On the Applicability of the Green Chemistry Principles to Sustainability of Organic Matter on Asteroids
Abstract
:1. Introduction
2. Aqueous Reactions of Water-Insoluble Organic Materials
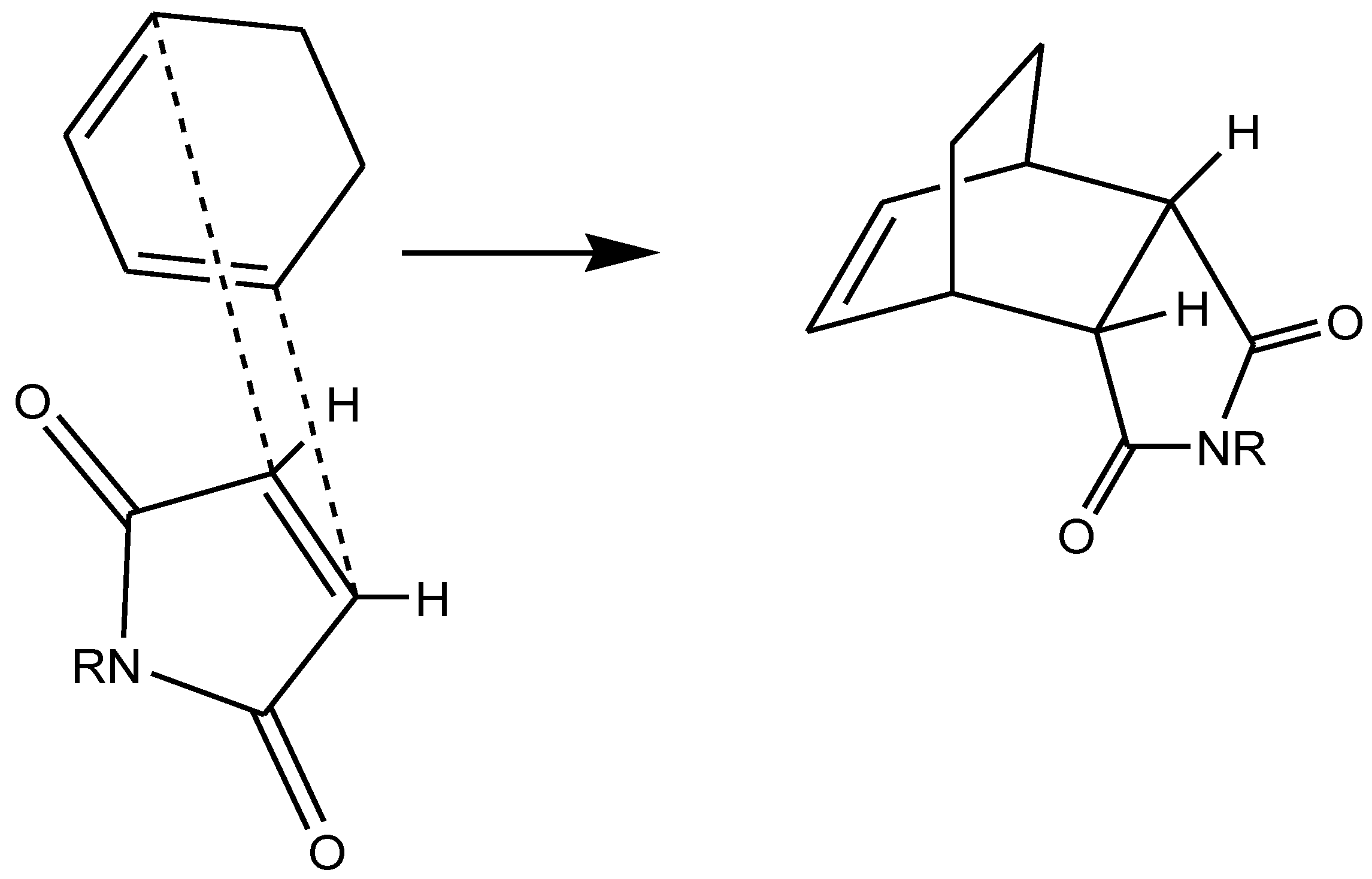

3. Solventless and Solid-State Organic Reactions
4. Conclusions
Acknowledgements
References
- Des Marais, D.J.; Nuth, J.A., III; Alamandola, L.J.; Boss, A.P.; Farmer, J.D.; Hoehler, T.M.; Jakosky, B.M.; Meadows, V.C.; Pohorille, A.; Runnegar, B.; Spormann, A.M. The NASA astrobiology roadmap. Astrobiology 2008, 8, 715–730. [Google Scholar]
- Kerridge, J.F. Formation and processing of organics in the early solar system. Space Sci. Rev. 1999, 90, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.M. Meteorite and Comet Chemistry. In Astrochemistry: From Astronomy to Astrobiology; Wiley: New York, NY, USA, 2006; Chapter 6; pp. 157–192. [Google Scholar]
- Cronin, J.R. Clues from the Origin of the Solar System: Meteorites. In The Molecular Origins of Life; Brack, A., Ed.; Cambridge University Press: Cambridge, UK, 1998; pp. 119–146. [Google Scholar]
- Schmitt-Kopplin, P.; Gabelica, Z.; Gougeon, R.D.; Fekete, A.; Kanawati, B.; Harir, M.; Gebefuegi, I.; Eckel, G.; Hertkorn, N. High molecular diversity of extraterrestrial organic matter in Murchison meteorite revealed 40 years after its fall. Proc. Nat. Acad. Sci. USA 2010, 107, 2763–2768. [Google Scholar] [CrossRef] [PubMed]
- Sephton, M.A. Organic compounds in carbonaceous meteorites. Nat. Prod. Rep. 2002, 19, 292–311. [Google Scholar] [CrossRef] [PubMed]
- Sephton, M.; Gilmour, I. Macromolecular Organic Materials in Carbonaceous Chondrites: A Review of Their Sources and Their Role in the Origin of Life on the Early Earth. In Impacts and the Early Earth; Gilmour, I., Keoberl, C., Eds.; Springer-Verlag: Berlin, Germany, 2000; pp. 27–49. [Google Scholar]
- Cronin, J. R.; Chang, S. Organic Matter in Meteorites: Molecular and Isotopic Analysis of the Murchison Meteorite. In The Chemistry of Life’s Origins; Greenberg, J.M., Mendoza-Gomez, C.X., Pirronello, V., Eds.; Kluwer Academic Publishers: Dodrecht, The Netherlands, 1993; pp. 209–258. [Google Scholar]
- Cronin, J.R.; Cooper, G.W.; Pizzarello, S. Characteristics and formation of amino acids and hydroxyl acids of the Murchison meteorite. Adv. Space Res. 1995, 15, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Cooper, G.; Kimmich, N.; Belisle, W.; Sarinana, J.; Brabham, K.; Garrel, L. Carbonaceous meteorites as a source of sugar-related organic compounds for the early Earth. Nature 2001, 414, 879–883. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.M. Prebiotic Chemistry. In Astrochemistry: From Astronomy to Astrobiology; Wiley: New York, NY, USA, 2006; Chapter 8; pp. 225–255. [Google Scholar]
- Lurquin, P.F. The Origins of Life and the Universe; Columbia University Press: New York, NY, USA, 2003. [Google Scholar]
- Fry, I. The Emergence of Life on Earth; Rutgers University Press: New Brunswick, NJ, USA, 2000. [Google Scholar]
- Luisi, P.L. The Emergence of Life; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Doxsee, K.M.; Hutchinson, J.E. Green Organic Chemistry, Strategies, Tools, and Laboratory Experiments; Thomson Brooks/Cole: Toronto, Canada, 2004. [Google Scholar]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Lancaster, M. Green Chemistry, an Introductory Text; Royal Society of Chemistry: Cambridge, UK, 2002. [Google Scholar]
- Lankey, R.L.; Anastas, P.T. Advancing Sustainability through Green Chemistry and Engineering; ACS Symposium Series 823; American Chemical Society: Washington, DC, USA, 2002. [Google Scholar]
- Laue, T.; Plagens, A. Named Organic Reactions, 2nd ed.; Wiley: Chichester, UK, 2005; pp. 88–95. [Google Scholar]
- Breslow, R. Hydrophobic effects on simple organic reactions in water. Acc. Chem. Res. 1991, 24, 159–164. [Google Scholar] [CrossRef]
- Blokzijl, W.; Blandamer, M.J.; Engberts, J.B.F.N. Diels-Alder reactions in aqueous solutions. Enforced hydrophobic interactions between Diene and Dienophile. J. Am. Chem. Soc. 1991, 113, 4241–4246. [Google Scholar] [CrossRef]
- Meijer, A.; Orro, S.; Engberts, J.B.F.N. Effect of the hydrophobicity of the reactants on Diels-Alder reactions in water. J. Org. Chem. 1998, 63, 8989–8994. [Google Scholar] [CrossRef]
- Voet, D.; Voet, J.G.; Pratt, C.W. Fundamentals of Biochemistry: Life at the Molecular Level; Wiley: Hoboken, NJ, USA, 2006; pp. 27–28. [Google Scholar]
- Wang, B.; Sutherland, I.O. Self-replication in a Diels-Alder reaction. Chem. Commun. 1997, 1495–1496. [Google Scholar] [CrossRef]
- Kindermann, M.; Stahl, I.; Reinhold, M.; Pankau, W.M.; von Kiedrowski, G. Systems Chemistry: Kinetic and Computational Analysis of a Nearly Exponential Organic Replicator. Angew. Chem. Int. Ed. Engl. 2005, 44, 6750–6755. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Toda, F. Solvent-free organic syntheses. Chem. Rev. 2000, 100, 1025–1074. [Google Scholar] [CrossRef] [PubMed]
- Toda, F. Solid state organic chemistry: Efficient reactions, remarkable yields, and stereoselectivity. Acc. Chem. Res. 1995, 28, 480–486. [Google Scholar] [CrossRef]
- Kaupp, G. Organic Solid-state Reactions with 100% Yield. In Topics in Current Chemistry; Springer Berlin/Heidelberg: Heidelberg, Germany, 2005; Volume 254, pp. 95–183. [Google Scholar]
- Cave, G.W.V.; Raston, C.L.; Scott, J.L. Recent advances in solventless organic reactions: Towards benign synthesis with remarkable versatility. Chem. Commun. 2001, 2159–2169. [Google Scholar] [CrossRef]
- Rothenberg, G.; Downie, A.P.; Raston, C.L.; Scott, J.L. Understanding solid/solid organic reactions. J. Am. Chem. Soc. 2001, 123, 8701–8708. [Google Scholar] [CrossRef] [PubMed]
- Toda, F.; Takumi, H.; Akehi, M. Efficient solid-state reactions of alcohols: Dehydration, rearrangement, and substitution. J. Chem. Soc. Chem. Commun. 1990, 1270–1271. [Google Scholar] [CrossRef]
- Tanaka, K. Solvent-Free Organic Synthesis, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2009. [Google Scholar]
- Doxsee, K.M.; Hutchinson, J.E. Green Organic Chemistry, Strategies, Tools, and Laboratory Experiments; Thomson, Brooks/Cole: Toronto, Canada, 2004; pp. 115–119. [Google Scholar]
- De Rosa, M.; Gambacorta, A.; Gliozzi, A. Structure, Biosynthesis, and Physicochemical Properties of Archaebacterial Lipids. Microbiol. Rev. 1986, 50, 70–80. [Google Scholar] [PubMed]
- Kates, M. Membrane Lipids of Archaea. In The Biochemistry of Archaea (Archaebacteria); Kates, M., Kushner, D.J., Matheson, A.T., Eds.; Elsevier: Amsterdam, The Netherlands, 1993; pp. 261–295. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kolb, V.M. On the Applicability of the Green Chemistry Principles to Sustainability of Organic Matter on Asteroids. Sustainability 2010, 2, 1624-1631. https://doi.org/10.3390/su2061624
Kolb VM. On the Applicability of the Green Chemistry Principles to Sustainability of Organic Matter on Asteroids. Sustainability. 2010; 2(6):1624-1631. https://doi.org/10.3390/su2061624
Chicago/Turabian StyleKolb, Vera M. 2010. "On the Applicability of the Green Chemistry Principles to Sustainability of Organic Matter on Asteroids" Sustainability 2, no. 6: 1624-1631. https://doi.org/10.3390/su2061624



