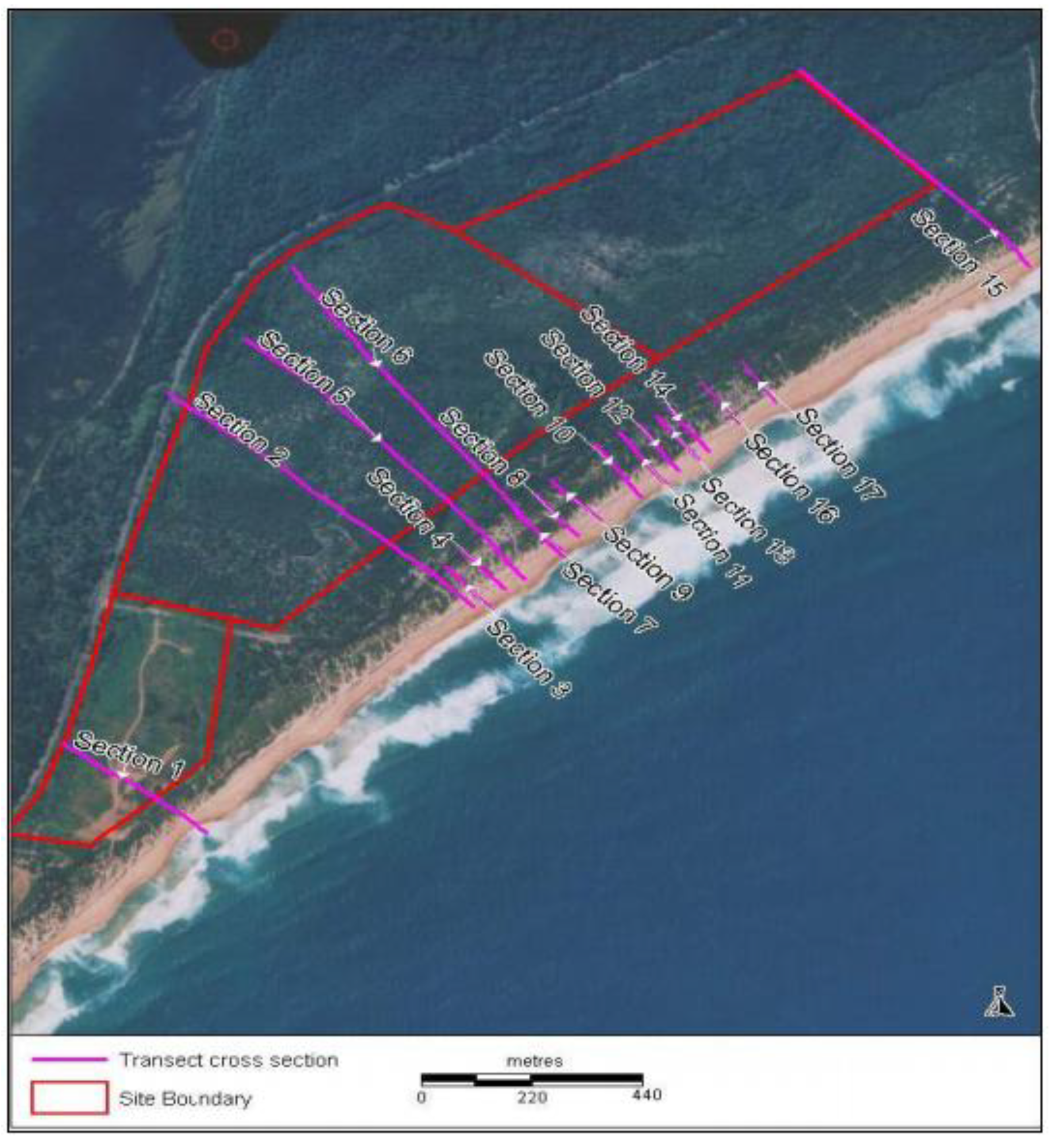
Reference Sites
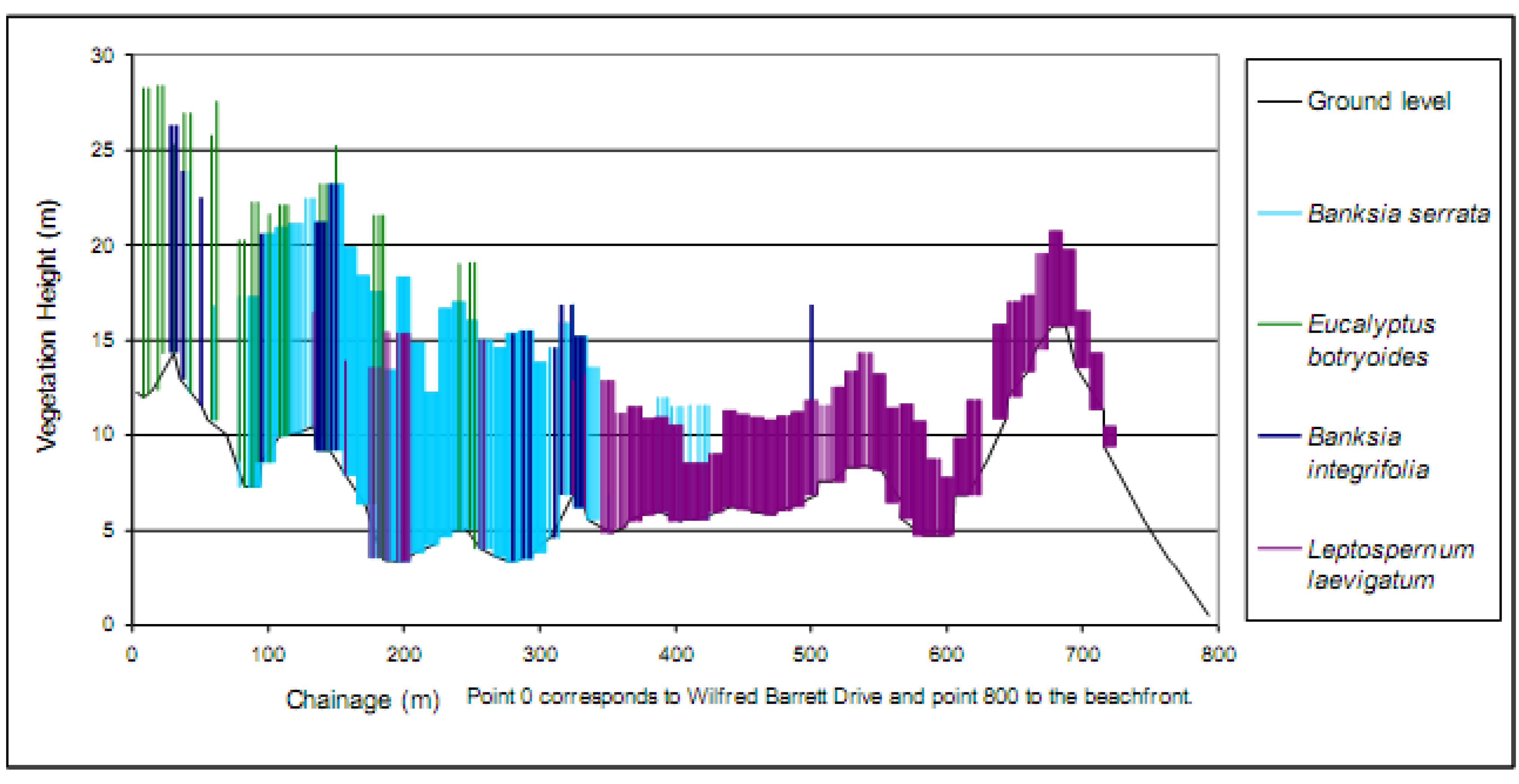

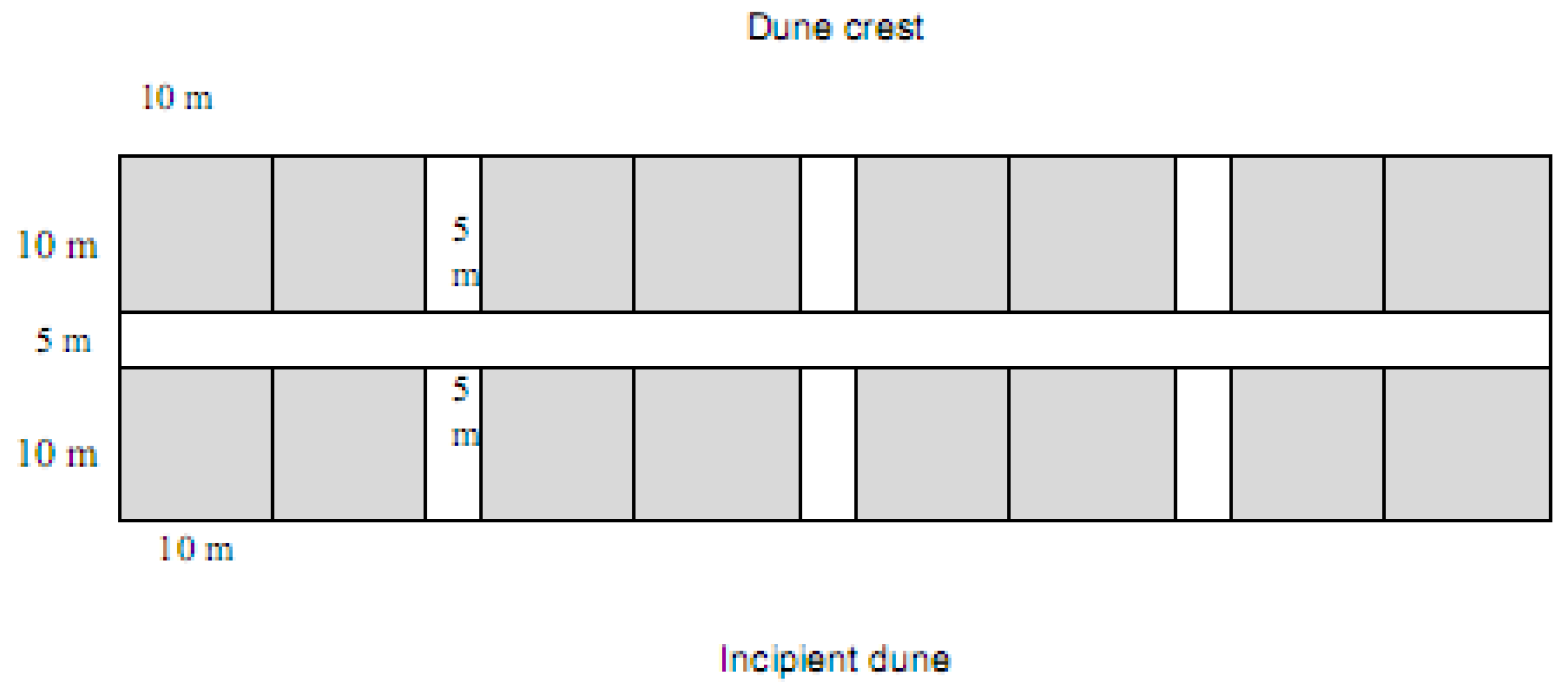
4. Rehabilitation Methods Employed at Magenta Shores
4.1. Landform Reconstruction

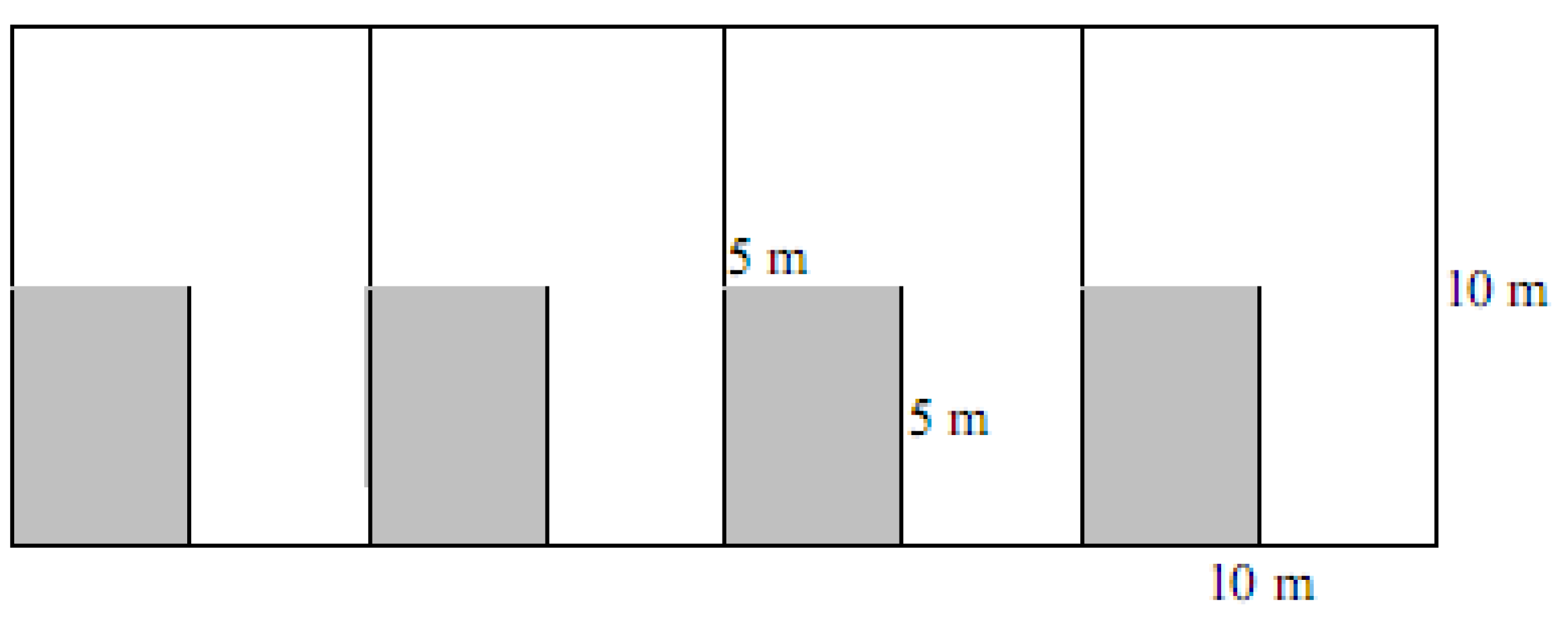
4.2. Spinifex Trials and Primary Planting

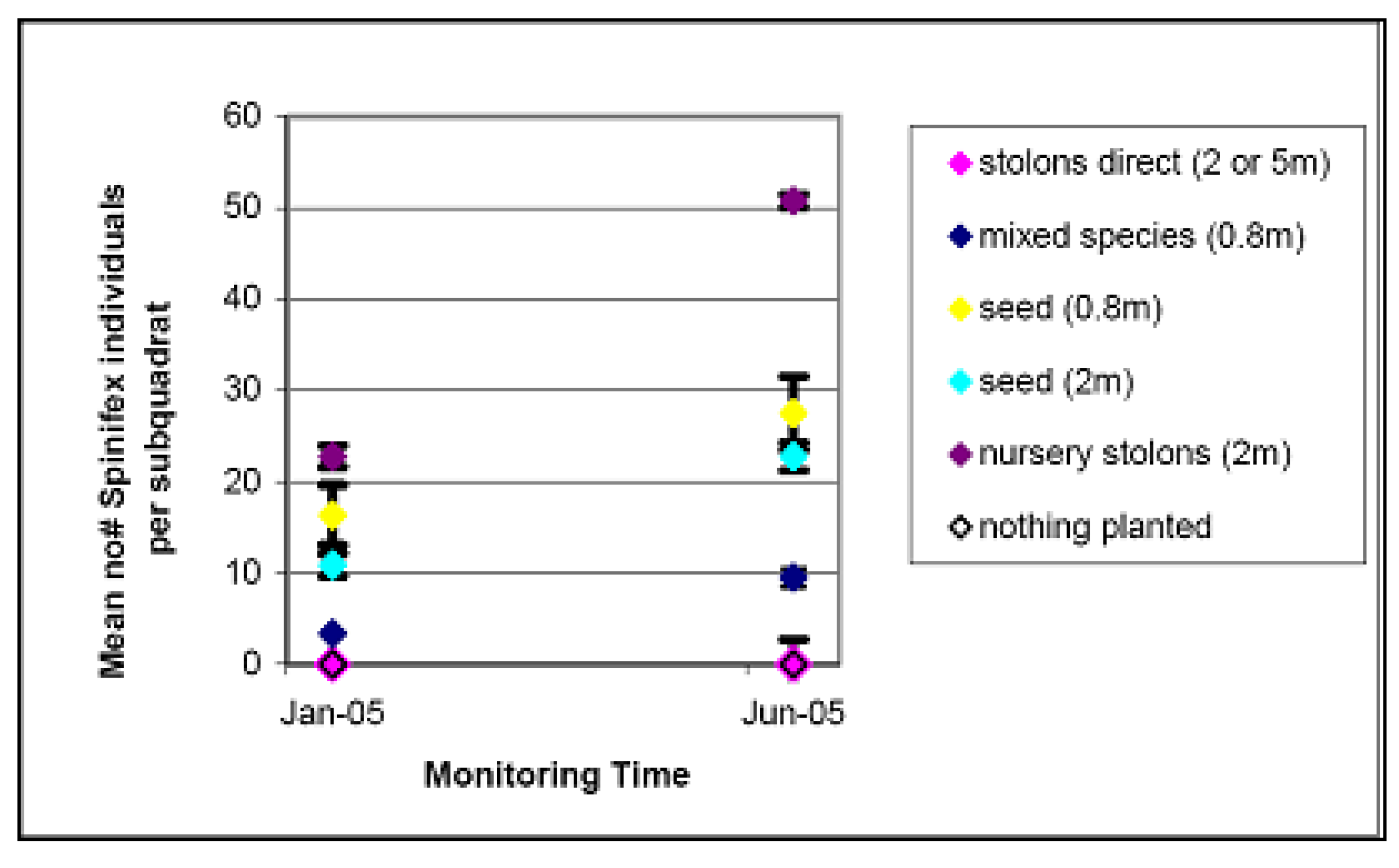
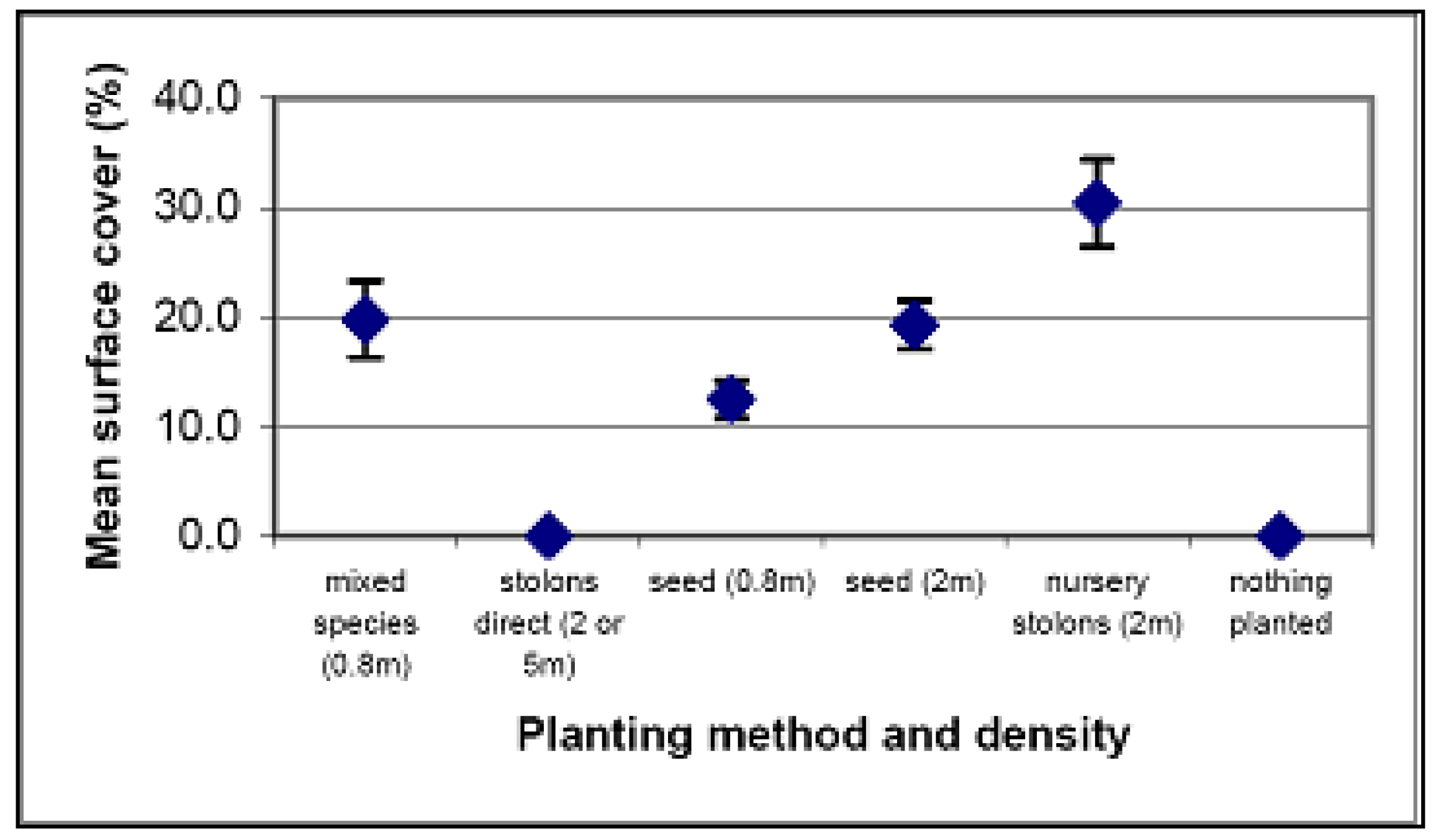


| Method | Cost per plant | Cost at 5 m density per 20 m × 20 m quadrat | Cost at 2 m density per 20 m × 20 m quadrat | Cost at 0.8 m density per 20 m × 20 m quadrat |
|---|---|---|---|---|
| Nursery grown stolons | $2.33 | NA—not trialled | $233 | NA—not trialled |
| Seed head | $1.60 | NA—not trialled | $160 | $1,000 |
| Direct planted stolons (including mixed planting) | $3.39 | $ 54.24 | $339 | $2,118.75 |
Relative cost of each method
4.3. Soil Fungi Testing
| Specimen No. | Plant Species | Hyphae present | Observations |
|---|---|---|---|
| 1 | Chrysanthemoides monilifera subsp. rotunda | Yes | Some hyphal penetration. Intracellular hyphae. |
| 2 | Scaevola calendulacea | Yes | Some penetration, no mycorrhizal structures. |
| 3 | Chrysanthemoides monilifera subsp. rotunda | Yes | Possible some penetration |
| 4 | Leucopogon parviflorus | Yes | Cell penetration, some hyphae coiling. |
| 5 | Leucopogon parviflorus | Yes | Cell penetration, some hyphae coiling, early ericoid mycorrhizal structures. |
| 6 | Chrysanthemoides monilifera subsp. rotunda | Yes | Possible some penetration. No mycorrhizal structures. |
| 7 | Spinifex sericeus | Yes | Possible some penetration. No mycorrhizal structures. |
| 8 | Spinifex sericeus | Yes | No penetration. |
| 9 | Unknown, near Leucopogon parviflorus | Yes | Root heavily colonised by hyphae. |
| 10 | Spinifex sericeus | Yes | Possible some penetration. No mycorrhizal structures. |
| 11 | Spinifex sericeus | Yes | Possible some penetration. No mycorrhizal structures. |
| 12 | Spinifex sericeus | Yes | Possible some penetration. No mycorrhizal structures. |
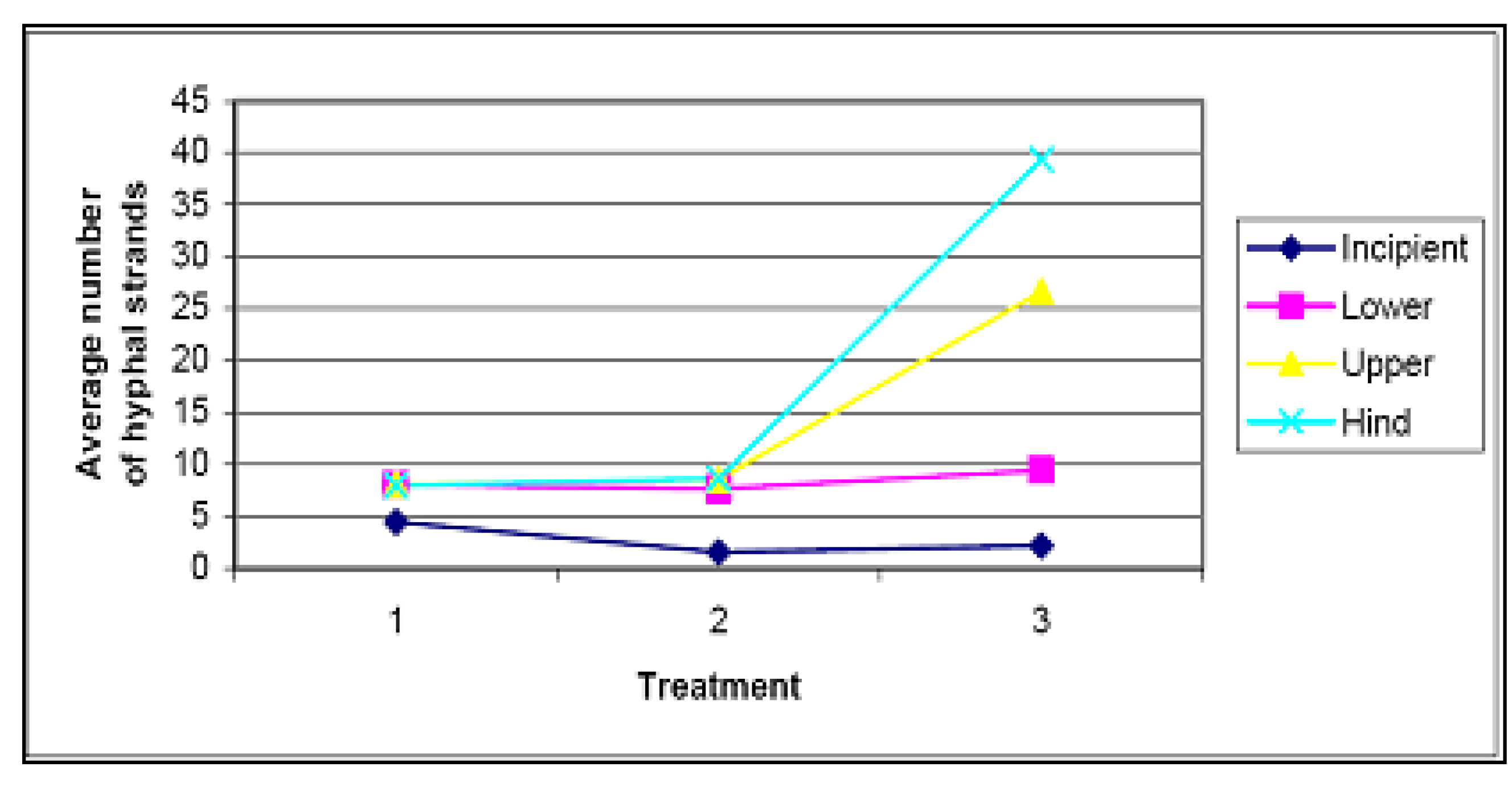
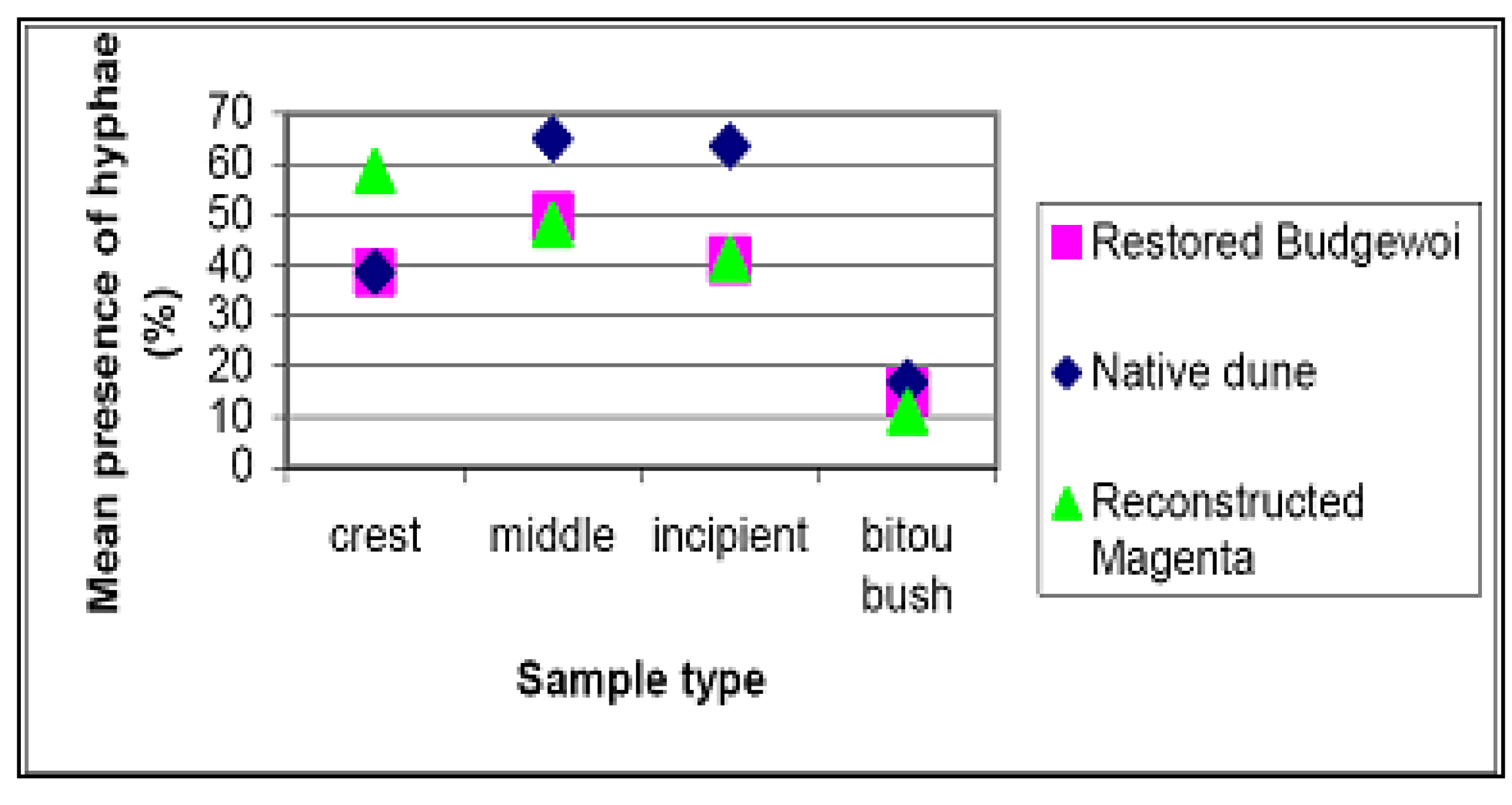
4.4. Weeding and Secondary Seeding and Planting

4.5. Fauna Habitat Values
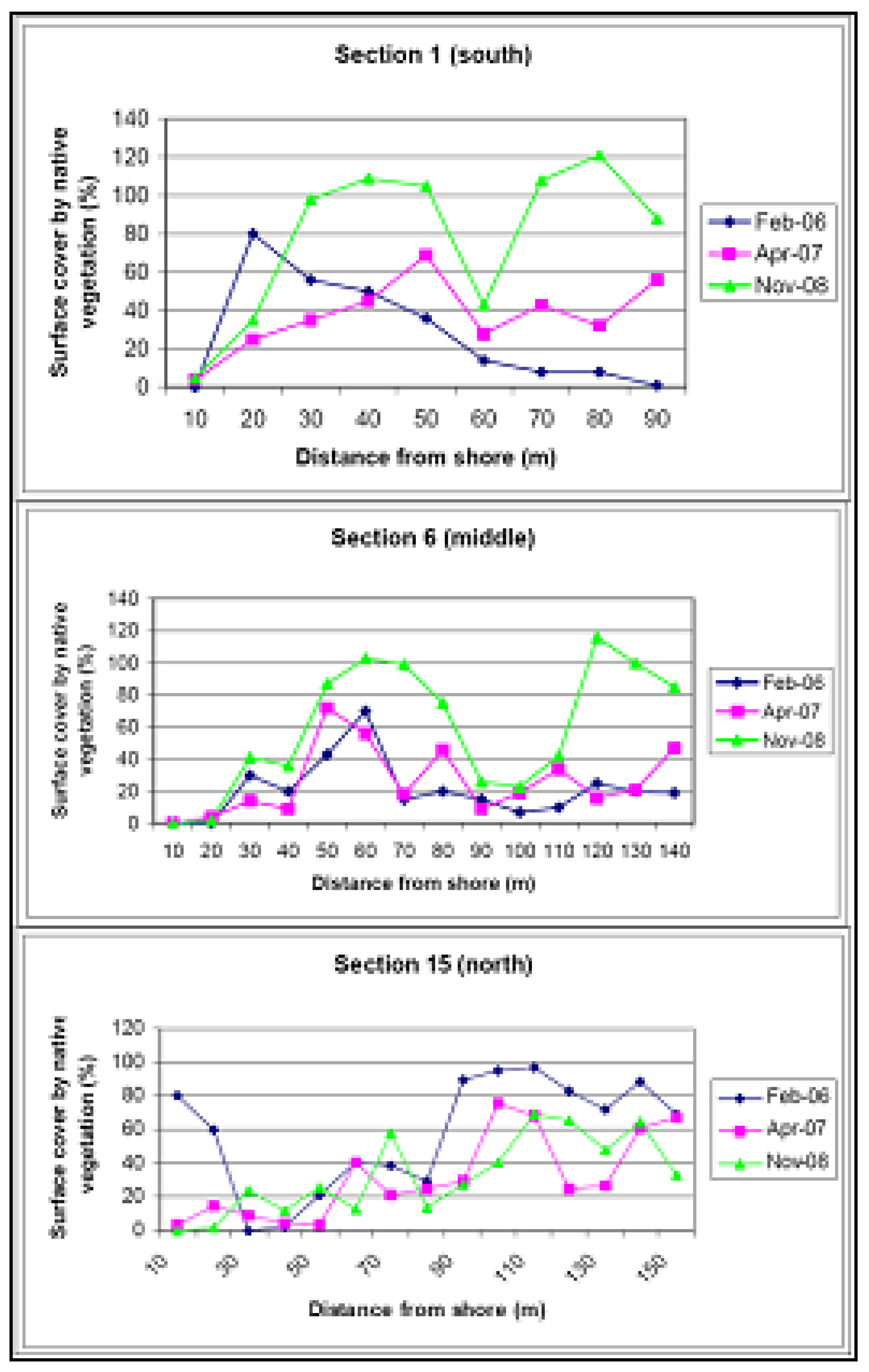
5. Establishment of a Diverse Local Native Sand Barrier Ecosystem
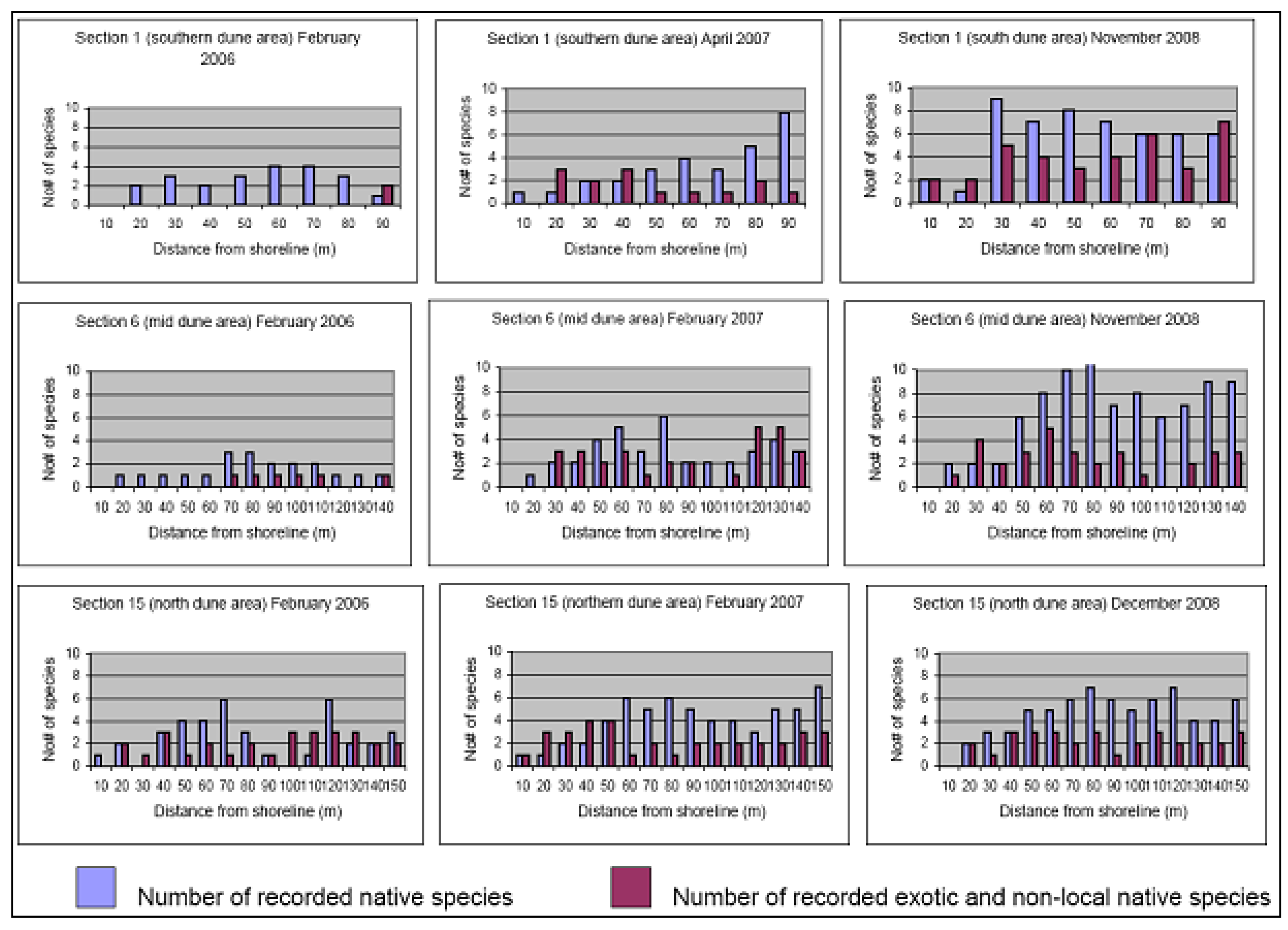


6. Conclusions and Recommendations




Acknowledgements
References
- Hazelton, P.; Clements, A. Construction of an environmentally sustainable development on a modified coastal sand mined and landfill site—Part 1. Planning and implementation. Sustainability 2009, 1, 319–334. [Google Scholar]
- McLachlan, A.; Brown, A.C. Ecology of Sandy Shores; Academic Press: Liverpool, UK, 2006. [Google Scholar]
- Barr, D.A. Restoration of coastal dunes after beach mining. J. Soil Conserv. Serv. New South Wales 1965, 21, 199–209. [Google Scholar] cited in Approved Threat Abatement Plan for Invasion of Native Plant Communities by Bitou Bush/Boneseed (Chrysanthemoides monilifera) 2006.
- Vranjic, J.A.; Woods, M.J.; Barnard, J. Soil-mediated effects on germination and seedling growth of coastal wattle (Acacia sophorae) by the environmental weed, bitou bush (Chrysanthemoides monilifera ssp. rotundata). Austral Ecol. 2000, 25, 445–453. [Google Scholar] [CrossRef]
- Weeds of National Significance: Bitou and Boneseed (Chrysanthemoides monilifera ssp. rotundata and monilifera) Strategic Plan; National Weeds Strategy Executive Committee: Launceston, Australia, 2000.
- Lindsay, E.A.; French, K. Litterfall and nitrogen cycling following invasion by Chrysanthemoides monilifera ssp. rotundata in coastal Australia. J. Appl. Ecol. 2005, 42, 556–566. [Google Scholar] [CrossRef]
- Coastal Dune Management, a Manual of Coastal Dune Management and Rehabilitation Technique; Coastal Unit, New South Wales Department of Land and Water Conservation: Newcastle, Australia, 2001.
- Hesp, P. The formation of sand “beach ridges” and foredunes. Search 1984, 15, 289–291. [Google Scholar]
- Maze, K.M.; Whalley, D.B. Germination, seedling occurrence and seedling survival of Spinifex sericeus R.Br (Poaceae). Aust. J. Ecol. 1992, 17, 189–194. [Google Scholar] [CrossRef]
- Geoscience Australia. In Geodesy—Australian Height Datum (AHD). Available online: http://www.ga.gov.au/geodesy/datums/ahd.jsp (accessed on 13 October 2009).
- Koske, R.E.; Polsen, W.R. Are VA mycorrhizae required for sand dune stabilization? Bioscience 1984, 34, 420–424. [Google Scholar] [CrossRef]
- Jehne, W.; Thompson, C.H. Endomycorrhizae in plant colonisation on coastal sand dunes at Cooloola, Queensland. Aust. J. Ecol. 1981, 6, 221–230. [Google Scholar] [CrossRef]
- Clough, K.K.; Sutton, J.C. Direct observation of fungal aggregates in sand dune soil. Can. J. Microbiol. 1978, 24, 333–335. [Google Scholar] [CrossRef] [PubMed]
- Kendrick, B. The Fifth Kingdom; Mycologue Publications: British Columbia, Canada, 2003. [Google Scholar]
- Logan, V.S.; Clarke, P.J.; Allaway, W.G. Mycorrhizas and root attributes of plants of coastal sand-dunes of New South Wales. Aust. J. Plant Physiol. 1989, 16, 141–146. [Google Scholar] [CrossRef]
- Perumal, J.V.; Maun, M.A. The role of mycorrhizal fungi in growth enhancement of dune plants following burial in sand. Funct. Ecol. 1999, 13, 560–566. [Google Scholar] [CrossRef]
- Landcare Notes. Boneseed and Bitou Bush; Department of Primary Industries, State of Victoria: Melbourne, Australia, 2008. Available online: http://www.dpi.vic.gov.au/DPI/nreninf.nsf/9e58661e880ba9e44a256c640023eb2e/41964f1cbae8c4a7ca25735a0020e6a3/$FILE/LC0181_Aug07.pdf (accessed on 25 February 2010).
- Climate Statistics for Australian Locations NORAH HEAD AWS, Site Number 61366; Bureau of Meteorology, Commonwealth of Australia: Melbourne, Australia, 2008. Available online: http://www.bom.gov.au/climate/averages (accessed on 19 May 2008).
- Harty, R.L.; McDonald, T.J. Germination behaviour in beach Spinifex (Spinifex hirsutus labill.). Aust. J. Bot. 1972, 20, 241–251. [Google Scholar] [CrossRef]
- Kurtböke, D.I.; Neller, R.J.; Bellgard, S.E. Mesophilic actinomycetes in the natural and reconstructed sand dune vegetation zones of Fraser island, Australia. Microb. Ecol. 2007, 54, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Brundrett, M.C. Mycorrhizal associations of australian plants. Mycorrhizal Associations: The Web Resource, Version 2.0; 2008. Available online: http://mycorrhizas.info/ozplants.html (accessed on 2 December 2009).
- Steinke, E.; Williams, P.G.; Ashford, A.E. The structure and fungal associates of mycorrhizas in Leucopogon parviflorus (Andr.) Lindl. Ann. Bot. 1996, 77, 413–420. [Google Scholar] [CrossRef]
- Pattinson, G.S.; Hammill, K.A.; Sutton, B.G; McGee, P.A. Growth and survival of seedlings of native plants in an impoverished and highly disturbed soil following inoculation with arbuscular mycorrhizal fungi. Mycorrhiza 2004, 14, 339–346. [Google Scholar] [CrossRef]
- Brockwell, J.; Searle, S.D.; Jeavons, A.C.; Waayers, M. Nitrogen fixation in Acacias: an untapped resource for sustainable plantations, farm forestry and land reclamation. Australian Centre for International Agricultural Research 2005. Available online: http://bingo.clarus.com.au/public/static/MN115_web.pdf (accessed on 2 December 2009).
- Duponnois, R.; Founoune, H.; Lesueur, D.; Thioulouse, J.; Neyra, M. Ectomycorrhization of six acacia auriculiformis provenances from Australia, Papua New Guinea and Senegal in glasshouse conditions: effect on the plant growth and on the multiplication of plant parasitic nematodes. Aust. J. Exp. Agric. 2000, 40, 443–450. [Google Scholar] [CrossRef]
- Agely, A.A.; Sylvia, D.M. Compatible host/mycorrhizal fungus combinations for micropropagated sea oats: II. Field evaluation. Mycorrhiza 2008, 18, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Duponnois, R.; Founoune, H.; Lesueur, D.; Thioulouse, J.; Neyra, M. Ectomycorrhization of six acacia auriculiformis provenances from Australia, Papua New Guinea and Senegal in glasshouse conditions: effect on the plant growth and on the multiplication of plant parasitic nematodes. Aust. J. Exp. Agric. 2000, 40, 443–450. [Google Scholar] [CrossRef]
- Agely, A.A.; Sylvia, D.M. Compatible host/mycorrhizal fungus combinations for micropropagated sea oats: II. Field evaluation. Mycorrhiza 2008, 18, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Ralph, M. Growing Australian Native Plants from Seed for Revegetation, Tree Planting and Direct Seeding; Bushland Horticulture: Fitzroy, Australia, 1997. [Google Scholar]
- Smith, E. Line in the sand. Central Coast Express Advocate. 10 June 2009. Available online: http://digitaledition.expressadvocate.com.au/?iid=26123 (accessed on 13 October 2009).
- Noone, R. Huge sand movement threatens beach homes. Central Coast Express Advocate, 28 May 2008. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Clements, A.; Simmonds, A.; Hazelton, P.; Inwood, C.; Woolcock, C.; Markovina, A.-L.; O’Sullivan, P. Construction of an Environmentally Sustainable Development on a Modified Coastal Sand Mined and Landfill Site—Part 2. Re-Establishing the Natural Ecosystems on the Reconstructed Beach Dunes. Sustainability 2010, 2, 717-741. https://doi.org/10.3390/su2030717
Clements A, Simmonds A, Hazelton P, Inwood C, Woolcock C, Markovina A-L, O’Sullivan P. Construction of an Environmentally Sustainable Development on a Modified Coastal Sand Mined and Landfill Site—Part 2. Re-Establishing the Natural Ecosystems on the Reconstructed Beach Dunes. Sustainability. 2010; 2(3):717-741. https://doi.org/10.3390/su2030717
Chicago/Turabian StyleClements, AnneMarie, Appollonia Simmonds, Pamela Hazelton, Catherine Inwood, Christy Woolcock, Anne-Laure Markovina, and Pamela O’Sullivan. 2010. "Construction of an Environmentally Sustainable Development on a Modified Coastal Sand Mined and Landfill Site—Part 2. Re-Establishing the Natural Ecosystems on the Reconstructed Beach Dunes" Sustainability 2, no. 3: 717-741. https://doi.org/10.3390/su2030717





