Real-Time Study of Noxious Gas Emissions and Combustion Efficiency of Blended Mixtures of Neem Biodiesel and Petrodiesel
Abstract
:1. Introduction
2. Experimental Methods and Conditions
2.1. Production of Neem Biodiesel
2.2. Instrumental Set-up
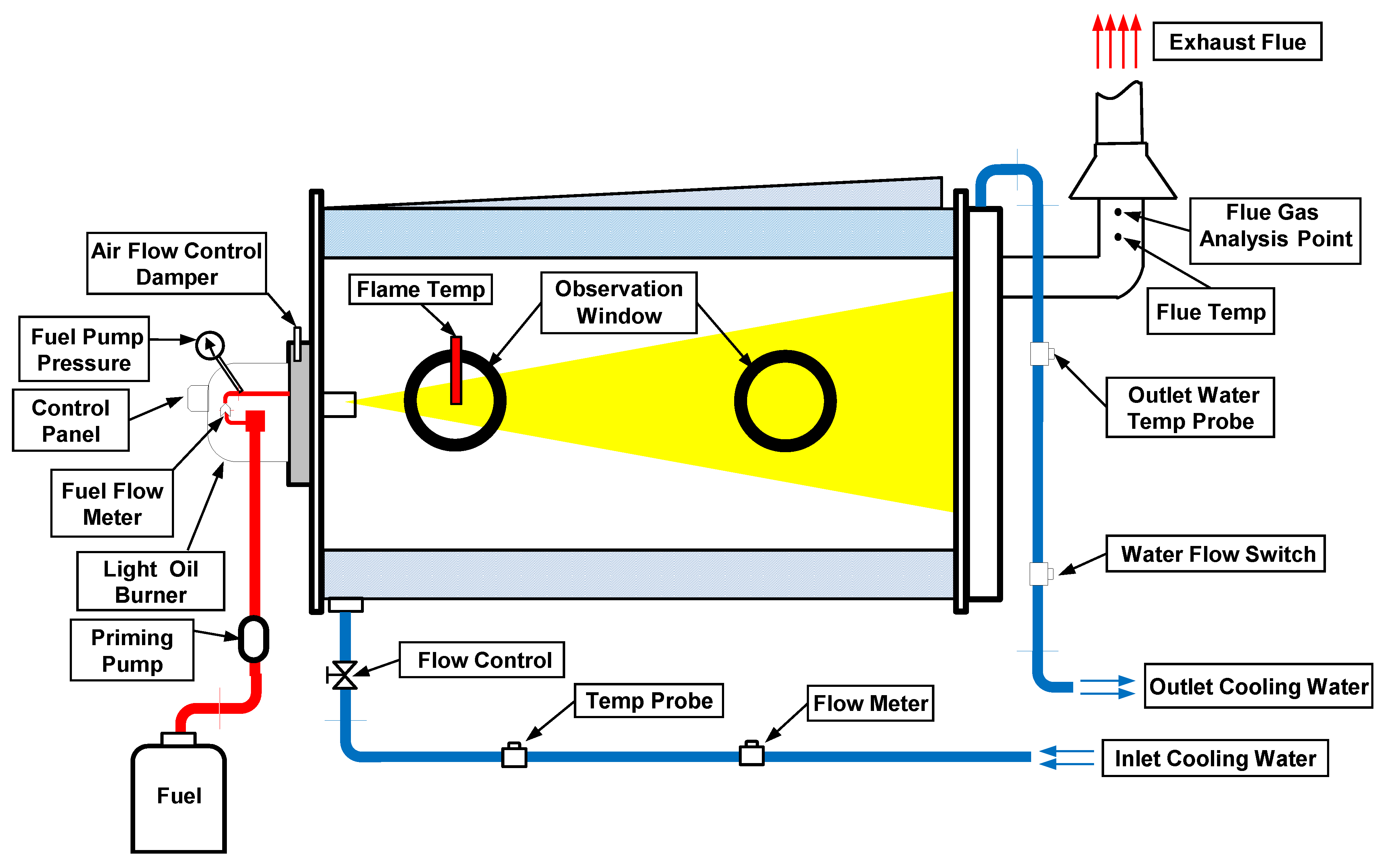
2.3. Analytical Procedure
2.4. Numerical Methodology




| Blend Ratio (Diesel/Biofuel) | %Cfuel | Qnet (kJ/kg) | K2 (%) |
|---|---|---|---|
| 100.0% | 86.80 | 44,800 | 15.51 |
| 95/5% | 86.30 | 44,364 | 14.73 |
| 90/10% | 85.80 | 43,928 | 13.96 |
| 85/15% | 85.30 | 43,492 | 13.18 |
| 75/25% | 84.30 | 42,620 | 11.63 |


3. Results and Discussion
3.1. Real-Time Study
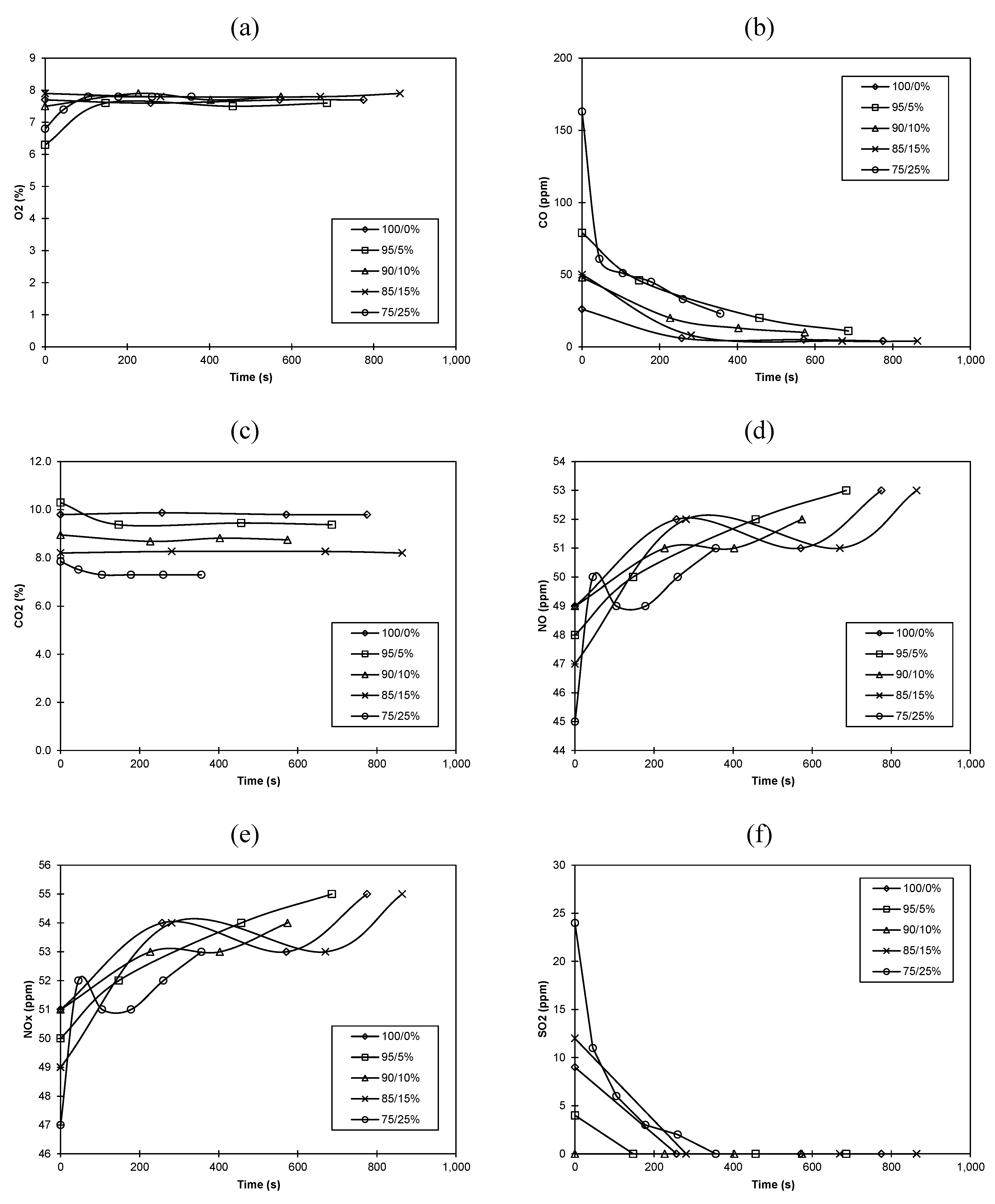
3.2. Combustion Efficiency/Temperature Considerations
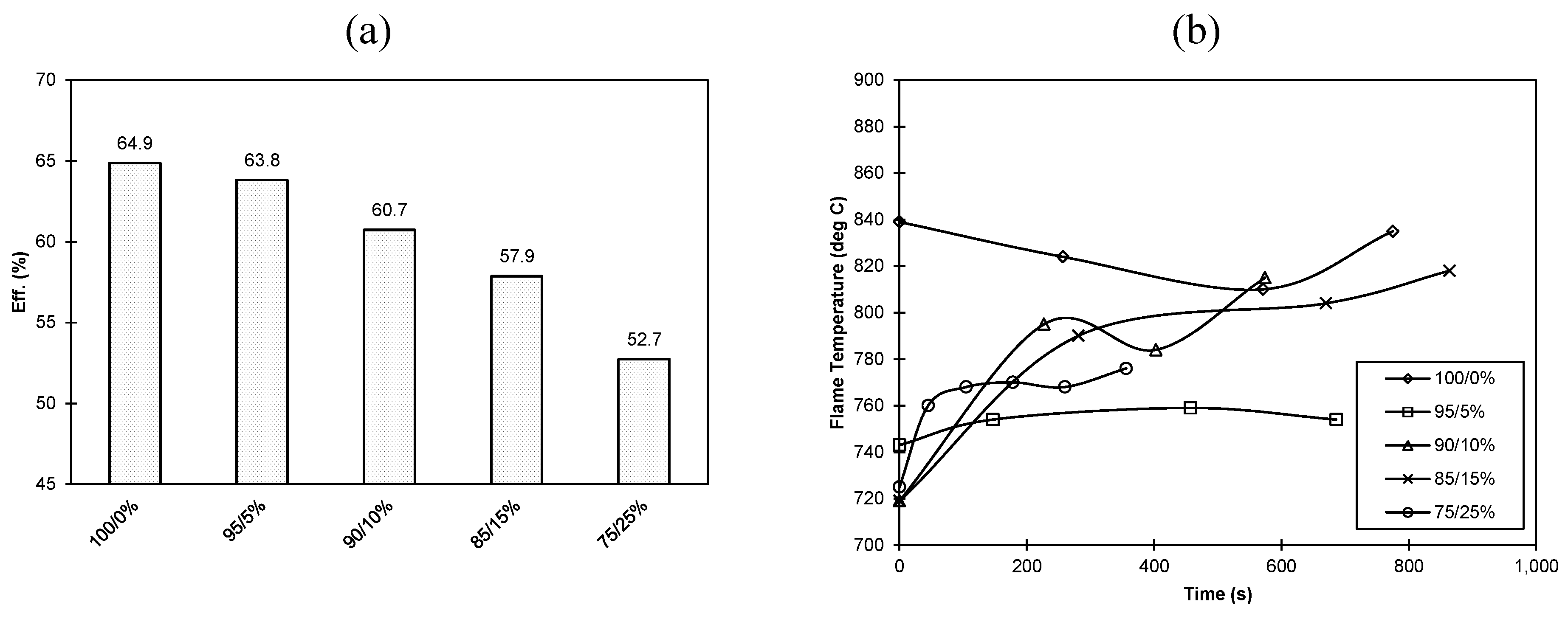
3.3. Potential Environmental Implications
4. Conclusions
Acknowledgments
References and Notes
- Pillay, A.E.; Elkadi, M.; Fok, S.C.; Stephen, S.; Manuel, J.; Khan, M.Z. A comparison of trace metal profiles of neem biodiesel and commercial biofuels using high-performance ICP-MS. Fuel 2012, 97, 385–389. [Google Scholar] [CrossRef]
- Sekhar, M.C.; Mamilla, V.R.; Mallikarjun, M.V.; Reddy, K.V. Production of biodiesel from neem oil. IJES 2009, 1, 295–302. [Google Scholar]
- Kalam, M.A.; Masjuki, H. Biodiesel from palm oil—An analysis of its properties and potential. Biomass Bioenerg. 2002, 23, 471–479. [Google Scholar]
- Freedman, B.; Pryde, E.; Mounts, T. Variables affecting the yields of fatty esters from transesterificatied vegetable oils. J. Am. Oil Chem. Soc. 1984, 61, 1638–1643. [Google Scholar] [CrossRef]
- Goering, C.E.; Schwab, A.W.; Daugherty, M.J.; Pryde, E.H.; Heakin, A.J. Fuel properties of eleven vegetable oils. T. ASAE 1982, 25, 1472–1483. [Google Scholar]
- Lin, B.-F.; Huang, J.-H.; Huang, D.-Y. Experimental study of the effects of vegetable oil methyl ester on DI diesel engine performance characteristics and pollutant emissions. Fuel 2009, 88, 1779–1785. [Google Scholar] [CrossRef]
- Schuchardta, U.; Serchelia, R.; Matheus, V. Transesterification of Vegetable Oils: A Review. J. Brazil. Chem. Soc. 1998, 9, 199–210. [Google Scholar]
- Muthu, H.; Sathyaselvabala, V.; Varathachary, K.; Kirupha Selvaraj, D.; Nandagopal, J.; Subramanian, S. Synthesis of biodiesel from neem oil using sulfated zirconia via transesterification. Braz. J. Chem. Eng. 2010, 27, 601–608. [Google Scholar]
- Banapurmatha, N.R.; Tewaria, P.G.; Yaliwalb, V.S.; Kambalimathc, S.; Basavarajappad, Y.H. Combustion characteristics of a 4-stroke CI engine operated on Honge oil, Neem and Rice Bran oils when directly injected and dual fuelled with producer gas induction. Renew. Energ. 2009, 34, 1877–1884. [Google Scholar] [CrossRef]
- Rao, G.L.N.; Saravanan, S. Role of biofuels in a sustainable environment—A technical study. Clean 2008, 36, 830–834. [Google Scholar]
- Nabi, M.; Shahadat, M.; Rahman, M.; Beg, M. Behavior of Diesel Combustion and Exhaust Emission with Neat Diesel Fuel and Diesel-Biodiesel Blends; SAE Technical Paper 2004-01-3034; SAE International: Englewood, CO, USA, 2004. [Google Scholar]
- Nabi, M.N.; Akhter, M.S.; Shahadat, M.M.Z. Improvement of engine emissions with conventional diesel fuel and diesel–biodiesel blends. Bioresource Technol. 2006, 97, 372–378. [Google Scholar] [CrossRef]
- Sundarapandian, I.; Devaradjane, I. Theoretical and Experimental Investigation of the Performance of Vegetable Oil Operated CI Engine; SAE Technical Paper 2007-32-0067; SAE International: Englewood, CO, USA, 2007. [Google Scholar]
- Singh, S.P.; Singh, D. Biodiesel production through the use of different sources and characterization of oils and their esters as the substitute of diesel: A review. Renew. Sustain. Energy Rev. 2010, 14, 200–216. [Google Scholar] [CrossRef]
- Aziz, A.A.; Said, M.F.; Awang, M.A. Performance of palm oil-based biodiesel fuels in a single cylinder direct injection engine. Palm Oil Develop. 2005, 42, 15–27. [Google Scholar]
- Anbumani, K.; Singh, A. Performance of mustard and neem oil blends with diesel fuel in C.I. engine. ARPN 2010, 5, 1–6. [Google Scholar]
- Hossain, A.K.; Davies, P.A. Plant oils as fuels for compression ignition engines: a technical review and life-cycle analysis. Renew. Energ. 2010, 35, 1–13. [Google Scholar] [CrossRef]
- Ilkilic, C.; Yucesu, H.S. Investigation of the effect of sunflower oil methyl esther on the performance of a diesel engine. Energ. Source. 2005, 27, 1225–1234. [Google Scholar] [CrossRef]
- Knothe, G.; Steidley, K. Kinematic viscosity of biodiesel fuel components and related compounds—influence of compound structure and comparison to petrodiesel fuel components. Fuel 2005, 84, 1059–1065. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Kumar, A.; Raheman, H. Biodiesel production from jatropha oil (Jatropha curcas) with high free fatty acids: An optimized process. Biomass Bioenerg. 2007, 31, 569–575. [Google Scholar] [CrossRef]
- BSI, BS 845-1:1987: Methods for Assessing Thermal Performance of Boilers for Steam, Hot Water and High Temperature Heat Transfer Fluids, 3rd ed; BSI: London, UK, 1999.
- Lindstad, T.; Syvertsen1, M.; Ishak, R.J.; Arntzen, H.B.; Grøntvedt, P.O. The Influence of Alkalis on the Boudouard Reaction. In Proceedings of the Tenth International Ferroalloys Congress, Cape Town, South Africa, 1–4 February 2004; SAIMM: Johannesburg, South Africa, 2004. [Google Scholar]
- De, A.K. Environmental Chemistry, 3rd ed; Wiley Eastern: New Delhi, India, 1994; pp. 103–119. [Google Scholar]
- Pillay, A.E.; Fok, S.C.; Elkadi, M.; Stephen, S.; Manuel, J.; Khan, M.Z.; Unnithan, S. Engine emissions and performances with alternative biodiesels: A review. JSD 2012, 5, 59–73. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pillay, A.; Molki, A.; Elkadi, M.; Manuel, J.; Bojanampati, S.; Khan, M.; Stephen, S. Real-Time Study of Noxious Gas Emissions and Combustion Efficiency of Blended Mixtures of Neem Biodiesel and Petrodiesel. Sustainability 2013, 5, 2098-2107. https://doi.org/10.3390/su5052098
Pillay A, Molki A, Elkadi M, Manuel J, Bojanampati S, Khan M, Stephen S. Real-Time Study of Noxious Gas Emissions and Combustion Efficiency of Blended Mixtures of Neem Biodiesel and Petrodiesel. Sustainability. 2013; 5(5):2098-2107. https://doi.org/10.3390/su5052098
Chicago/Turabian StylePillay, Avin, Arman Molki, Mirella Elkadi, Johnson Manuel, Shrinivas Bojanampati, Mohammed Khan, and Sasi Stephen. 2013. "Real-Time Study of Noxious Gas Emissions and Combustion Efficiency of Blended Mixtures of Neem Biodiesel and Petrodiesel" Sustainability 5, no. 5: 2098-2107. https://doi.org/10.3390/su5052098




