2.1. A Summary of the History of the Four Most Important Diseases in Buffalo
The Cape Buffalo (
Syncerus caffer) is a large bovid that historically roamed in large numbers across the major parts of southern Africa. They are ecologically important for their role as bulk grazers but their numbers have significantly declined in the past century due to overexploitation and disease pandemics [
6]. Apart from its importance in a multispecies ecosystem, buffalo are also important from an ecotourism and economic perspective [
7]. Unfortunately, 80% of South Africa’s total buffalo population are carriers of one of the four major diseases that can be passed on from wild to domesticated species [
8]. Although buffalo are susceptible to a number of different diseases (the most important of these will be discussed further on), foot-and-mouth disease (FMD) was the first to come under scrutiny [
7]. This disease is known as an “indigenous disease” as the Southern African Territories serotypes (SAT-1, SAT-2 and SAT-3) of the FMD virus were identified for the first time in southern Africa in the 1940s [
9]. Such indigenous diseases generally do not pose a threat to wildlife populations due to the evolutionary development of unique coping mechanisms [
7]. However, FMD has been under continuous scrutiny due to the economic implications of the disease, as it is passed on directly and indirectly from buffalo to cattle. The transmission of the disease is through an unknown mechanism with some authors hypothesizing its spread via aerogenous transmission under certain climatic and epidemiological conditions, while others maintain that the disease is spread through close contact between animals [
10,
11]. Most important, many countries do not want to import the disease via agricultural products [
12]. Although the disease is not fatal, it has severe effects on South Africa’s ungulate populations because of the extreme measures adopted to control it [
13]. The export of agricultural products of animal origin is strictly regulated and banned if an outbreak of FMD occurs. Some authors and researchers view FMD as one of the major impediments on the continent of Africa that prevents access to lucrative export markets for animal products [
12,
13].
FMD is caused by an
Aphthovirus in the family
Picornaviridae. FMD virus (FMDV) is endemic in the Kruger National Park because a long-term carrier state exists in buffalo [
13]. Research has shown that free-ranging buffalo under natural conditions are true maintenance hosts of FMDV and that infection with one of the three Southern African Territories (SAT) serotypes that cause FMDV, occurs between the early ages of three to seven months after maternally inferred immunity is diminished and almost always in the total absence of outward clinical symptoms [
4,
14]. After infection, the virus has been known to persist in the pharyngeal region for as long as five years with no clinical symptoms presenting as the animal then becomes a carrier of the disease [
1,
15,
16]. Transmission between buffalo and cattle is thought to occur during close contact and usually when buffalo are in the acute phases of the disease [
4,
17]. Although the disease is characterized by a high morbidity (in other words, a high number of animals affected) and a low mortality (a low number of animals that actually die from the disease), an outbreak outside a controlled area may have detrimental effects on the livestock industry, especially the export of animals, animal products and agricultural products [
12]. In Europe especially, the effects of foot-and-mouth disease outbreaks have historically been great, and vast amounts of money have been spent to control the disease. As a result, the European Export Community is wary of reimporting the disease, especially exotic strains, and has instituted strict requirements on the import of animal products [
2].
Since the 1970s, the testing for FMDV has been required by government for any buffalo to be moved within South Africa. At the time, other diseases were not investigated and animals that tested negative for FMDV could be moved freely between areas [
3]. However, by the early 1980s, it became apparent that many of the animals infected by FMDV were also infected with Corridor disease (CD) and the disease control regulations started to change [
3].
CD is one of three related diseases caused by
Theileria parva [
18]. The brown ear ticks
Rhipicephalus appendiculatus,
R. duttoni and
R. zambeziensis are considered to be the main vectors of
T. parva, which causes East Coast fever, CD and January disease in cattle [
17,
18]. CD results when buffalo derived
T. parva is transmitted via ticks from buffalo to cattle, which results in mortality, although evidence has shown that cattle that survive this infection then go on to become immune to it [
19,
20,
21,
22]. Indeed, Yusufmia
et al. (2010) [
23] found evidence that suggests that cattle may also be subclinical carriers of
T. parva. However, because in the majority of cases the disease is acutely fatal in cattle, it is considered one the most important diseases transmitted from buffalo to cattle and has contributed greatly to the need for confinement of buffalo to well-fenced areas and stringent veterinary control measures [
2,
17]. CD is also an indigenous disease and although the disease was first documented in Zimbabwe, it gets its name from the fact that it was recognized in South Africa in 1955 in cattle found in the corridor formed by the historic Hluhluwe and iMfolozi game parks [
7,
11]. Since then, recognized Corridor disease-endemic areas in South Africa include the Kruger National Park, Hluhluwe–iMfolozi Park and areas surrounding and in between these regions [
19,
22].
In the 1980s,
bovine tuberculosis (BTB) was also diagnosed in buffalo in the Hluhluwe-iMfolozi Park, which presented a new threat to the game and agricultural industry [
24]. In June 1990, the first buffalo bull in Kruger National Park was diagnosed with BTB, which prompted an investigation into the BTB status of all Kruger Park buffalo [
25]. By the late 1990s, numerous buffalo herds in both the Kruger National Park and the Hluhluwe–iMfolozi Park were found to be infected with BTB and it is thought that the disease was introduced by imported European cattle breeds mainly during the 18th and 19th centuries [
24,
25]. Subsequent outbreak investigations indicated that the disease was likely to have been transmitted from domesticated cattle to buffalo in the Kruger National Park in the southeast corner of the park between 1950 to 1960 [
2,
23,
24]. BTB is therefore known as a foreign, so-called alien livestock disease which is caused by the bacterium
Mycobacterium bovis and can spread quite rapidly from buffalo to cattle and
vice versa. As it had previously been introduced into the country, it had successfully been established in buffalos which then served as a wildlife maintenance host [
7,
23]. Once an animal is infected, it will spread spontaneously through the herd when cough droplets from infected animals are inhaled by healthy animals as well as through food and water contamination [
1]. Although infected buffalo have been known to live with BTB for several years before clinical signs develop, the disease is fatal and has been seen to spill over into other wildlife species such as baboon, lion, cheetah and kudu when it reaches a certain level within a buffalo herd. It has been described as a chronic, progressively debilitating condition [
25].
Another disease found in African buffalo that is transferable to livestock and that is now common practice to test for is bovine brucellosis, which is also classified as a foreign livestock disease [
7]. It is caused by the bacterium
Brucella abortus, one of six species recognized within the genus
Brucella [
26,
27]. It is usually transmitted orally via contact with fetal membranes, post-parturition discharges and milk as well as through the semen of infected males [
26,
27,
28]. The disease causes abortion in the latter half of gestation and is also known as “contagious abortion.” This may result in massive economic losses, as well as pose a potential barrier to international trade [
29]. The disease presents with very noticeable outward symptoms as it leads to highly swollen carpal joints, known as “hygromata” or “house maid knees syndrome.” In South Africa, no breeding is allowed with buffalo that have tested positive for brucellosis and aborted fetuses have to be handled with care as the disease is transmissible to humans [
1].
2.2. The History of Disease-Free Buffalo Breeding Projects in South Africa
In 1996, the South African National Parks Board (SANPARKS) requested permission for and started the first official pilot studies for breeding “disease-free” buffalo. Part of the reason was that SANPARKS wanted to increase “disease-free” buffalo numbers because many buffalo herds had gone extinct from their former ranges due to over-hunting, the rinderpest epidemic (caused by the Rinderpest virus or RPV) and exclusion from cattle ranching areas. They also aimed to preserve the most heterogeneous buffalo genetics from other parks other than Kruger Park [
30]. These pilot studies were designed to produce “disease-free” calves from “diseased” parent stock to provide official conservation organizations and the wildlife ranching industry with clean “disease-free” buffalo from controlled areas that did not pose any disease risk [
6]. The studies made use of diseased parent stock from the Kruger National Park as it had the largest gene pool within its herds and the aims were to preserve this buffalo genotype if ever a depopulation decision was made to control BTB as well as to supply other parks with “disease-free” buffalo of the Kruger genotype [
3]. There were two pilot studies approved, one that left calves with their diseased mothers until their maternally derived immunity vanished and a privately funded project where calves were taken from their mothers at birth and fostered by surrogate dairy cows. After the success of these pilot studies, numerous other similar projects were launched and, in 1998, a formal Buffalo Advisory Committee was formed to control disease-free buffalo breeding projects through official registration of such projects as well as the monitoring of movement of animals from controlled to noncontrolled areas, among other things [
3]. In 2001, a meeting was held by the Committee in Hoedspruit after a number of “breakthrough” infections indicated that the breeding projects did not sufficiently lower the risk of diseases spreading. At this meeting, it was decided that these disease-free buffalo breeding activities would be phased out by December 2011 as by such time, enough “disease-free” stock should have been produced. Before the projects were phased out, there were 28 official “disease-free” buffalo breeding projects in South Africa [
6]. As the demand for “disease-free” buffalo increased, so did the price of these animals and many farmers started such breeding projects for the potentially large economic benefits they held [
31]. Today there are over 130 registered farms (defined as farms containing only buffalo that are “disease-free”) in the province of Mpumalanga, South Africa alone. A comprehensive and complete list of farms registered within South Africa as “disease-free” buffalo farms can be found on the South African Department of Agriculture, Forestry and Fisheries website under Disease Control under Food and Veterinary Services [
8].
2.3. The “Disease-Free” Breeding System
In their natural environments, breeding would occur in accordance with the availability of nutritionally adequate feed [
32]. However, in captivity, buffalo breed throughout the year although most births occur during the summer months [
33]. Similar to any other agricultural animal breeding practice, the breeding stock for a “disease-free” buffalo breeding herd is chosen based on specific principles and standards. Bulls are selected on temperament and semen quality as well as horn length, the latter being an important economical factor for the hunting industry as many hunters select trophy animals based on this trait. Cows are selected primarily based on fertility and both cows and bulls have to be free of BTB and brucellosis to be selected. The breeding stock can either be purchased directly from farmers, at wildlife auctions or through reputable wildlife capture operators. “Diseased” stock can be as much as a third of the price of “disease-free” stock because of the large financial costs involved in using such stock to breed “disease-free” calves [
1]. When “diseased” stock are captured directly from the field, the test for brucellosis is done in the field while the animals are immobilized and animals that test positive are released. Thereafter, animals are tested while in captivity for BTB (using the intradermal skin test) and animals that test positive are euthanized. If any animals test positive, the entire herd captured is rejected [
30].
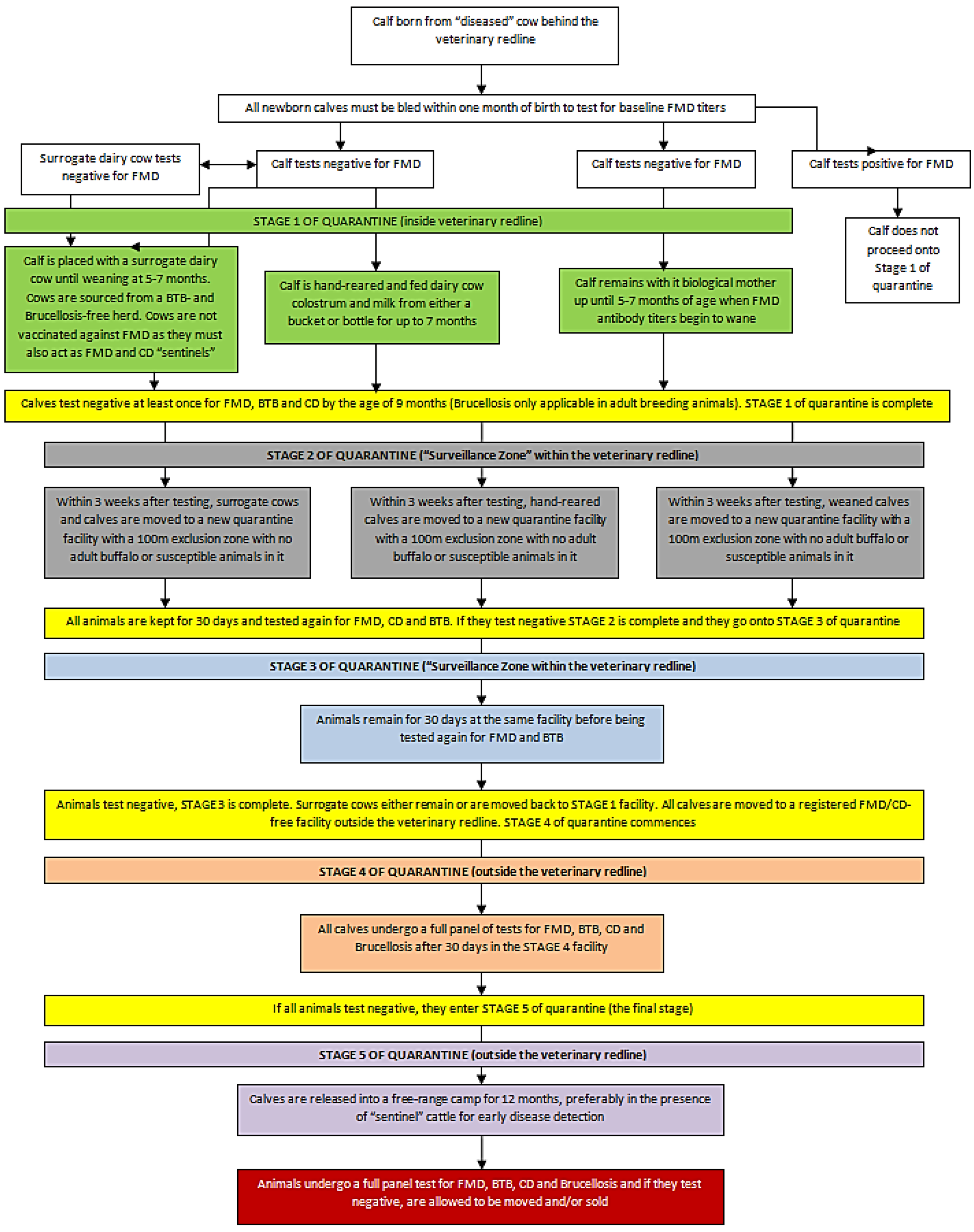
Figure 1.
The different breeding systems used to breed “disease-free” buffalo calves from “diseased” parent stock.
Figure 1.
The different breeding systems used to breed “disease-free” buffalo calves from “diseased” parent stock.
There are three basic systems that are used to obtain the “disease-free” calves, each system with its own advantages and pitfalls (refer to
Figure 1). The first two systems both make use of either foster dairy cows to act as surrogate mothers to buffalo calves or of feeding the calves with bottles or buckets. All three systems make use of intensive breeding practices so that breeding cows are kept in sheds built according to exact specifications, each with at least one quarantine station (
i.e., a box within a box). Such intensive breeding systems make it possible for the breeder to remove calves from their mothers at specific times, depending on the breeding system implemented [
1,
25].
In the first system, the calves are taken away immediately from the buffalo cow after birth and fed cattle colostrum. Thereafter, it is either hand-reared or placed with a surrogate dairy cow. The benefits that this system holds include a severe decrease in the risk of FMDV (which may be contracted once the colostrum-derived FMDV antibody titers begin to wane after several months) and CD being contracted by the calf [
27]. Even if ticks manage to break through the tick-control efforts, they are unlikely to carry the
T. parva parasite as there would be no animals with CD in the surrounding areas. The calves also tend to become more tame and easier to handle, and no lactational suppression of estrus results in a shorter calving interval for the buffalo cow. The latter fact is supported by Skinner
et al. (2006), who reported more peaks in percentage calves born in captivity and attributed this to both the high nutritional value of the feed given as well as the fact that calves were taken away immediately from their mothers [
32]. However, this system also holds the disadvantage of being highly labor and management intensive as the early detection of parturition is critical and calves need to be given good quality cattle colostrum at the correct dosage to ensure adequate early disease resistance [
27]. Additionally, although the calves are tamer, they are still dangerous and a number of incidents with calves/young animals attacking humans have been noted.
The second system makes use of the same principles as the first, except that calves are allowed to drink the colostrum naturally produced by their mothers before being taken away. This means the calf stays with its mother for roughly the first 48 hours of its life before being removed and either hand-reared using a bucket or bottle, or is placed with a foster cow. Making use of this method means that calves will have positive titers to FMDV for a variable period, thereby delaying the completion of stage 1 quarantine and their removal date from the infected zone facility. However, it also ensures that calves gain protection against diseases from the buffalo’s colostrum as well as learning to suckle strongly before being taken away [
27].
The choice between the first two methods is often the personal preference of the breeder, and calves are taken away from their mothers by pushing the mothers into a different pen and closing the gate before the much slower calf can catch up. If this method is unsuccessful, the mother may also be tranquilized via a projectile dart so that the calf can be removed. Once the calf is removed, it is washed and drenched with either pyrethroids or formamidines for treatment against any external parasites [
22]. The calf is also weighed, the sex determined, and it is given an ear tag and microchip for identification. All this data is kept as part of a calf register for future use. Calves that have been removed from their mothers are then placed in groups of five to eight animals and either hand-reared or fostered by dairy cows at a ratio of two to three calves per cow. Jersey cows are the preferred foster mothers, due to their good mothering abilities and high milk yield [
1].
Using both the aforementioned systems, breeders also have the option to hand-rear calves that have been removed from their diseased mothers. In this case, the calves are hand-reared on whole cow’s milk from registered disease-free dairies using either buckets or bottles. A disadvantage of doing this is that it is highly labor intensive and calves may imprint on the humans feeding them as they tend to become tame quite easily, resulting in aberrant behavior later in life. This behavior may only become apparent once the grown calves are released into a larger herd as they may become aggressive and dangerous due largely to no inherent fear of humans [
27].
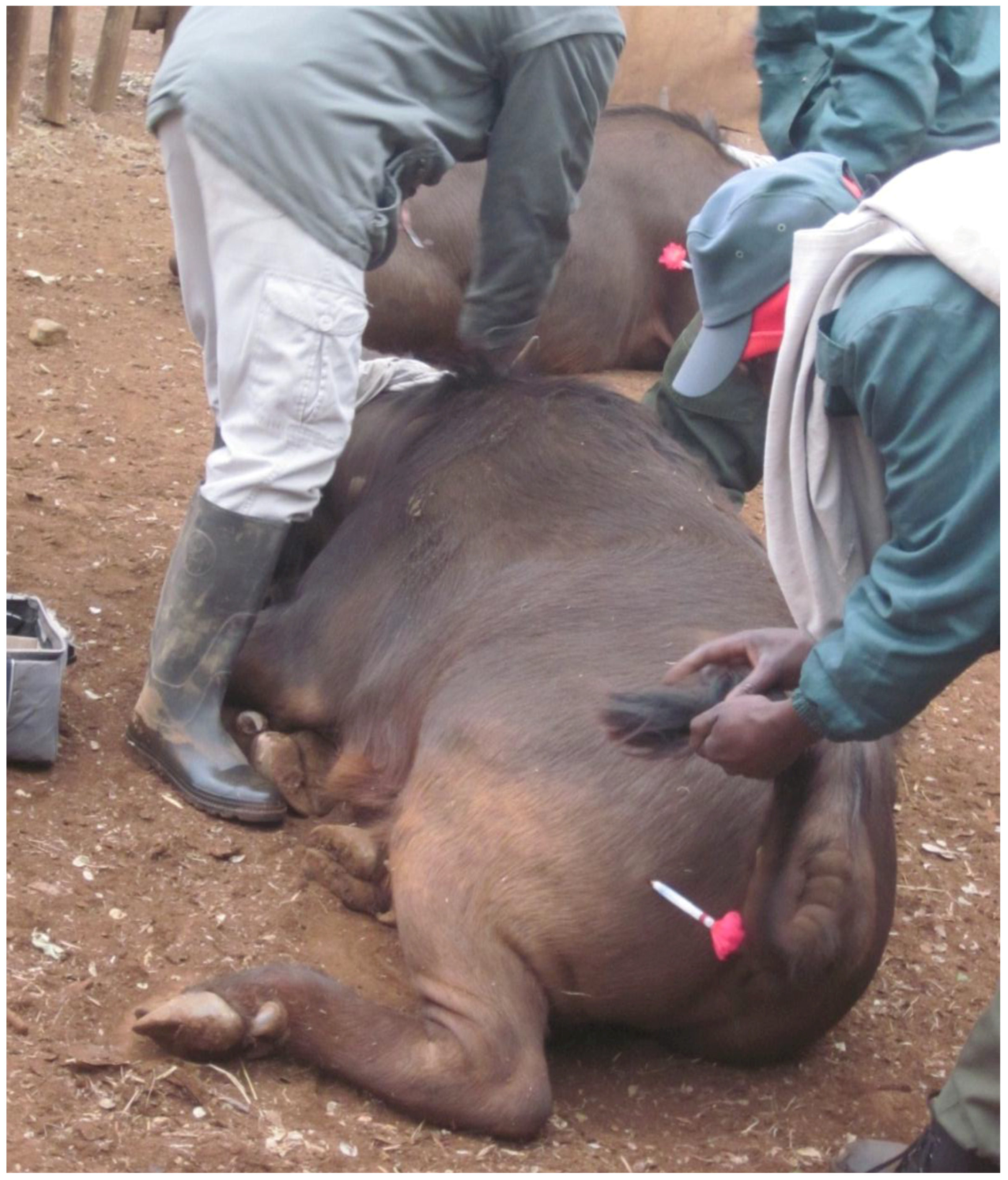
Figure 2.
A buffalo calf with a foster jersey dairy cow. The calf has just been darted to draw blood for testing.
Figure 2.
A buffalo calf with a foster jersey dairy cow. The calf has just been darted to draw blood for testing.
The alternative is to place the calves with foster dairy cows, which is often the preferred method used by breeders (
Figure 2). The dairy cows are selected based on a number of traits. Firstly, the cow has to be in good health, and be BTB- and brucellosis-free, along with other health traits such as no history of mastitis, strong feet,
etc. To prevent mastitis from occurring, it is generally recommended that cows be selected with a peak milk production of less than 25 liters per day. Buffalo milk has a butterfat content of 6–8% [
34], therefore, it is recommended to select cows with a high butterfat production rate so as to compensate for the replacement of buffalo milk. Also, buffalo calves nurse between the mother’s hind legs so cows need to be selected with long teats to assist the calf in suckling. Jersey cows tend to make good surrogate mothers and seldom reject calves that attempt to suckle from behind [
35]. Cows that have had their second parturition tend to make better foster mothers, as well, as they are more comfortable and less nervous than first-time mothers because they have already been handled in the milking parlor. Lastly, since cows have to be tested every three months and are likely to be handled on a daily basis, it is important that they are also selected for temperament, so as to ease handling procedures and avoid injuries to calves and workers [
1].
An alternative to hand-rearing and using surrogate dairy cows to raise buffalo calves is a third system whereby calves are left with their biological mothers, who originate from potentially “diseased” stock, after birth. They therefore obtain immunity naturally from buffalo colostrum as well as being exposed to normal buffalo herd behavior so that behavioral problems later on in life are prevented [
27]. However, this system is seldom recommended as it holds numerous disadvantages. Studies suggest that the FMDV virus is present in the semen of bulls so that there is the possibility that it may be passed on to the cow during mating and then onto the calf through the milk if the mother was mated while still lactating [
1,
36]. Calves also tend to grow slower than their counterparts raised on Jersey milk and, as mentioned before, the intercalving period for the cows become longer if their calves are kept with them because of lactation anestrus. Also, as the calf’s passive immunity wanes, there is a finite risk of a breakthrough FMDV infection, as well as the risk of a
Theileria infection if there is a breakdown in the tick control, since calves will still be in the presence of their
Theileria infected mothers [
27]. Lastly, if conditions are overcrowded and often wet, outbreaks of diseases such as
Escherichia coli and coccidiosis that lead to diarrhea and mortality in young calves may occur [
1]. Calves that are left with their biological mothers are usually weaned betweenthe ages of five and seven months, or at 100 kg of weight when the colostrums-derived FMDV antibody titers have started to wane significantly. Prior to this, this maternally derived immunity prevents calves from becoming infected with FMDV [
30]. However, fostered calves may be weaned earlier before they start to grow horns and become big and strong enough to injure their jersey surrogates [
27].
2.3.1. Quarantine Procedures for Breeding of “Disease-Free” Buffalo
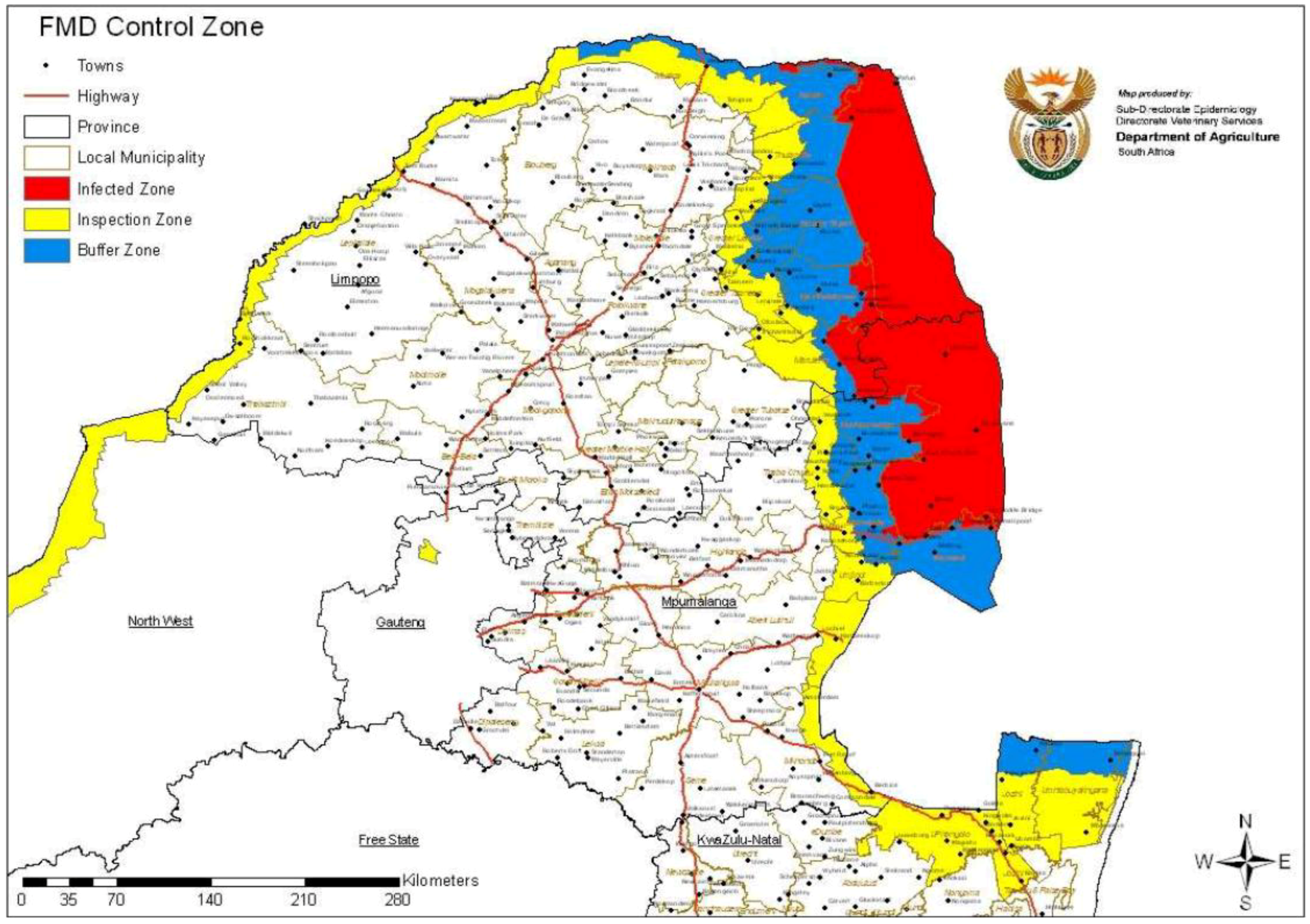
The veterinary redline area (infected zone) in South Africa is classified as the area in and around the Kruger National Park, including Mozambique and a 10- to 15-km inspection zone bordering Swaziland, Zimbabwe and Botswana (free movement of animals from the inspection zone to the free zone is prohibited) where FMDV is most prevalent (
Figure 3) [
1,
34]. This control area is defined by natural geographical borders, roads and fences [
37,
38]. Adjacent to the western and southern borders of this infected zone is the buffer zone, which has two sections: a portion where animals are vaccinated twice yearly (referred to as the buffer zone with vaccination (BZV)) and a portion where animals are not vaccinated but where increased surveillance and movement control are implemented, known as the buffer zone without vaccination (BZNV)) [
4]. Buffalo that occur behind the redline and that are infected with one of the four diseases previously discussed, are often referred to as “dirty buffalo” and movement of such buffalo is strictly regulated to prevent diseases spreading to unaffected animals [
39]. However, diseased animals inside the redline area that test negative for brucellosis and BTB are used as breeding stock in “disease-free” breeding projects. While using these latter animals for breeding, they are all kept in quarantine and the property must fall behind the veterinary redline as it is illegal to move animals from behind the redline to outside the redline area unless they have been bred as “disease-free” following the strict protocol discussed below [
39].
Figure 3.
Disease control zones in South Africa according to the South African Department of Agriculture, Forestry and Fisheries [
8].
Figure 3.
Disease control zones in South Africa according to the South African Department of Agriculture, Forestry and Fisheries [
8].
All parent stock must test negative for BTB and, if they are sourced from a unknown BTB area, all animals must undergo an additional two tests for BTB after the initial three-month quarantine period before they will be considered BTB-free and allowed to join a BTB-free herd for breeding. If animals are sourced from a known BTB area, they have to undergo at least five negative BTB tests over a period of fifteen months before being considered BTB-free and the first of these tests can only be done three months post-capture [
39]. As mentioned, all breeding animals must also test negative for brucellosis, and cows have to test negative at least three times, as well as be tested once a year thereafter, usually two to twelve weeks postcalving. Foster dairy cows may not be vaccinated against FMDV so that they can fulfill their additional roles as “sentinels” for the disease [
27].
When a calf is born from a FMDV or a potential FMDV mother, it is taken away within 24 hours for testing, sexing and tagging (
Figure 1). This first testing must be performed at least 100 m from the “diseased” parents and all surrogate mothers must be tested parallel to the calves. If the calf is taken away permanently from its mother, it has to be bled within one month for baseline FMDV titers and the results will indicate whether the calf ingested any of its mother’s colostrum. If the test indicated no buffalo colostrum was ingested, then stage 1 of the quarantine process begins and calves also have to test negative at least once for Corridor disease and BTB. Once they do, stage 1 of the quarantine is complete [
30]. Those calves that have ingested or been allowed to drink their biological mother’s colostrum, must be bled at five to seven months of age (when they are weaned) to test for residual FMDV titers. If they test positive, this is not considered an outbreak as the occurrence falls within the veterinary redline area. Financial implications due to the low marketability of such calves are carried by the commercial operator. However, if they test negative then they also have to be tested for Corridor disease and BTB, and once they test negative for all three diseases, stage 1 of their quarantine is complete. On completion of stage 1, all calves and surrogate cows are moved within three weeks of sampling to a quarantine facility within the FMDV surveillance zone under the supervision of the local state veterinarian. All the calves and foster cows now have the same disease status and the quarantine facility will have no adult buffalo in it and will be surrounded by a 100 m exclusion zone with no susceptible animals in it. This is the second stage of quarantine and all animals will remain at this facility for 30 days and all calves and foster cows will be bled again for FMDV, BTB and Corridor disease testing. If they test negative, they enter stage 3 of quarantine and must remain for a further 30 days in the same facility before being bled again for FMDV and BTB. Once they test negative again, stage 3 is completed and foster cows either remain in the surveillance zone facility or are moved back to the primary facility in the infected zone while the calves are moved under the supervision of the state veterinarian to a suitable facility that is registered as FMDV/Corridor disease free in an area outside the veterinary redline [
39]. This is the fourth stage of quarantine for the buffalo calves and after 30 days at the new facility, all the calves have to be re-bled for a full panel test for FMDV, BTB, Corridor disease and brucellosis. This last testing must occur before the calves are 11 months old and once they test negative, they enter the fifth and final stage of quarantine. At this stage, calves are released into a free-range camp for a retention period of 12 months during which time they are exposed to brown-ear ticks and are preferably in the presence of FMDV “sentinel” cattle, which act as an early detection system for the disease [
1,
35]. The sentinel cattle are much tamer than the buffalo and thus require no specialized equipment for immobilization when blood sampling is done and are thus far easier to test for disease status in the absence of outward symptoms. After this 12-month period, all animals undergo a full panel test again for all four diseases and, if they test negative, are allowed to be moved/sold under the supervision of the state veterinarian. In Corridor disease-only breeding projects in the vector-free areas, the last two stages of quarantine do not apply if animals are destined to be sold to farms within the vector-free area [
1,
25,
28].
Disease-free buffalo are thereafter traded freely on the South African game species market and make up a large percentage of the profit margin at annual game auctions [
40].
2.3.2. Testing Procedures
Diagnostic testing of infectious diseases may often be difficult in wildlife if they are taxonomically far removed from livestock. However, buffalo fall within the subfamily Bovidae, which thus provides a basis for the adaptation and development of diagnostic tests for BTB, FMDV, CD and Brucellosis. Although a number of diagnostic tests are currently being used to test for these diseases, these tests still require validation to prove fitness of purpose as stated by the World Organization for Animal Health (OIE) [
7].
2.3.2.1. Bovine Tuberculosis
For BTB, the standard method of detection is to inject a small amount of the antigen into the skin of the animal and then to measure the immune response. This is usually done by measuring skin thickness 72 hours postinjection to determine if any swelling has occurred (
Figure 4). This method is also known as the comparative intradermal test, or skin test [
27,
41]. This test is used as the golden standard in South Africa but may lose some accuracy if there is a high level of environmental exposure to other sensitizing organisms. According to the World Organization for Animal Health (OIE), purified protein derivatives (PPD) products have replaced heat-concentrated synthetic medium tuberculins because of their higher specificity and easier standardization, and no less than 2000 International Units (IU) of bovine PPD should be used when performing the skin test [
42].
Figure 4.
Immobilized buffalo being tested for BTB using the skin test.
Figure 4.
Immobilized buffalo being tested for BTB using the skin test.
2.3.2.2. Brucellosis
In the case of brucellosis, any clinical signs such as abortion or vaginal discharge should be treated as suspicious. A number of serological tests are suitable for detection of
Brucella, including the Rose Bengal test and buffered plate agglutination test, the complement fixation test (CFT), the enzyme-linked immunosorbent assay (ELISA) or the fluorescence polarization assay [
43]. However, no single test is appropriate in all epidemiological situations and, in African buffalo, the complement fixation test is considered suitable, although Rose Bengal reactions are also treated with suspicion [
27].
2.3.2.4. Corridor Disease
To test if an animal is infected with Corridor disease, the animal must be tested using the indirect immunofluorescent antibody (IFA) test or polymerase chain reaction (PCR)/probe assay. Disadvantages of the IFA test include cross-reactivity between different species, subjectivity in the interpretation of the results and difficulty in standardization [
46]. If animals are not subject to a continuous tick challenge, it may also happen that the antibodies are not detected and as a result a number of other techniques have also been developed for detection of CD using PCR and DNA probes. Smear examination may also be used, although this can only detect
Theileria piroplasms and can as such not be used to make a completely accurate diagnosis of CD [
27,
46].
2.3.3. Rationale Behind the Extensive Protocol Used for “Disease-Free” Breeding Projects
The purpose of this extensive protocol is to ensure that the buffalo are free of the four diseases: FMD, CD, TB, and brucellosis. The test for FMD is very specific and very sensitive. However, it does not distinguish between maternal immunity and acquired immunity. Research has proven that the maternal immunity will have completely dissipated after nine months, hence the animals have to stay in quarantine for this period for the sake of FMD. The brucellosis is also sensitive and specific and because no positive maternal animals are allowed to participate, it is almost guaranteed that this test is always negative. TB, however, has a less sensitive and specific test and, therefore, the test is only considered as a herd test and not an individual test. For this reason, the protocol requires five negative tests of which the first four is taken at three-month intervals, and the last test only a year later. This will hopefully pick up any new infections should there be TB in the animals. For CD, the test is extremely sensitive, but not that specific. CD is tick transmitted and, before the last test, the animals must be exposed to the vectors for a period of one year, which will allow time for infection of all animals should the disease be present. There has been no definitive proof of any transplacental infections for any of these diseases. Unfortunately, calves are removed from the program the minute they test positive for any of these diseases, and their outcomes are unknown [
47].