Colloidal Mobilization and Fate of Trace Heavy Metals in Semi-Saturated Artificial Soil (OECD) Irrigated with Treated Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Leaching Experiments
2.2. Analytical Procedures
3. Results
3.1. Mobility Profiles in Soil
3.2. Leachates Characterization
3.3. Spectroscopic Characterization of Released Soil Colloids
4. Discussion
4.1. Colloidal Mobilization of HMs
4.2. Role and Nature of Colloidal Aggregates
4.3. Comparison of Results with the Literature
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ma, X.; Xue, X.; González-Mejía, A.; Garland, J.; Cashdollar, J. Sustainable water systems for the city of tomorrow—A conceptual framework. Sustainability 2015, 7, 12071. [Google Scholar] [CrossRef]
- Chen, W.; Bai, Y.; Zhang, W.; Lyu, S.; Jiao, W. Perceptions of different stakeholders on reclaimed water reuse: The case of beijing, china. Sustainability 2015, 7, 9696–9710. [Google Scholar] [CrossRef]
- Ilias, A.; Panoras, A.; Angelakis, A. Wastewater recycling in greece: The case of thessaloniki. Sustainability 2014, 6, 2876–2892. [Google Scholar] [CrossRef]
- Lu, J.; Wilson, P.C.; Wen, X.; Jin, Q.; Wu, J. Harmful chemicals in the environment: Measurement, fate, and remediation. J. Chem. 2015, 2015, 723749. [Google Scholar] [CrossRef]
- Nunes, J.; Ramos-Miras, J.; Lopez-Piñeiro, A.; Loures, L.; Gil, C.; Coelho, J.; Loures, A. Concentrations of available heavy metals in mediterranean agricultural soils and their relation with some soil selected properties: A case study in typical mediterranean soils. Sustainability 2014, 6, 9124. [Google Scholar] [CrossRef]
- Dixit, R.; Malaviya, D.; Pandiyan, K.; Singh, U.B.; Sahu, A.; Shukla, R.; Singh, B.P.; Rai, J.P.; Sharma, P.L.; Lade, H.; et al. Bioremediation of heavy metals from soil and aquatic environment: An overview of principles and criteria of fundamental processes. Sustainability 2015, 7, 2189. [Google Scholar] [CrossRef]
- Weng, L.; Fest, E.P.; Fillius, J.; Temminghoff, E.J.; van Riemsdijk, W.H. Transport of humic and fulvic acids in relation to metal mobility in a copper-contaminated acid sandy soil. Environ. Sci. Technol. 2002, 36, 1699–1704. [Google Scholar] [CrossRef] [PubMed]
- McGechan, M.; Lewis, D. Sw—Soil and water: Transport of particulate and colloid-sorbed contaminants through soil, part 1: General principles. Biosyst. Eng. 2002, 83, 255–273. [Google Scholar] [CrossRef]
- Kleber, M.; Eusterhues, K.; Keiluweit, M.; Mikutta, C.; Mikutta, R.; Nico, P.S. Mineral–organic associations: Formation, properties, and relevance in soil environments. Adv. Agron. 2015, 130, 1–140. [Google Scholar]
- Ryan, J.N.; Elimelech, M. Colloid mobilization and transport in groundwater. Colloids Surf. A Physicochem. Eng. Asp. 1996, 107, 1–56. [Google Scholar] [CrossRef]
- Pontoni, L.; van Hullebusch, E.D.; Fabbricino, M.; Esposito, G.; Pirozzi, F. Assessment of trace heavy metals dynamics during the interaction of aqueous solutions with the artificial oecd soil: Evaluation of the effect of soil organic matter content and colloidal mobilization. Chemosphere 2016, 163, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Piccolo, A. The supramolecular structure of humic substances: A novel understanding of humus chemistry and implications in soil science. Adv. Agron. 2002, 75, 57–134. [Google Scholar]
- De Jonge, L.W.; Kjaergaard, C.; Moldrup, P. Colloids and colloid-facilitated transport of contaminants in soils. Vadose Zone J. 2004, 3, 321–325. [Google Scholar]
- Pédrot, M.; Dia, A.; Davranche, M.; Bouhnik-Le Coz, M.; Henin, O.; Gruau, G. Insights into colloid-mediated trace element release at the soil/water interface. J. Colloid Interface Sci. 2008, 325, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, K.-P.; Maibach, H.I. Oecd guidelines for testing chemicals. In Dermatoxicology, 7th ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 303–305. [Google Scholar]
- Pescod, M. Wastewater Treatment and Use in Agriculture; Nations, Ed.; Fao Irrigation and Drainage Paper 47 United; FAO: Rome, Italy, 1992. [Google Scholar]
- American Public Health Association; American Water Works Association. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- Yamashita, Y.; Jaffé, R. Characterizing the interactions between trace metals and dissolved organic matter using excitation-emission matrix and parallel factor analysis. Environ. Sci. Technol. 2008, 42, 7374–7379. [Google Scholar] [CrossRef] [PubMed]
- Boguta, P.; D’Orazio, V.; Sokołowska, Z.; Senesi, N. Effects of selected chemical and physicochemical properties of humic acids from peat soils on their interaction mechanisms with copper ions at various phs. J. Geochem. Explor. 2016, 168, 119–126. [Google Scholar] [CrossRef]
- Boguta, P.; Sokołowska, Z. Interactions of zn(II) ions with humic acids isolated from various type of soils. Effect of ph, zn concentrations and humic acids chemical properties. PLoS ONE 2016, 11, e0153626. [Google Scholar] [CrossRef] [PubMed]
- Warrence, N.J.; Bauder, J.W.; Pearson, K.E. Basics of Salinity and Sodicity Effects on Soil Physical Properties; Departement of Land Resources and Environmental Sciences, Montana State University-Bozeman: Bozeman, MT, USA, 2002. [Google Scholar]
- Duan, R.; Sheppard, C.D.; Fedler, C.B. Short-term effects of wastewater land application on soil chemical properties. Water Air Soil Pollut. 2010, 211, 165–176. [Google Scholar] [CrossRef]
- Kretzschmar, R.; Sticher, H. Transport of humic-coated iron oxide colloids in a sandy soil: Influence of Ca2+ and trace metals. Environ. Sci. Technol. 1997, 31, 3497–3504. [Google Scholar] [CrossRef]
- Moreno-Jiménez, E.; Beesley, L.; Lepp, N.W.; Dickinson, N.M.; Hartley, W.; Clemente, R. Field sampling of soil pore water to evaluate trace element mobility and associated environmental risk. Environ. Pollut. 2011, 159, 3078–3085. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fabbricino, M.; Benedetti, M.F.; Korshin, G.V. Spectroscopic in situ examination of interactions of rare earth ions with humic substances. Water Res. 2015, 68, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.C.; Mills, R.B.; Evans, R.D.; Dillon, P.J. Kinetics of metal-fulvic acid complexation using a stopped-flow technique and three-dimensional excitation emission fluorescence spectrophotometer. Anal. Chem. 2004, 76, 110–113. [Google Scholar] [CrossRef]
- Lam, B.; Simpson, A.J. Direct 1h nmr spectroscopy of dissolved organic matter in natural waters. Analyst 2008, 133, 263–269. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, J.F.; Zachara, J.M. Subsurface transport of contaminants. Environ. Sci. Technol. 1989, 23, 496–502. [Google Scholar] [CrossRef]
- Klitzke, S.; Lang, F. Hydrophobicity of soil colloids and heavy metal mobilization. J. Environ. Qual. 2007, 36, 1187–1193. [Google Scholar] [CrossRef] [PubMed]
- Covelo, E.F.; Vega, F.A.; Andrade, M.L. Competitive sorption and desorption of heavy metals by individual soil components. J. Hazard. Mater. 2007, 140, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Flogeac, K.; Guillon, E.; Aplincourt, M. Surface complexation of copper(II) on soil particles: Epr and xafs studies. Environ. Sci. Technol. 2004, 38, 3098–3103. [Google Scholar] [CrossRef] [PubMed]
- Grybos, M.; Davranche, M.; Gruau, G.; Petitjean, P. Is trace metal release in wetland soils controlled by organic matter mobility or fe-oxyhydroxides reduction? J. Colloid Interface Sci. 2007, 314, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Temminghoff, E.J.; van der Zee, S.E.; de Haan, F.A. Copper mobility in a copper-contaminated sandy soil as affected by pH and solid and dissolved organic matter. Environ. Sci. Technol. 1997, 31, 1109–1115. [Google Scholar] [CrossRef]
- Sposito, G. The Chemistry of Soils; Oxford University Press: Oxford, UK, 2008. [Google Scholar]
- Kelleher, B.P.; Simpson, A.J. Humic substances in soils: Are they really chemically distinct? Environ. Sci. Technol. 2006, 40, 4605–4611. [Google Scholar] [CrossRef] [PubMed]
- Renella, G.; Landi, L.; Nannipieri, P. Degradation of low molecular weight organic acids complexed with heavy metals in soil. Geoderma 2004, 122, 311–315. [Google Scholar] [CrossRef]
- Schwab, A.; Zhu, D.; Banks, M. Influence of organic acids on the transport of heavy metals in soil. Chemosphere 2008, 72, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Gerritse, R.G. Column-and catchment-scale transport of cadmium: Effect of dissolved organic matter. J. Contam. Hydrol. 1996, 22, 145–163. [Google Scholar] [CrossRef]
- Plassard, F.; Winiarski, T.; Petit-Ramel, M. Retention and distribution of three heavy metals in a carbonated soil: Comparison between batch and unsaturated column studies. J. Contam. Hydrol. 2000, 42, 99–111. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, L. Cadmium transport mediated by soil colloid and dissolved organic matter: A field study. J. Environ. Sci. 2010, 22, 106–115. [Google Scholar] [CrossRef]
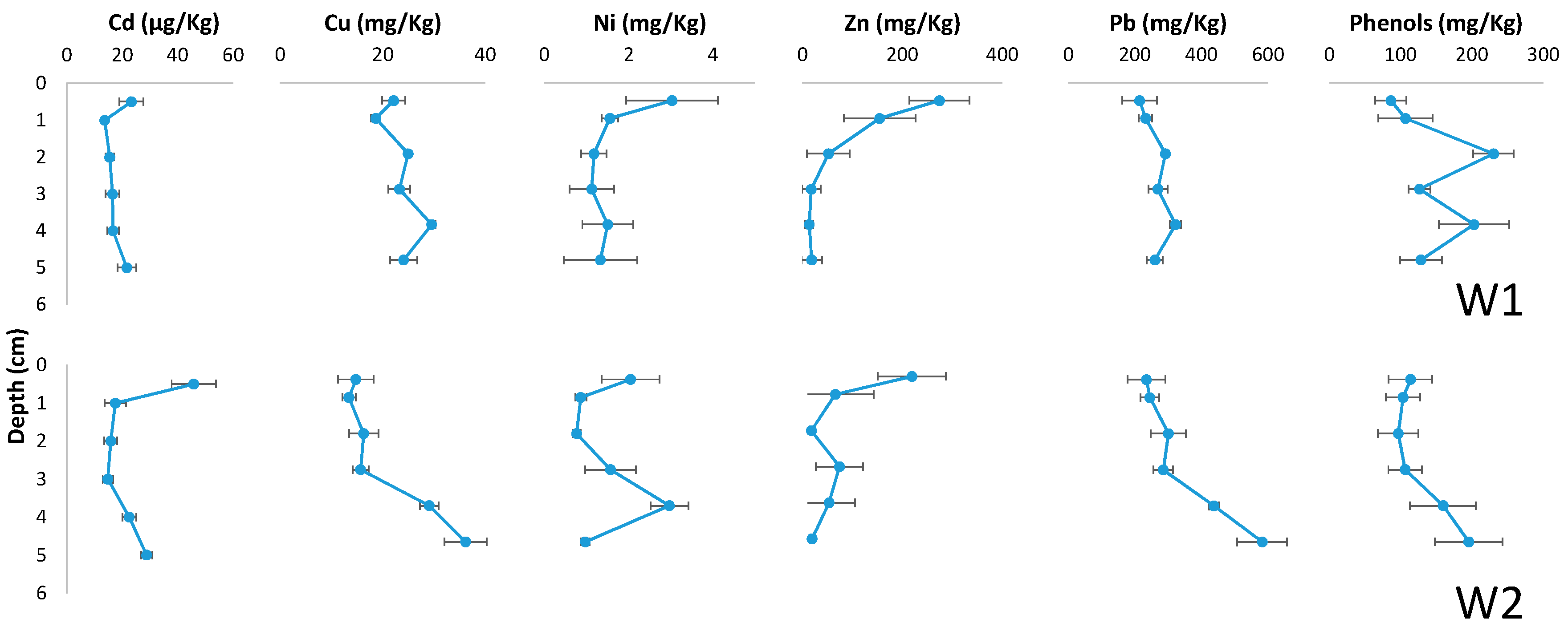
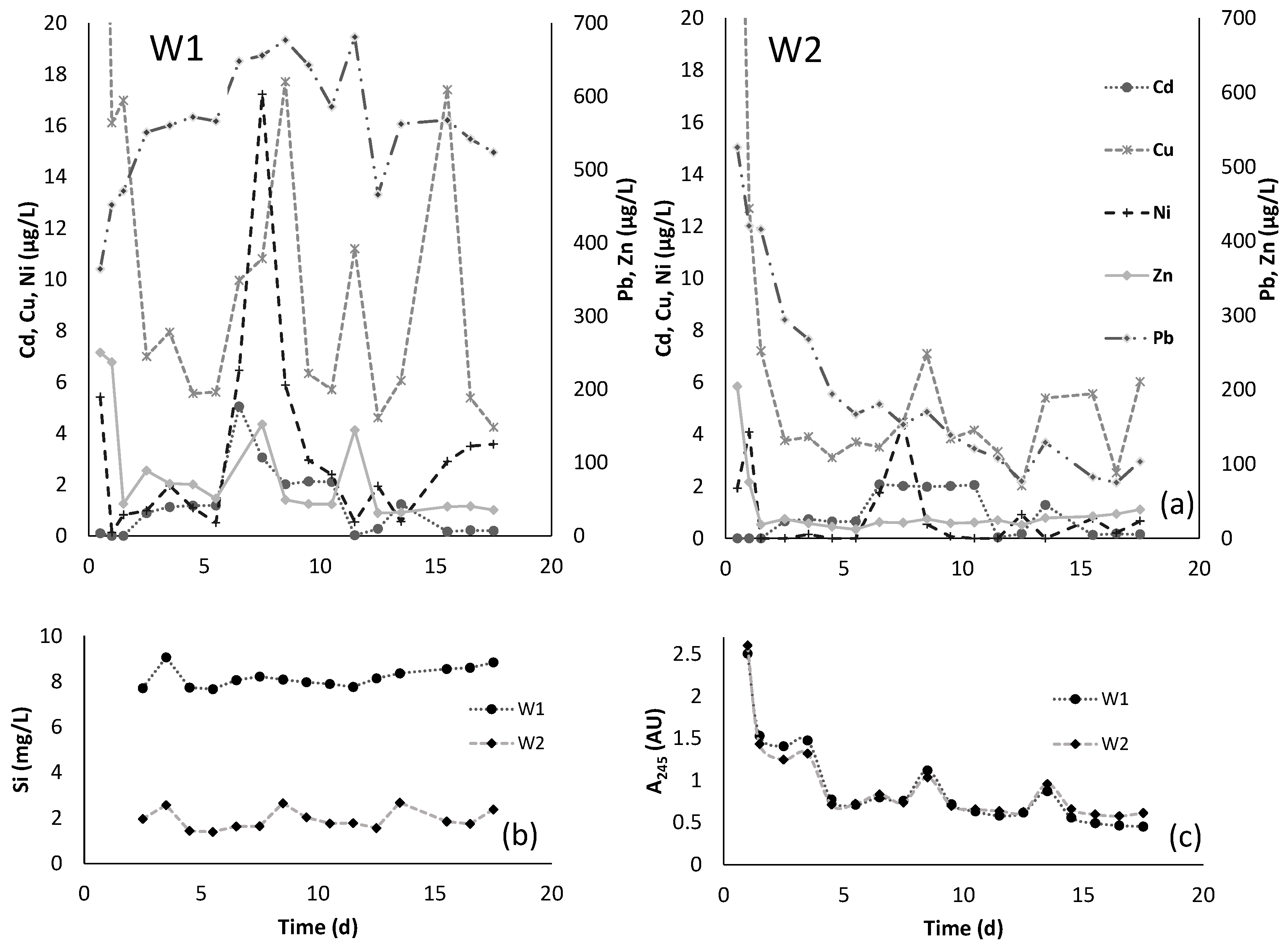
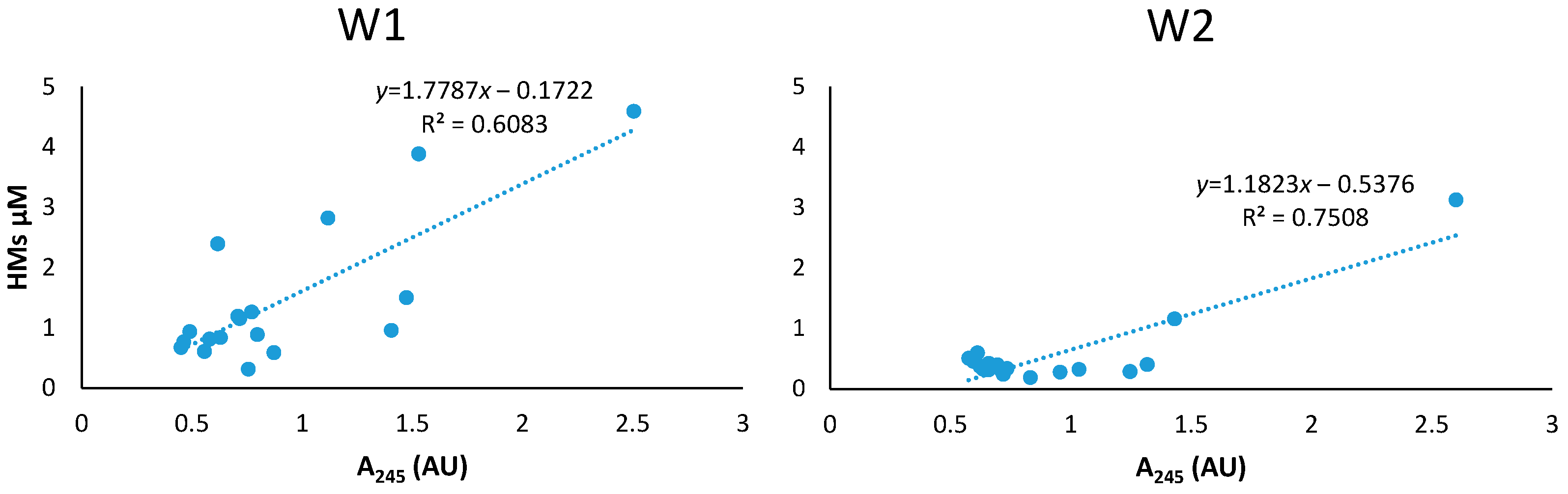

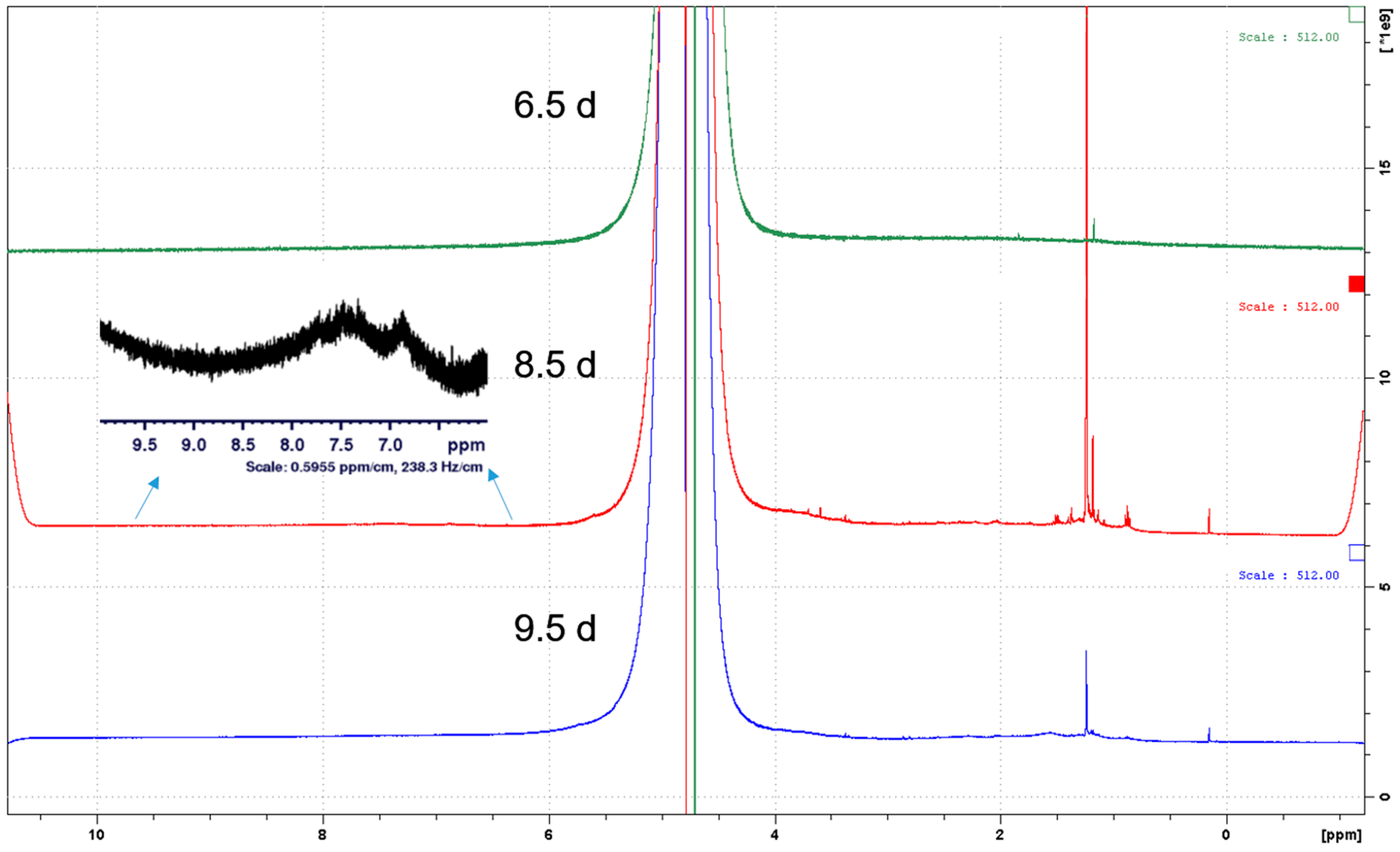
| Parameter | OECD Soil (mg/Kg) | W1 (µg/L) | W2 (µg/L) |
|---|---|---|---|
| Al | 118 | - | |
| Ca | 1.11 × 105 | - | |
| Cd | 0.0363 | 0.232 | 0.25 |
| Co | <0.5 | - | |
| Cr | ND | - | |
| Cu | 23.827 | 8.73 | 10 |
| K | 8.62 × 104 | - | |
| Mg | 1.34 × 105 | - | |
| Mn | 153 | - | |
| Na | 3.5 × 105 | - | |
| Ni | 1.525 | 1.31 | 1.5 |
| Pb | 499.3 | ND | - |
| Si | 2530 | ||
| Sr | 928 | - | |
| Zn | 89.0 | 24.82 | 25 |
| pH | 7.6 | 6.8 | 6.8 |
| DOM (as TOC) | 6.71 × 103 | - |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pontoni, L.; Van Hullebusch, E.D.; Pechaud, Y.; Fabbricino, M.; Esposito, G.; Pirozzi, F. Colloidal Mobilization and Fate of Trace Heavy Metals in Semi-Saturated Artificial Soil (OECD) Irrigated with Treated Wastewater. Sustainability 2016, 8, 1257. https://doi.org/10.3390/su8121257
Pontoni L, Van Hullebusch ED, Pechaud Y, Fabbricino M, Esposito G, Pirozzi F. Colloidal Mobilization and Fate of Trace Heavy Metals in Semi-Saturated Artificial Soil (OECD) Irrigated with Treated Wastewater. Sustainability. 2016; 8(12):1257. https://doi.org/10.3390/su8121257
Chicago/Turabian StylePontoni, Ludovico, Eric D. Van Hullebusch, Yoan Pechaud, Massimiliano Fabbricino, Giovanni Esposito, and Francesco Pirozzi. 2016. "Colloidal Mobilization and Fate of Trace Heavy Metals in Semi-Saturated Artificial Soil (OECD) Irrigated with Treated Wastewater" Sustainability 8, no. 12: 1257. https://doi.org/10.3390/su8121257





