1. Introduction
The growing attention that the scientific community has paid in the last decades to the corrosion phenomena is related to the huge economic, social and environmental losses that result from the spread of damage on several metal constructions.
During the 1970s and 80s, several studies on the cost of corrosion were carried out. It was shown that the impact of corrosion on the domestic economy of various countries accounted for up to 3% of the GDP [
1]. When the damage is greatly enlarged, expensive maintenance and replacement are required for the safe and reliable use of constructions and, even if the serviceability of the structure is not compromised, the deterioration of appearance produces reduced value of constructions.
From the structural point of view, the loss of thickness of the cross section due to corrosion attack leads to a smaller resistant area, producing a decrease in the structural performance in terms of strength, stiffness and ductility. In some cases, the local failure of a component or joint could affect the stability of the whole structure. In addition, in case of cyclic loads, the corrosion phenomenon can produce a significant reduction in the fatigue strength, mainly in zones with high stress concentrations [
2].
In order to prevent failures due to corrosion, a durable design of metal structures is necessary, both for ultimate and serviceability limit states.
In this regard, the main European structural design codes [
3,
4] provide only general protective measures, such as the use of weathering or stainless steel, of surface protection systems and of structural redundancy. It should be also noted that no predictive models that are able to estimate the corrosion depth of structural material are provided, but only general recommendations concerning the minimum thickness to be used for structural components are reported.
Nevertheless, several models concerning the evaluation of the damage produced by corrosion are available in the literature, as are standards, even if they refer to different parameters and conditions.
The object of this study is to present a report on the predictive models relevant to atmospheric corrosion which are available in the literature in order to evaluate their suitability with respect to use in a durability design procedure based on a lifetime safety factor method [
5]. The procedure is composed of different phases: the analysis of environmental loads, selection of influencing parameters and the evaluation of thickness loss by means of corrosion models and related safety factors.
With this aim, corrosion rates and classification methods of environmental corrosivity considered in standards are reported in the following. Moreover, several models are shown that take into account the different factors affecting the evolution of damage, such as the presence of chlorides, sulfates and pollution in the atmosphere.
Finally, a comparison among the considered dose response curves corresponding to selected corrosion classes and metals is presented and the main differences are described with respect to degradation rates reported by ISO standards.
2. Corrosion of Metals and Alloys
The corrosion phenomena of metals and alloys involve mainly two elements: the material and its environment. In particular, corrosion is defined as the deterioration of a material, usually a metal, that results from a reaction with its environment [
6], causing the degradation of both.
Corrosion attacks can manifest in different forms, and several classification system are provided by the international scientific literature.
A first classification is made on the basis of the reaction that takes place on the metal surface. In this case, it is possible to distinguish between chemical and electrochemical corrosion, the latter involving at least one cathodic-anodic reaction.
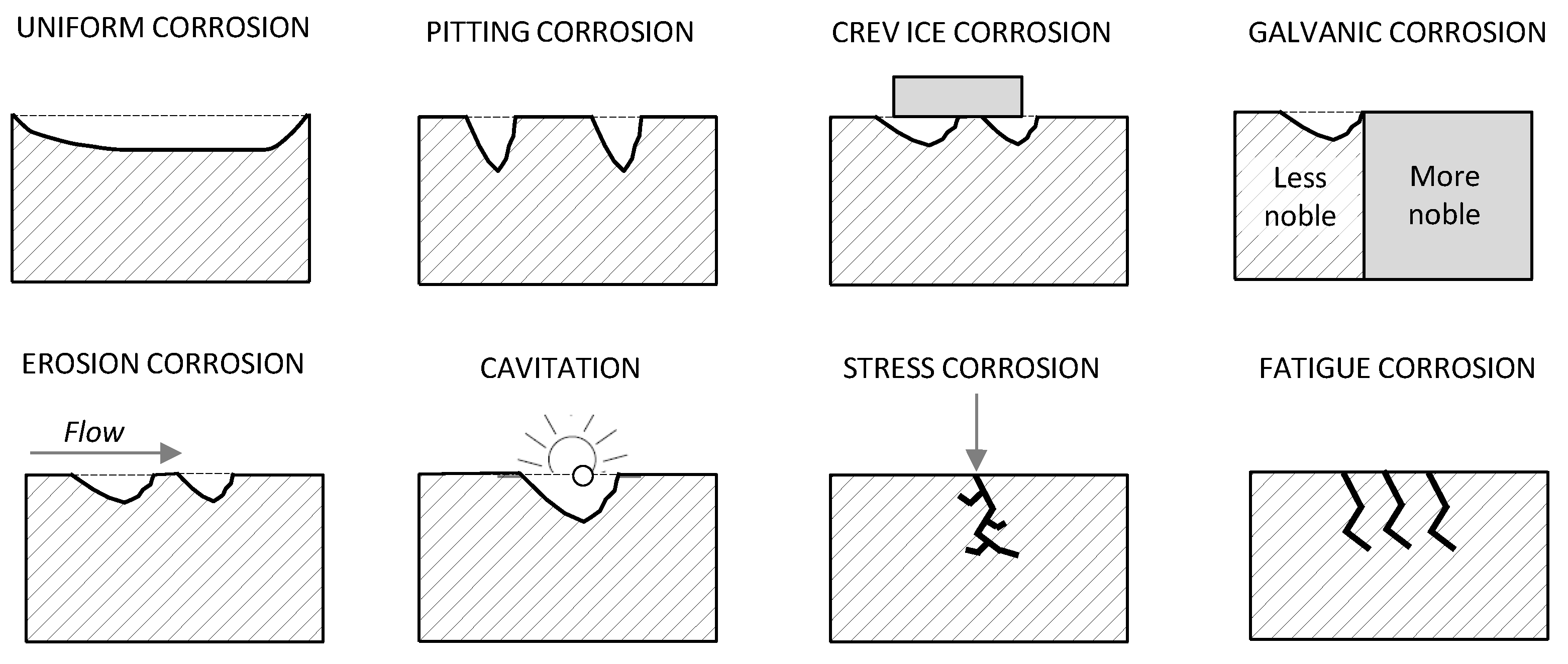
Another traditional classification system [
6,
7,
8] characterizes corrosion phenomena according to their appearance and the basic forms of corrosion, which could be uniform or localized (
Figure 1), are defined as follows:
General corrosion: corrosion that is diffused more or less homogeneously over the surface of a material;
Uniform corrosion: a kind of general corrosion that proceeds at the same rate on the metal surface;
Pitting corrosion: localized corrosion that is restricted to a small area, taking the form of pits;
Crevice corrosion: localized corrosion of a metal surface at, or close, to an area that is protected by another material;
Erosion corrosion: a conjoint action involving a corrosive flowing which leads to accelerated loss of material;
Cavitation corrosion: development and rapid collapse of cavities or bubbles;
Galvanic corrosion: corrosion of a metal due to contact with a more noble metal in a corrosive electrolyte;
Fatigue corrosion: cracking of metal caused by repeated stresses in a corrosive environment;
Stress corrosion: brittle cracking caused by the parallel occurrence of an applied stress and a specific environment.
Finally, on the basis of the corrosive environment, degradation mechanisms are categorized in microbial and bacterial corrosion, gaseous corrosion, marine corrosion, underground corrosion and atmospheric corrosion. The first one is usually associated with the presence of micro-organisms and/or bacteria on the metal surface that occurs in fresh- and seawater as well as in soils. Gaseous corrosion occurs when a dry gas is the main corrosive agent and there is not a liquid layer on the surface. Marine corrosion refers to seawater acting in an immersion and/or a splash zone, while underground corrosion is related to corrosion in soils.
Figure 1.
The main forms of corrosion (modified after Roberdge [
9]).
Figure 1.
The main forms of corrosion (modified after Roberdge [
9]).
This paper focuses on the atmospheric corrosion of metal structures in outdoor atmospheres, which represents one of the most severe forms of corrosion, resulting in huge economic and structural performance losses.
The atmospheric corrosion phenomena comprise three different forms of corrosion: dry, damp and wet corrosion. Damp and wet corrosion occur in indoor and outdoor atmospheres, as a consequence of wet and dry cycles induced by rainfall and condensation, which produce thin water films at the metal surface. In damp corrosion, a thin film of electrolyte is formed on the metal surface by adsorption of water molecules when a critical humidity level is reached, while wet films are associated flowing with water [
9].
Atmospheric corrosion is mainly an electrochemical process that occurs in the presence of thin film electrolytes formed on the metal surface. The attack proceeds by balancing anodic oxidation reaction, which involves the dissolution of the metal in the electrolytic film, and cathodic reactions, involving the oxygen reduction reaction. The anodic process controls the overall rate of atmospheric corrosion and it is represented by Equation (1):
The electrolyte films tend to form only when a certain critical humidity level is reached. For example, in the case of iron, a threshold value for humidity of about 60% in a pure atmosphere can be defined. Corrosion rate can be neglected for lower values of relative humidity (RH) while for higher ones it rapidly increases.
The overall rate of the metal dissolution process is strongly influenced by the formation of corrosion products, their solubility in the water film and the formation of passive coatings as well as by the corrosiveness of the environment. These phenomena processes are influenced by several factors, which can be divided into two different classes: the
endogenous factors, which are related to the metal itself (the effective electrode potential of a metal in a solution, the composition of the metal, the chemical and physical homogeneity of the surface,
etc.), and the
exogenous factors that are connected to the atmospheric composition [
2].
Among these factors, important practical environmental variables are the time of wetness (TOW) and the concentration of contaminants, such as sulfur dioxide and chlorides.
The TOW represents the average period of time during which the electrolyte is on the corroding surface [
9], for practical purpose it could also be defined as the number of hours during which the relative humidity is greater than 80% and the average temperature T > 0 °C [
10]. The negative effect of the humidity is exacerbated by the concentration of the atmospheric pollutants, mainly sulfurs and chlorides.
Chlorides in the atmosphere can definitely increase the corrosion rate, such as in the case of marine environments, where the critical humidity level is about 30–40% [
11]. The main effect of chlorides is to prevent formation of protective oxide films on the metal surface, thus increasing the overall corrosion rate.
As far as sulfurs are concerned, when the relative humidity is above 60–70%, even small concentrations dissolving in the thin electrolyte layer stimulate both the anodic and the cathodic reactions [
9]. According to Vernon [
12], when the RH is above 80%, a concentration of 0.01% of sulfur dioxide in the atmosphere leads to a sharp increase in the corrosion rate if compared to the exposure in pure air.
3. Standards and Codes
With respect to corrosion phenomena, the European structural design codes [
3,
4,
13] provide only general recommendations and basic principles that are mainly concerned with the use of coating protective systems, the choice of corrosion resistant materials and structural redundancy. Such specifications aim to prevent the initiation and propagation of the corrosion attacks by means of design detailing, surface protective systems [
14] and proper inspection and maintenance programs, but no references are reported to models able to predict the damage effects during service life.
The EN 1993–1–1 [
4] states few common principles, such as the opportunity of providing corrosion protection measures by means of surface protection systems, improving the use of weathering and stainless steel and by structural redundancy. It should be noted that in this case, no references are made to models able to estimate the corrosion depth.
Nevertheless, in the new Italian structural code [
15], an important innovation has been introduced concerning corrosion, which is expressly included among the different loads acting on constructions. Corrosion is classified as a type of entropic load, which comprises deteriorating actions, caused by natural degradation mechanism of materials and environmental loads and affecting the structural integrity. With respect to durability, it is recommended to adopt a 2 mm extra thickness in an aggressive environment if maintenance and inspection cannot be performed for design life up to 100 years.
The European Standard EN 12500–2000 [
16], edited by the European Committee for Standardization (CEN), defines the procedure to be adopted for the classification, determination and estimation of corrosiveness of atmospheric environments by assessing the mass loss of standard samples, after one year exposure. The procedure is described in detail in the next section. Important references to the corrosion of metals and alloys are reported in the almost 40 standards compiled by the ISO Technical Committee TC 156. The TC working group 4 worked on categorization of the corrosivity of environment in terms of practical environmental variables, atmospheric corrosion testing and classification of corrosivity of atmosphere.
In particular, EN ISO 9223 [
10] provides the classification of the corrosivity of atmospheres on the basis of three key factors: the TOW (time of wetness), and the deposition rate of chlorides and sulfur dioxide concentration. It defines five corrosivity classes, ranging from C1 to C5.
EN ISO 9224 [
17] provides guiding values for the corrosivity categories of atmospheres. EN ISO 9225 [
18] is concerned with the methods of measurement of pollution data, while EN ISO 9226 [
19] gives the corrosivity rates of standard specimens for the evaluation of corrosivity of atmospheres. In EN ISO 14713 [
20], specific recommendations are provided for each corrosivity class with respect to different coating typologies. In particular, the life duration of zinc and aluminum coatings are related both to thickness loss and corrosiveness of environment.
Other references can be found in international standards, but a design procedure has still to be codified for predicting and preventing the potential damage that a specific environment could lead to for both coatings and structural materials during the entire service life.
4. Corrosivity of Atmospheric Environments
As far as the corrosivity of atmospheres is concerned, EN 12500 [
16] defines five outdoor environments on the basis of the presence of corrosive agents in the air, namely:
Rural atmosphere: countryside and small towns, minor corrosive agent contamination (carbon dioxide, chlorides, artificial fertilizers).
Urban atmosphere: densely populated areas, few industrial activities, medium corrosive agent contamination (sulfur dioxides);
Industrial atmosphere: intensive industrial activities, high corrosive agent contamination (sulfur dioxides);
Marine atmosphere: areas close to the sea, or internal zones strongly affected by airborne salinity. Corrosion effects are influenced by topographic conditions, prevailing wind direction.
Marine Industrial atmosphere: complex environment, areas close to both the sea and industrial districts, or internal zones located in the prevalent wind direction. Medium and/or high corrosive agent contamination (sulfur dioxides, chlorides).
The methodology for the quantitative evaluation of the corrosivity of a specific environment standard is provided in EN 12500 [
16] on the basis of guiding values of the mass loss of standard flat specimens (rectangular shape, 50 × 100 mm) of four materials (carbon steel, copper, zinc, aluminum), after one year of exposure. The corrosivity classification is defined according to
Table 1.
Table 1.
Mass loss (g/m2) for one year field test exposure in the five corrosivity classes C1–C5, the order being from the least to the most corrosive. (EN 12500/2000).
Table 1.
Mass loss (g/m2) for one year field test exposure in the five corrosivity classes C1–C5, the order being from the least to the most corrosive. (EN 12500/2000).
| MASS LOSS g/m2 | Corrosiveness category |
| Carbon Steel | Zinc | Copper | Aluminum |
| ≤10 | ≤0.7 | ≤ 0.9 | Negligible | Very low | C1 |
| 10–200 | 0.7–5 | 0.9–5 | ≤0.6 | Low | C2 |
| 200–400 | 5–15 | 5–12 | 0.6–2 | Medium | C3 |
| 400–650 | 15–30 | 12–25 | 2–5 | High | C4 |
| 650–1,500 | 30–60 | 25–50 | 5–10 | Very high | C5 |
Table 2.
Corrosivity qualitative classification. An extract of EN 12500.
Table 2.
Corrosivity qualitative classification. An extract of EN 12500.
| Corrosiveness category | Typical outdoor atmospheric environments |
|---|
| C1 | Very low | Dry or cold zones; very low pollutants contamination; time of wetness very low, e.g., desert, Antarctic zone. |
| C2 | Low | Temperate zone; low pollution (SO2 [µg/m3] < 12), e.g., rural areas and small towns.
Dry or cold zones; short damp periods, e.g., desert, sub-artic zones. |
| C3 | Medium | Temperate zones; medium pollutant contamination (12 < SO2 [µg/m3] < 40); low chloride influences, e.g., urban areas, coastal area characterized by low chloride deposition rate.
Tropical zones with low pollution. |
| C4 | High | Temperate zones; high pollution levels (40 < SO2 [µg/m3] < 80); important chloride influences, e.g., polluted urban areas, industrial areas, costal areas (no splashing zones), de-icing salt influence.
Tropical zones with medium pollution level. |
| C5 | Very high | Temperate zones; very high pollution levels (80 < SO2 [µg/m3] < 250); strong chloride deposition rates, e.g., industrial zones, coastal and sea areas (no splashing zones).
Tropical zones with high pollution levels and/or strong chloride influences. |
Although it is strongly recommended to follow this procedure, a qualitative classification of the corrosiveness of atmosphere has been established for cases where field test data are not available (
Table 2).
As an alternative to this qualitative classification, it is possible to refer to ISO 9223 [
10] corrosivity chart, where each class is defined by the guiding values of TOW, sulfur dioxide concentration and the chloride deposition rate. The time of wetness is defined as the number of hours per year during which RH is greater than 80% and the average temperature exceeds 0 °C. Five TOW classes are identified (T1–T5), ranging from the indoor environment with climate control (TOW ≤ 10 hours/year) to the most corrosive damp climate (TOW ≥ 5,500 hours/year). Finally, four sulfur dioxide (P0–P3) and chloride (S0–S1) deposition rates classes have been stated for the classification of environmental corrosivity. An application of ISO 9223[
10] is presented in
Table 3.
Table 3.
An application of the ISO 9223 chart to carbon steel for TOW = T3.
Table 3.
An application of the ISO 9223 chart to carbon steel for TOW = T3.
| TOW = T3 |
|---|
| Sulfur dioxide concentration [µg/m3] or deposition rate [mg/m2day] | P3 | C2 | C2 | C3 | C4 |
| P2 | C1–2 | C1–2 | C2–3 | C3–4 |
| P1 | C1 | C1 | C2 | C3–4 |
| P0 | C1 | C1 | C2 | C3–4 |
| | S0 | S1 | S2 | S3 |
| Chloride deposition rate [mg/m2day] |
5. Corrosion Modeling
Several models concerning the evaluation of the damage produced by atmospheric corrosion are available in the literature. They are formulated according to different approaches, which depend on the objectives of the model itself. Such models can be classified as
first level and
second level models. The first ones are based on laws of physics and chemistry [
2]. In this case, the dissolution of metal and the formation of corrosion products are evaluated at microscope level in the sense of current [
9]. The second level models are useful for engineering applications and allow evaluation of the corrosion rate as a function of mass and/or thickness loss with time, being obtained from the observation and the interpolation of experimental data.
In the following, a review of the second level corrosion models for structural applications is presented. In this case, the relationship between the corrosion rate and the levels of pollutants is expressed in combination with different climatic parameters [
21]. Considered variables affecting the corrosion rate over time are the time of wetting, the frequency and duration of drying out periods, relative humidity, temperature and temperature variation, and the composition of the atmosphere. The corrosion rate is usually expressed as the mass loss per unit area per unit time, or as the rate of penetration, by means of the thickness loss. It is important to note that the thickness loss reported in corrosion studies is usually the average of the thickness losses of the exposed and groundward surfaces of a specimen [
22].
Corrosion models usually describe the corrosion depth as a function of time in the form of a power model:
where d(t) = corrosion depth [µm, g/m
2], t = exposure time [years], A = corrosion rate in the first year of exposure, B = corrosion rate long-term decrease.
Because of the formation of corrosion products on the metal surface, the initial corrosion rate usually decreases over a long-term period. If B is smaller than 0.5, the corrosion products show protective, passivating characteristics, otherwise B is greater than 0.5. Some simplified models have been proposed, which take into account the environmental effects influencing the corrosion rate by means of constant values of coefficients A and B. Such models express the thickness loss only as a function of time and are usually calibrated on data obtained from short- and long-term field test exposure. As a consequence, if they are used for environments which differ from the one where the model has been calibrated, the predicted value of the corrosion rate is often inaccurate. A first attempt to develop general models has been provided by International Standard ISO 9224 [
17], which specify the long term corrosion rates for standard structural materials in the five corrosivity classes C1–C5. According to the Standard, the average corrosion rate of each material follows a bi-linear law. During the first 10 years, the corrosion depth is given by the formula:
where
d1(t) = corrosion depth after the first 10 years of exposure (micrometers);
rav = average corrosion rate (micrometers per year);
t = time at which the exposure ends.
After 10 years of exposure, the corrosion rate is assumed to be constant with time and the thickness loss is given by the formula:
where
d(t) = corrosion depth for the considered time interval (micrometers);
rlin = steady state corrosion rate (micrometers per year);
t=time in the linear region of the curve of uniform corrosion as function of time.
The standard provides the guiding values of both
rav and
rlin for carbon steel, weathering steel, zinc, copper and aluminum. In
Figure 2, a representation of corrosivity band for carbon and weathering steel is shown.
Figure 2.
An application of ISO 9224. Thickness loss as a function of time for carbon steel (a) and weathering steel (b) for different corrosiveness classes.
Figure 2.
An application of ISO 9224. Thickness loss as a function of time for carbon steel (a) and weathering steel (b) for different corrosiveness classes.
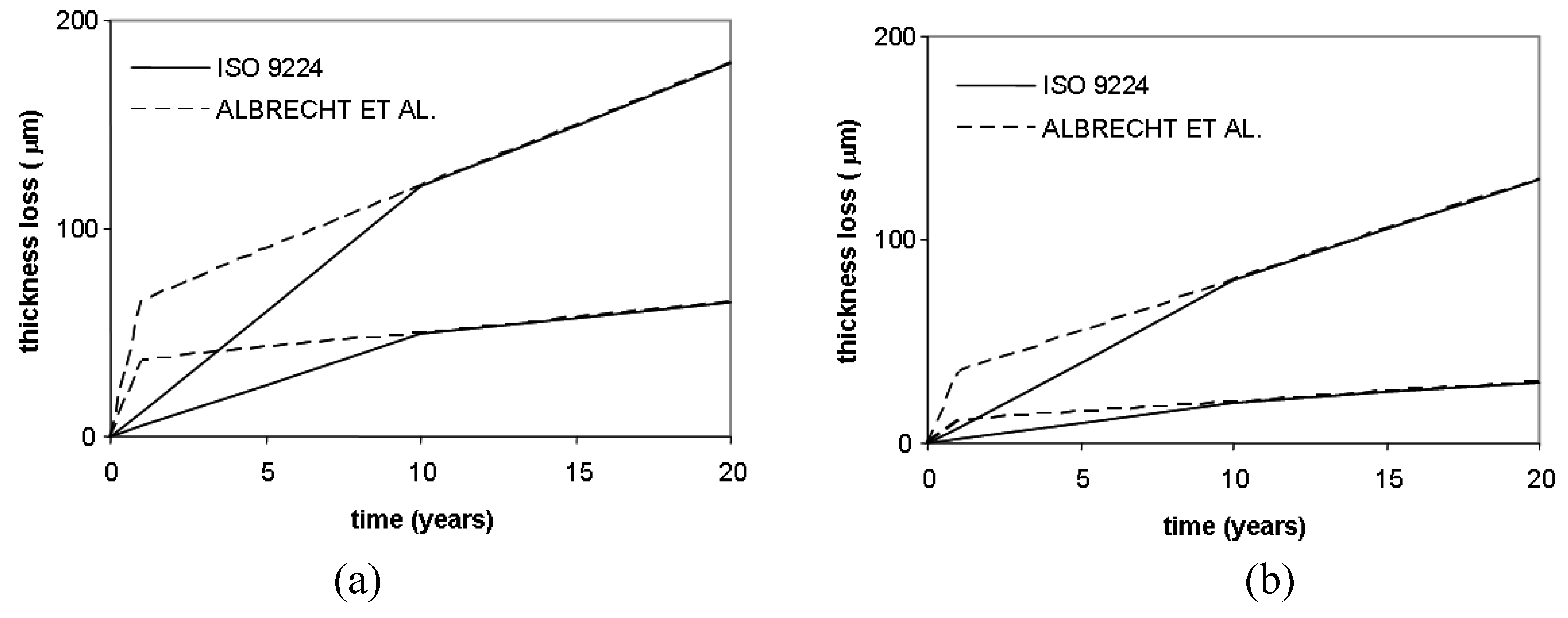
Some adjustment to these corrosion laws have been reported in Albrecht and Hall [
22], where the authors proposed a new bi-linear model that accounts for a modified corrosion rate during the first year of exposure and a steady state during the subsequent years. An application of the modified bi-linear law for carbon and weathering steel for the upper and lower bound in the medium corrosivity category C3 is reported in
Figure 3.
Figure 3.
Thickness loss as a function of time for carbon steel (a) and weathering steel (b) for corrosivity class C3.
Figure 3.
Thickness loss as a function of time for carbon steel (a) and weathering steel (b) for corrosivity class C3.
Recently, different models have been developed with the aim to generalize the corrosion loss over time for different environments, reporting the climate and pollutants variables as independent factors. In the following, two of these models are presented.
Several dose-response functions have been developed within the International Cooperative Programme (ICP) on “Effects on Materials, including Historic and Cultural Monuments” in the framework of the UN ECE convention on long range transboundary air pollution [
21]. These functions have been formulated for different metal materials (
Table 4) and are based on both long-term exposures and trend analysis based on repeated one-year measurements, taking also into account the unsheltered (Equations 5–8) or sheltered (Equation 9) exposure. The degradation of metal over time is expressed by means of mass loss (ML) as a function of climatic parameters (Rh; T), gaseous pollutants (SO
2, O
3) and precipitation parameters (Rain, H
+ Cl
–) as reported in
Table 4. Further details can be found in Kucera [
21].
Klinesmith
et al. [
23] developed a model for the atmospheric corrosion of carbon steel, zinc, copper and aluminum, taking into account the effects of four environmental variables (TOW; sulfur dioxide, salinity and temperature). The general form of the degradation model is the following:
where y = corrosion loss (μm); t = exposure time (years); TOW = time-of-wetness (h/year); SO
2 = sulfur dioxide concentration (μg/m
3); Cl is chloride deposition rate (mg/m
2 /day); T = air temperature (°C); and A, B, C, D, E, F, G, H, J, T
0 = empirical coefficients whose numerical values can be found in Klinesmith
et al. [
23].
Table 4.
Dose response function, ICP Materials.
Table 4.
Dose response function, ICP Materials.
| Material | Mass loss [g/m2] | Equation No. |
|---|
| Weathering steel (unsheltered) | ML = 34[SO2]0.33exp{0.020Rh + f(T)}t0.33 | (5) |
| Zinc | ML = 1.4[SO2]0.22exp{0.018Rh + f(T)}t0.85 + 0.029Rain[H+]t | (6) |
| Aluminum | ML = 0.0021[SO2]0.23Rh exp{f(T)}t1.2 + 0.000023Rain[Cl–]t | (7) |
| Copper | ML = 0.0027[SO2]0.32[O3]0.79Rh·exp{f(T)}t0.78 + 0.050Rain[H+]t0.89 | (8) |
| Weathering Steel (sheltered) | ML = 8.2[SO2]0.24exp{0.025Rh + f(T)}t0.66 | (9) |
| where: t = exposure time (years); Rh = relative humidity (%); T = average annual temperature (°C); f(T) = a (T – 10) when T < 10 °C, otherwise b (T – 10), with a, b being constant values depending on the specific metal; SO2 = sulfur dioxide concentration (μg/m3); O3 = ozone concentration (μg/m3); Rain = average annual rainfall precipitation (mm); H+ = hydrogen ion concentration in precipitation (mg/L); Cl– = chloride ion concentration in precipitation (mg/L). |
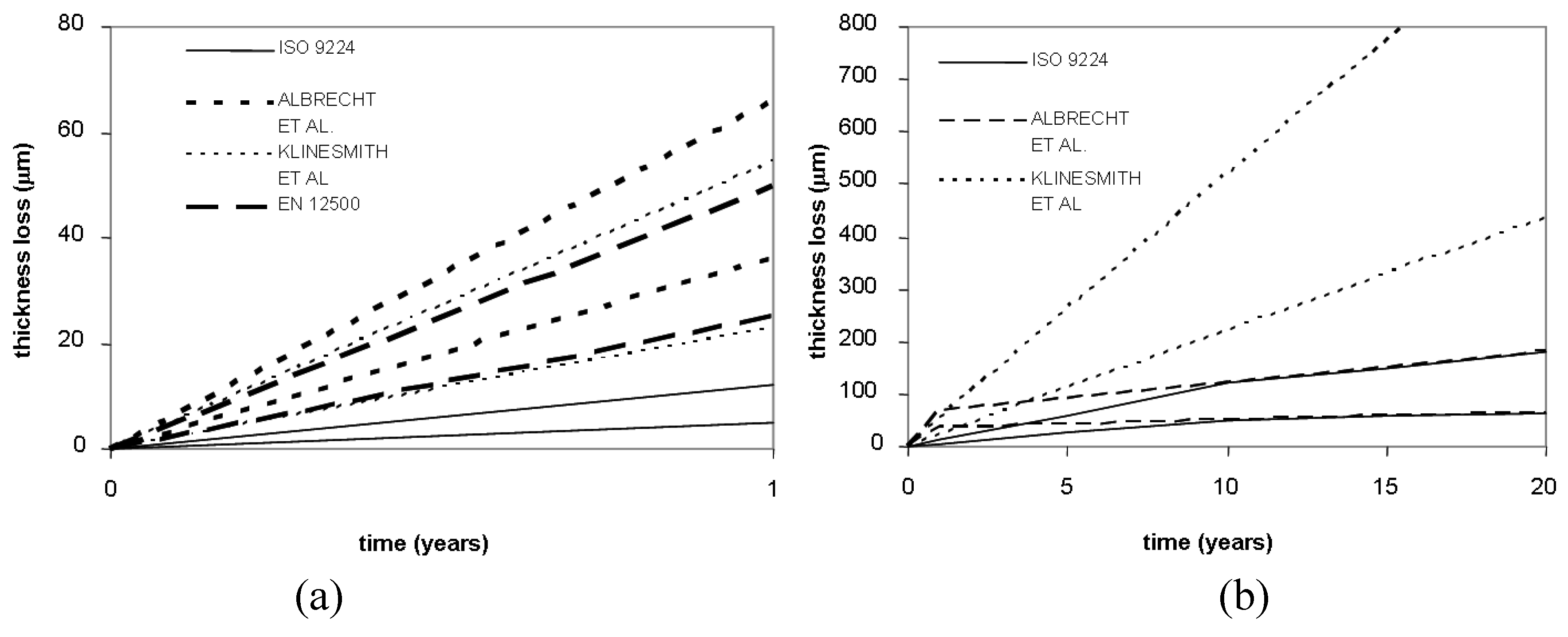
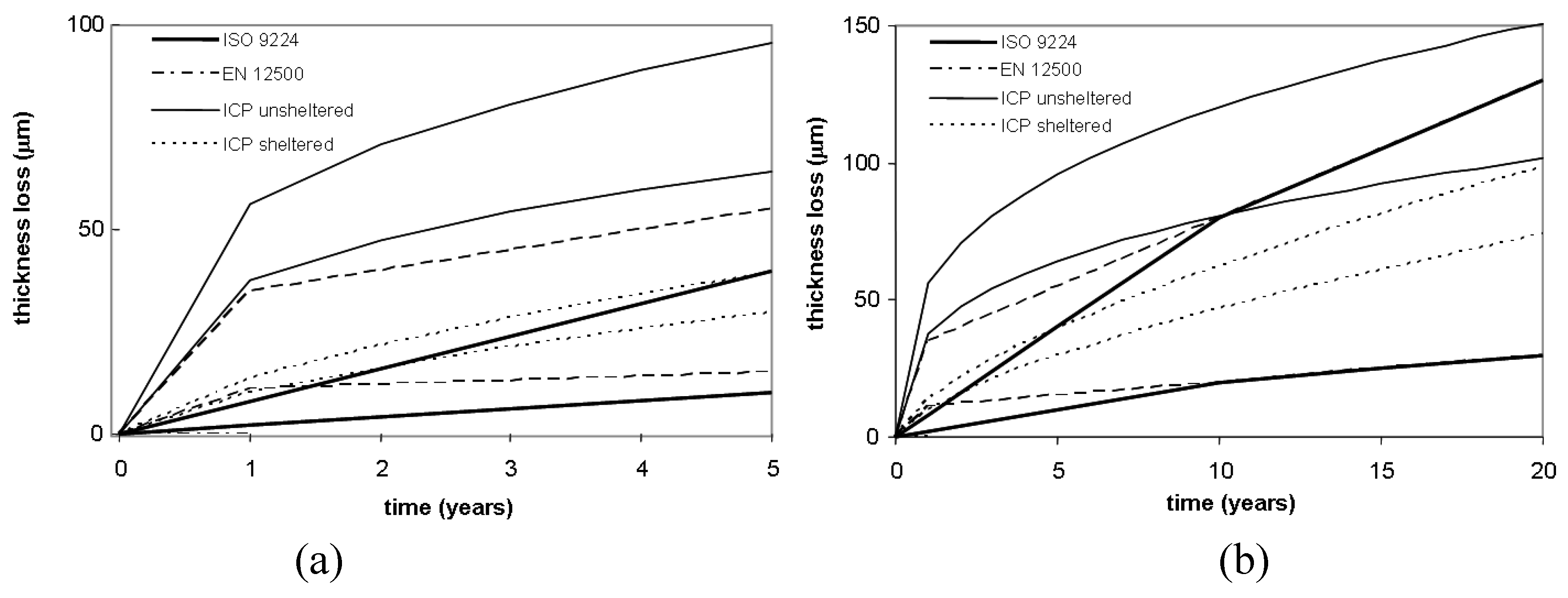
In order to evaluate the differences among the selected corrosion models, a comparison has been carried out for carbon and weathering steel. The considered corrosivity class is C3, which is characterized, according to EN12500 (
Table 2), by a temperate climate, medium pollutant contamination and low chloride influences. The TOW selected levels range from 2,500 to 4,200, while sulfur dioxides and chlorides have been chosen within class P1 and S1 respectively, as defined in ISO 9223. In
Figure 4 and
Figure 5, the comparison among selected models and corrosion rates is shown.
A good agreement among standards and selected corrosion models can be observed in the case of weathering steel for both short- and long-term exposures.
On the contrary, as far as carbon steel is concerned, it can be noted that Standard ISO 9224 underestimates the corrosion loss for both short- and long-term exposures, as also observed by Albrecht and Hall [
22]. In this regard, it should be observed also that the corrosion rates for carbon steel provided by ISO 9224 are similar to the ones given for weathering steel, which instead is characterized by higher resistance to atmospheric corrosion.
Figure 4.
An application of the selected model to carbon steel for 1 year (a) and 20 years (b), corrosivity class C3.
Figure 4.
An application of the selected model to carbon steel for 1 year (a) and 20 years (b), corrosivity class C3.
Figure 5.
An application of the selected model to weathering for five years (a) and 20 years (b), corrosivity class C3.
Figure 5.
An application of the selected model to weathering for five years (a) and 20 years (b), corrosivity class C3.
Indeed, as far as Klinesmith’s corrosion model is concerned, the thickness loss values predicted for one year exposure are very close to EN 12500, but in the long-term, the models provides an average thickness loss of about 1.7 mm for a design life of 50 years, which is approximately 10-times more than the previsions given by the other models.