Hydrogeochemistry of Groundwater and Arsenic Adsorption Characteristics of Subsurface Sediments in an Alluvial Plain, SW Taiwan
Abstract
:1. Introduction
2. Materials and Methods
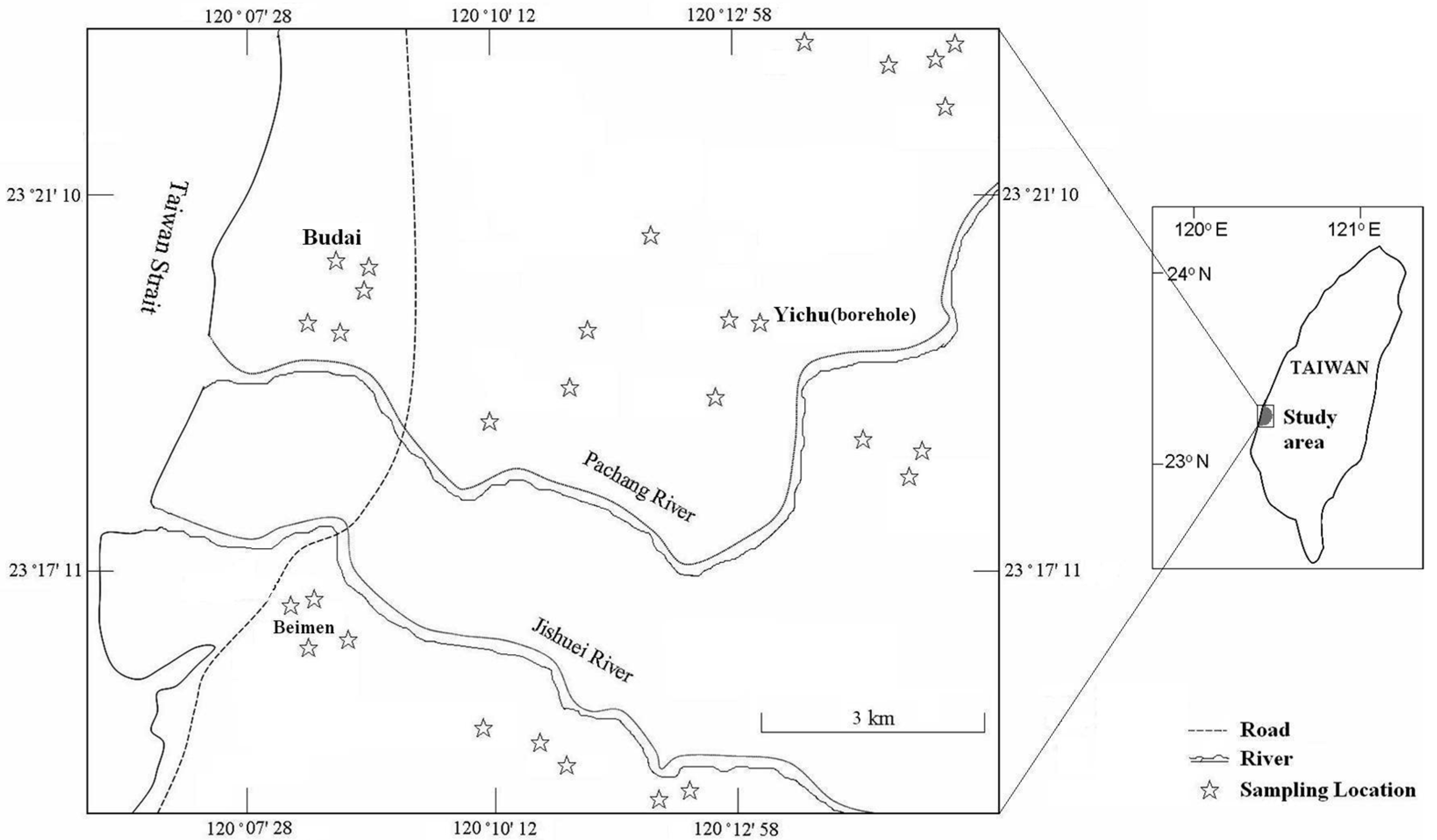
2.1. Geologic Setting
2.2. Drilling and Sediment Collection
2.3. Core Sediments Characterization
2.4. Groundwater Sampling and Analysis
2.5. Sequential Extraction Procedures
2.6. Arsenic Speciation
2.7. Batch Study for As Adsorption
3. Results and Discussion
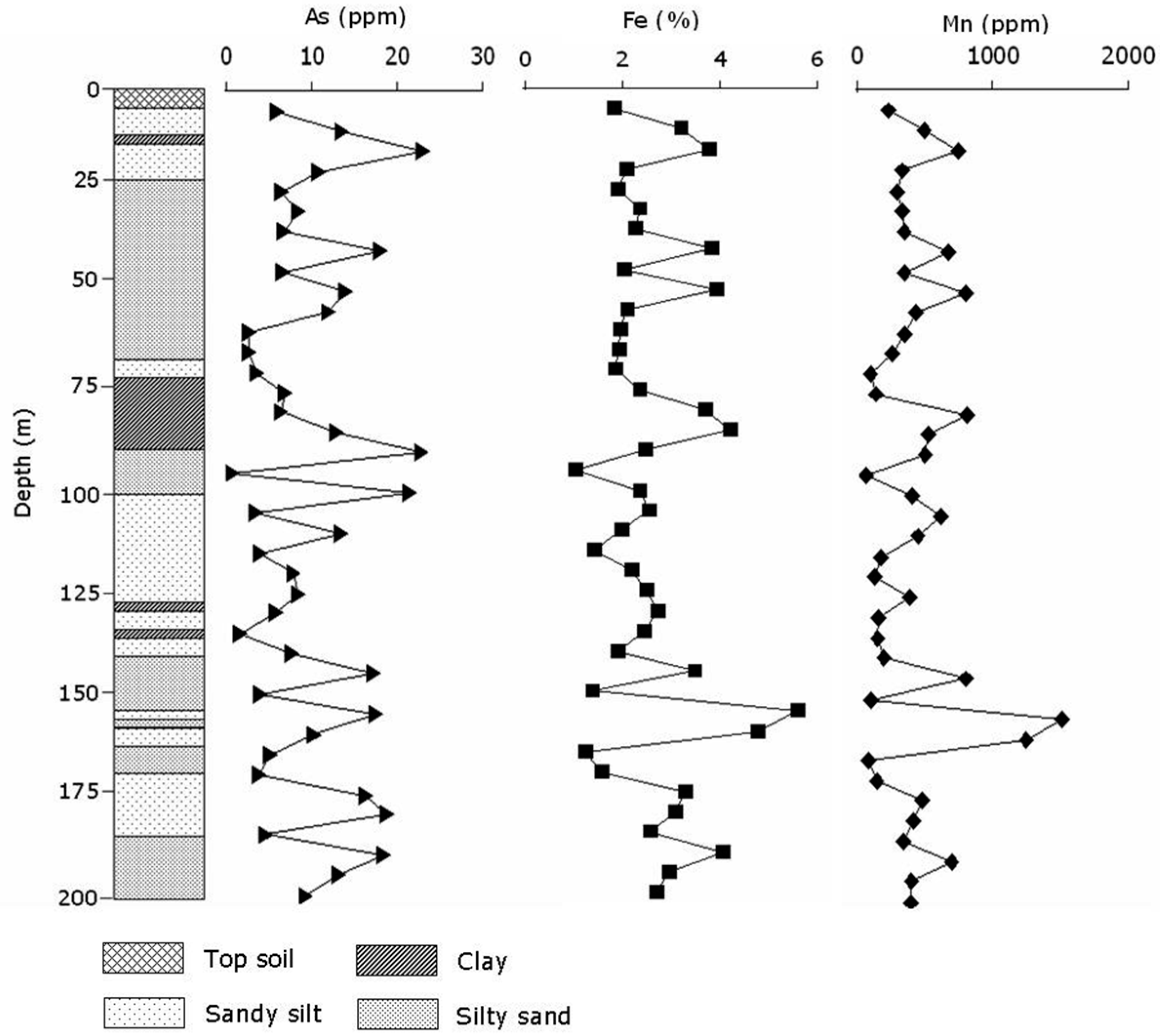
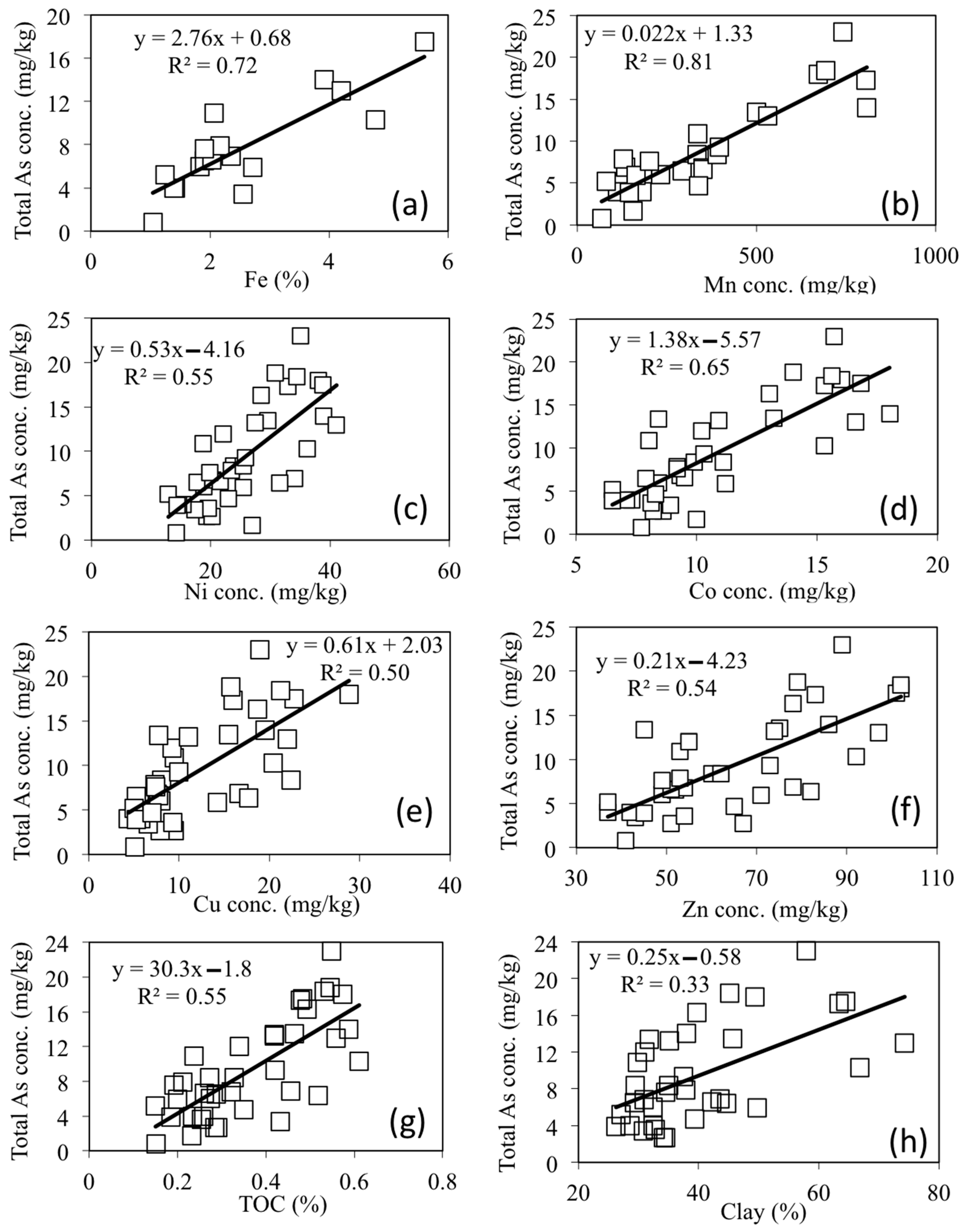
3.1. Chemical Characterization of Sediments
3.2. Hydrogeochemistry and Occurrences of As in Groundwater
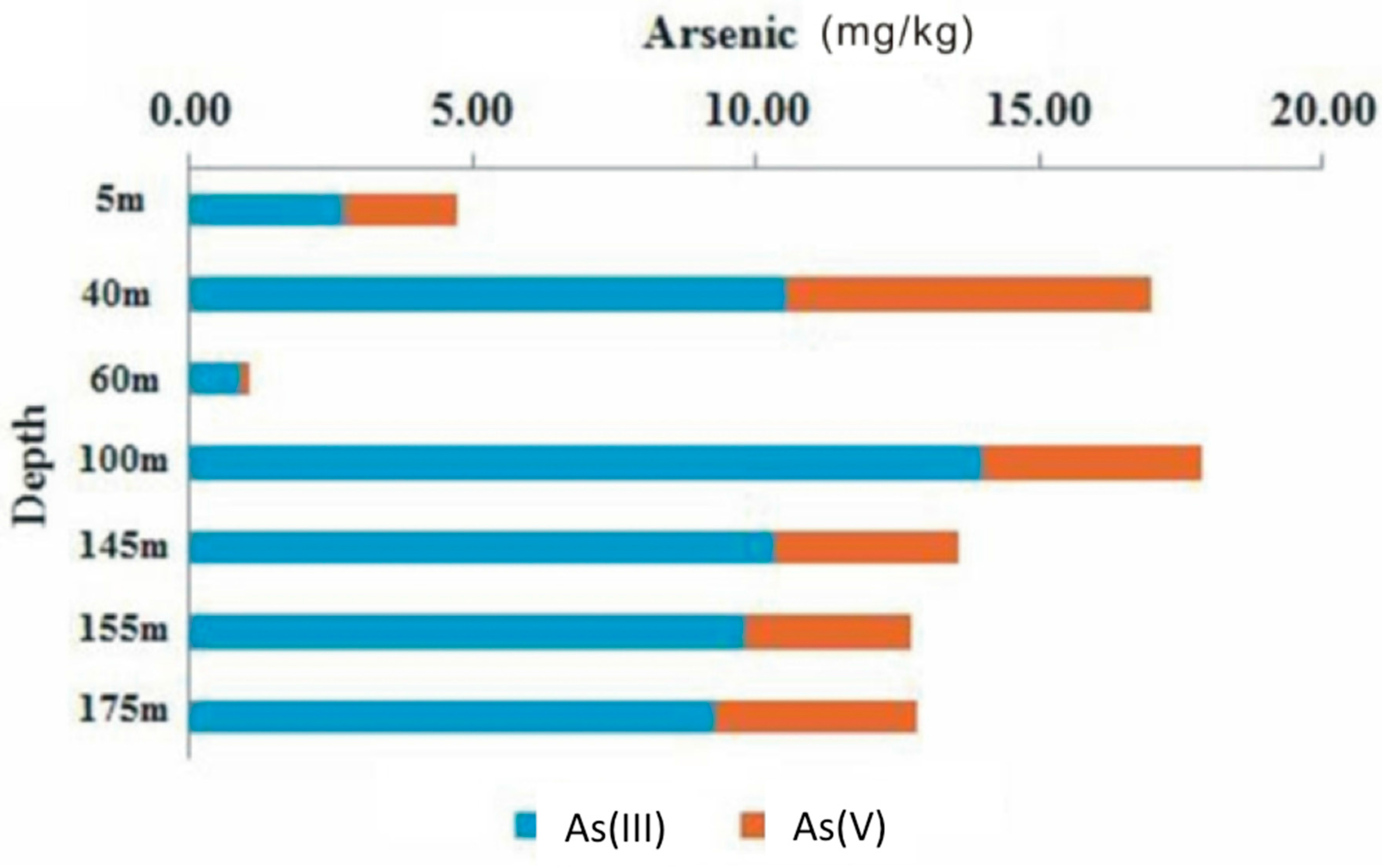
3.3. Solid Phase Partitioning of As and Speciation
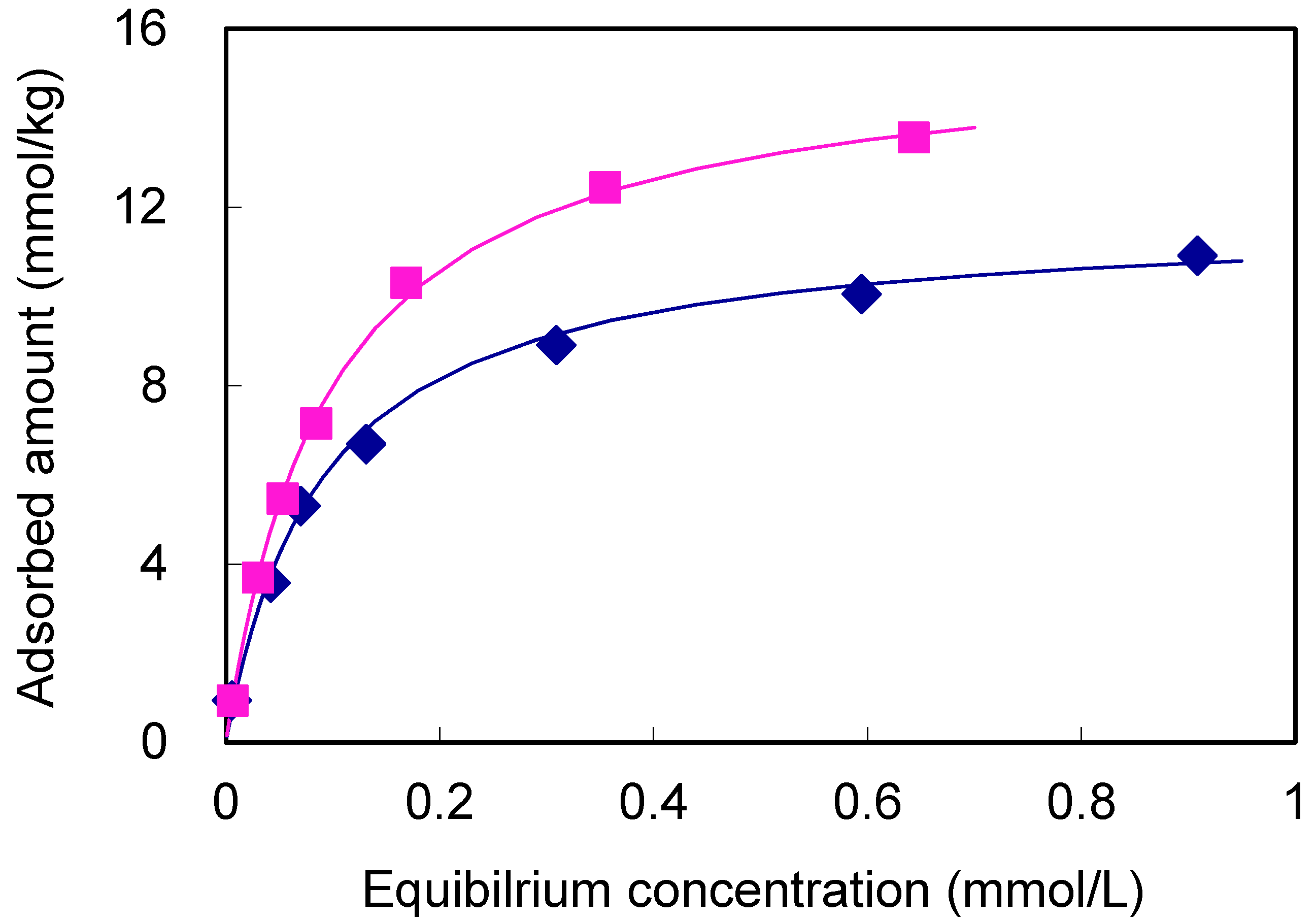
3.4. Adsorption Characteristics of Arsenic in Sediment and Its Implication on Mobilization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tseng, W.-P. Effects and dose-response relationships of skin cancer and blackfoot disease with arsenic. Environ. Health Perspect. 1977, 19, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-L.; Dzeng, S.R.; Yang, M.-H. Arsenic species in groundwaters of Blackfoot Disease area, Taiwan. Environ. Sci. Technol. 1994, 28, 877–881. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C. Accumulation and Release of Arsenic in Sediments from Hsindong and Jinhu in Chianan Plain, Taiwan. Master’s Thesis, Department of Geosciences, National Taiwan University, Taipei, Taiwan, 2003. [Google Scholar]
- Wang, S.-W.; Liu, C.-W.; Jang, C.-S. Factors responsible for high arsenic concentrations in two groundwater catchments in Taiwan. Appl. Geochem. 2007, 22, 460–476. [Google Scholar] [CrossRef]
- Nath, B.; Jean, J.-S.; Lee, M.-K.; Yang, H.-J.; Liu, C.-C. Geochemistry of high arsenic groundwater in Chia-Nan plain, Southwestern Taiwan: Possible sources and reactive transport of arsenic. J. Contam. Hydrol. 2008, 99, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Reza, R.A.H.M.; Jean, J.-S.; Yang, H.-J.; Lee, M.-K.; Hsu, H.-F.; Liu, C.-C.; Lee, Y.-C.; Bundschuh, J.; Lin, K.-H.; Lee, C.-Y. A comparative study on arsenic and humic substances in alluvial aquifers of Bengal delta plain (NW Bangladesh), Chianan plain (SW Taiwan) and Lanyang plain (NE Taiwan): Implication of arsenic mobilization mechanisms. Environ. Geochem. Health 2011, 33, 235–258. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-B.; Lin, Y.-P.; Liu, C.-W.; Tan, Y.-C. Mapping of spatial multi-scale sources of arsenic variation in groundwater on Chia-Nan floodplain of Taiwan. Sci. Total Environ. 2006, 370, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Saunders, J.A.; Lee, M.-K.; Uddin, A.S.; Mohammad, S.; Wilkin, R.; Fayek, M.; Korte, N. Natural arsenic contamination of Holocene alluvial aquifers by linked glaciation, weathering, and microbial processes. Geochem. Geophys. Geosyst. 2005, 6, 1–7. [Google Scholar] [CrossRef]
- Sengupta, S.; Sracek, O.; Jean, J.-S.; Lu, H.-Y.; Wang, C.-H.; Palcsu, L.; Liu, C.-C.; Jen, C.-H.; Bhattacharya, P. Spatial variation of groundwater arsenic distribution in Chianan Plain, SW Taiwan: Role of local hydrogeological factors and geothermal sources. J. Hydrol. 2014, 518, 393–409. [Google Scholar] [CrossRef]
- Sadiq, M. Arsenic chemistry in soils: An overview of thermodynamic predictions and field observations. Water Air Soil Pollut. 1997, 93, 117–136. [Google Scholar] [CrossRef]
- Keon, N.E.; Swartz, C.H.; Brabander, D.J.; Harvey, C.; Hemond, H.F. Validation of an arsenic sequential extraction method for evaluating mobility in sediment. Environ. Sci. Technol. 2001, 35, 2778–2784. [Google Scholar] [CrossRef] [PubMed]
- O’day, P.A.; Vlassopoulos, D.; Root, R.; Rivera, N. The influence of sulfur and iron on dissolved arsenic concentrations in the shallow subsurface under changing redox conditions. Proc. Natl. Acad. Sci. USA 2004, 101, 13703–13708. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.-L.; Liu, C.-W.; Wang, S.-W.; Jang, C.-S.; Lin, K.-H.; Vivian-Liao, H.-C.; Liao, C.-M.; Chang, F.-J. Assessing the characteristics of groundwater quality of arsenic contaminated aquifers in the blackfoot disease endemic area. J. Hazard. Mater. 2011, 185, 1458–1466. [Google Scholar] [CrossRef] [PubMed]
- Yu, X. Humic acids from endemic arsenicosis areas in Inner Mongolia and from the Blackfoot-disease areas in Taiwan: A comparative study. Environ. Geochem. Health 2001, 23, 27–42. [Google Scholar]
- Gee, G.W.; Or, D. Particle-size analysis. In Soil Science Society of America Book Series: Methods of Soil Analysis. Part 4. Physical Methods; Dane, J.H., Topp, G.C., Eds.; Soil Science Society of America: Madison, WI, USA, 2002; Volume 5, pp. 255–293. [Google Scholar]
- Matera, V.; Le Hecho, I.; Laboudigue, A.; Thomas, P.; Tellier, S.; Astruc, M. A methodological approach for the identification of arsenic bearing phases in polluted soils. Environ. Pollut. 2003, 126, 51–64. [Google Scholar] [CrossRef]
- Georgiadis, M.; Cai, Y.; Solo-Gabriele, H.M. Extraction of arsenate and arsenite species from soils and sediments. Environ. Pollut. 2006, 141, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Loeppert, R.H.; Jain, A.; El-Haleem, M.A.A.; Biswas, B.K. Quantity and speciation of arsenic in soils by chemical extraction. In Biogeochemistry of Environmentally Important Trace Elements; Cai, Y., Braids, O.C., Eds.; American Chemical Society: Washington, DC, USA, 2002; p. 436. [Google Scholar]
- McArthur, J.M.; Banerjee, D.M.; Hudson-Edwards, K.A.; Mishra, R.; Purohit, R.; Ravenscroft, P.; Cronin, A.; Howarth, R.J.; Chatterjee, A.; Talukder, T.; et al. Natural organic matter in sedimentary basins and its relation to arsenic in anoxic ground water: The example of West Bengal and its worldwide implications. Appl. Geochem. 2004, 19, 1255–1293. [Google Scholar] [CrossRef]
- Li, Z.; Hong, H.; Jean, J.-S.; Koski, A.J.; Liu, C.-C.; Reza, S.A.H.M.; Randolph, J.J.; Kurdas, S.R.; Friend, J.H.; Antinucci, S.J. Characterization on arsenic sorption and mobility of the sediments of Chia-Nan Plain, where Blackfoot disease occurred. Environ. Earth Sci. 2011, 64, 823–831. [Google Scholar] [CrossRef]
- Jeong, C.H. Effect of land use and urbanization on hydrochemistry and contamination of groundwater from Taejon area, Korea. J. Hydrol. 2001, 253, 194–210. [Google Scholar] [CrossRef]
- Luo, J.; Ye, Y.; Gao, Z.; Wang, Y.; Wang, W. Trace element (Pb, Cd, and As) contamination in the sediments and organisms in Zhalong Wetland, Northeastern China. Soil Sediment Contam. Int. J. 2016, 25, 395–407. [Google Scholar] [CrossRef]
- Du, Y.; Meng, F.; Fu, W.; Wang, Z. Distribution, speciation and bioaccumulation of Hg and As in mariculture sediments from Dongshan Bay, China. Soil Sediment Contam. Int. J. 2016, 25, 489–504. [Google Scholar] [CrossRef]
- Acharya, S.K.; Lahiri, S.; Raymahashay, B.C.; Bhowmik, A. Arsenic toxicity of groundwater in parts of the Bengal basin in India and Bangladesh: The role of quarternary stratigraphy and holocene sea-level fluctuation. Environ. Geol. 2000, 39, 1127–1137. [Google Scholar] [CrossRef]
- Oh, S.Y.; Yoon, M.K. Chemical extraction of arsenic and heavy metals from contaminated soils under high temperature and pressure conditions in abandoned mines in South Korea. Soil Sediment Contam. Int. J. 2015, 24, 423–436. [Google Scholar] [CrossRef]
- Klaus, P.R.; Jain, A.; Loeppert, R.H. Arsenite and arsenate adsorption on ferrihydrite: Kinetics, equilibrium, and adsorption envelopes. Environ. Sci. Technol. 1998, 32, 344–349. [Google Scholar]
- Woolson, E.A. Emission, cycling and effects of arsenic in soil ecosystems. In Biological and Environmental Effects of Arsenic; Fowler, B.A., Ed.; Elsevier Science Publisher B.V.: Amsterdam, The Netherlands; New York, NY, USA; Oxford, UK, 1983; pp. 51–120. [Google Scholar]
- Hung, J.-J.; Lu, C.-C.; Huh, C.-A.; Lin, J.-T. Geochemical controls on distributions and speciation of As and Hg in sediments along the Gaoping (Kaoping) Estuary–Canyon system off southwestern Taiwan. J. Mar. Syst. 2009, 76, 479–495. [Google Scholar] [CrossRef]
- Liu, C.-W.; Lin, K.-H.; Kuo, Y.-M. Application of factor analysis in the assessment of groundwater quality in a blackfoot disease area in Taiwan. Sci. Total Environ. 2003, 313, 77–89. [Google Scholar] [CrossRef]
- Hong, H.; Yin, K.; Lai, X.; Du, Y.; Li, Z.; Jean, J.-S. Occurrence of Arsenic in Mudstone of the Endemic Blackfoot Disease Region, Taiwan. In Arsenic in Geosphere and Human Diseases; Taylor & Francis Group: London, UK, 2010; pp. 556–557. [Google Scholar]
- Stollenwerk, K.G.; Breit, G.N.; Welch, A.H.; Yount, J.C.; Whitney, J.W.; Foster, A.L.; Uddin, M.N.; Majumder, R.K.; Ahmed, N. Arsenic attenuation by oxidized aquifer sediments in Bangladesh. Sci. Total Environ. 2007. [Google Scholar] [CrossRef] [PubMed]


| Sample Depth (m) | As (mg/kg) | Fe (%) | Mn (mg/kg) | Ni (mg/kg) | Co (mg/kg) | Pb (mg/kg) | Zn (mg/kg) | Cu (mg/kg) | Ca (%) | Al (%) | P (%) | Mg (%) | K (%) | NH4 (mg/kg) | Cl− (mg/kg) | NO3− (mg/kg) | HCO3− (mg/kg) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 | 6 | 1.83 | 234 | 18.8 | 8.4 | 9.1 | 49 | 8 | NA | NA | NA | NA | NA | 2.29 | 0.41 | 105.4 | 57.42 |
| 10 | 13.5 | 3.19 | 500 | 29.6 | 13.2 | 18.1 | 75 | 15.5 | 0.41 | 1.09 | 0.036 | 0.43 | 0.18 | 2.04 | 2.56 | 6.98 | 115.0 |
| 15 | 23 | 3.77 | 742 | 35.1 | 15.7 | 22.3 | 89 | 18.9 | 0.93 | 1.56 | 0.045 | 0.8 | 0.2 | 3.47 | 0.81 | 10.65 | 63.82 |
| 20 | 2.07 | 337 | 18.7 | 8 | 12 | 53 | 9.5 | 1.05 | 1.71 | 0.049 | 0.92 | 0.2 | 2.58 | 0.62 | 16.92 | 64.49 | |
| 25 | 1.9 | 294 | 17.7 | 7.9 | 8.3 | 52 | 5.3 | 0.74 | 0.95 | 0.032 | 0.48 | 0.12 | 2.51 | 0.35 | 6.07 | 67.97 | |
| 30 | 2.35 | 334 | 23.8 | 9.9 | 11.1 | 60 | 8.1 | 0.75 | 0.98 | 0.031 | 0.52 | 0.12 | 2.99 | 1.27 | 0.776 | 81.91 | |
| 35 | 2.26 | 351 | 20.4 | 9.3 | 11 | 54 | 7.6 | 0.82 | 1.16 | 0.038 | 0.62 | 0.13 | 3.36 | 1.11 | 0.745 | 77.33 | |
| 40 | 3.81 | 672 | 38.1 | 16 | 28.2 | 102 | 28.8 | 0.9 | 1.08 | 0.042 | 0.62 | 0.13 | 3.81 | 11.29 | 0.454 | 134.2 | |
| 45 | 2.03 | 351 | 21.6 | 9.5 | 12.2 | 51 | 7.7 | 1.23 | 1.8 | 0.049 | 1.05 | 0.17 | BDL | 9.04 | 0.217 | 92.42 | |
| 50 | 3.92 | 808 | 38.9 | 18 | 21.9 | 86 | 19.5 | 1.01 | 0.99 | 0.069 | 0.62 | 0.13 | 5.66 | 7.54 | 0.195 | 50.54 | |
| 55 | 2.1 | 431 | 22.1 | 10.2 | 11.5 | 55 | 9.2 | 1.2 | 1.83 | 0.052 | 1.07 | 0.21 | 7.90 | 3.57 | 31.67 | 71.16 | |
| 60 | 1.96 | 354 | 19.4 | 8.6 | 10.4 | 51 | 9.5 | 1.09 | 1 | 0.035 | 0.63 | 0.13 | 7.90 | 6.26 | 1.857 | 83.47 | |
| 65 | 1.92 | 262 | 20.2 | 8.2 | 10.3 | 67 | 7.9 | 0.93 | 1.04 | 0.043 | 0.55 | 0.17 | 3.39 | 1.84 | 41.11 | 65.74 | |
| 70 | 1.86 | 99 | 19.6 | 8.1 | 8.6 | 54 | 9.3 | 0.8 | 0.98 | 0.036 | 0.52 | 0.12 | 3.65 | 0.34 | 4.29 | 81.33 | |
| 75 | 2.36 | 136 | 34 | 17.4 | 18.4 | 78 | 16.6 | 0.12 | 1.05 | 0.036 | 0.37 | 0.14 | 10.73 | 1.15 | 189.3 | 81.33 | |
| 80 | 3.69 | 815 | 31.6 | 14 | 18.8 | 82 | 17.7 | 0.18 | 1.44 | 0.043 | 0.51 | 0.13 | 2.78 | 0.71 | 82.06 | 86.42 | |
| 85 | 4.21 | 531 | 41.1 | 16.6 | 21.8 | 97 | 22 | 0.57 | 1.68 | 0.055 | 0.71 | 0.14 | 5.61 | 0.76 | 74.12 | 36.6 | |
| 90 | 2.46 | 504 | 21.9 | 10.4 | 12.1 | 57 | 10 | 0.67 | 2.03 | 0.046 | 0.96 | 0.18 | 2.72 | 0.33 | 73.09 | 34.31 | |
| 95 | 1.04 | 69 | 14.2 | 7.7 | 8.4 | 41 | 5.1 | 0.1 | 1.25 | 0.038 | 0.36 | 0.15 | 5.27 | 0.64 | 5.45 | 31.47 | |
| 100 | 2.35 | 411 | 21.1 | 9.8 | 12 | 52 | 8.5 | 0.07 | 0.58 | 0.013 | 0.19 | 0.1 | 3.09 | 0.58 | 0.184 | 76.45 | |
| 105 | 2.55 | 622 | 17.4 | 8.9 | 9.9 | 43 | 6.6 | 0.14 | 1.22 | 0.051 | 0.33 | 0.14 | 3.21 | 0.39 | 3.113 | 75.08 | |
| 110 | 1.98 | 448 | 18.7 | 8.4 | 10.3 | 45 | 7.7 | 0.17 | 0.88 | 0.026 | 0.29 | 0.12 | 2.99 | 0.23 | 59.77 | 87.39 | |
| 115 | 1.42 | 181 | 15.4 | 7.3 | 8.1 | 42 | 5.8 | 0.45 | 0.85 | 0.025 | 0.38 | 0.11 | 6.45 | 0.32 | 77.08 | 129.62 | |
| 120 | 2.17 | 129 | 23.4 | 9.2 | 11.3 | 53 | 7.3 | 0.36 | 0.78 | 0.022 | 0.33 | 0.12 | 4.6 | 0.18 | 60.81 | 17.08 | |
| 125 | 2.48 | 391 | 25.5 | 11.1 | 12.3 | 62 | 22.4 | 0.11 | 0.99 | 0.032 | 0.42 | 0.13 | 1.59 | 0.32 | 76.34 | 34.56 | |
| 130 | 2.72 | 164 | 25.5 | 11.2 | 13.4 | 71 | 14.2 | 0.82 | 1.22 | 0.045 | 0.56 | 0.14 | 1.58 | 0.31 | 32.26 | 130.1 | |
| 135 | 2.43 | 157 | 26.9 | 10 | 16.1 | 85 | 13.3 | 0.08 | 1.54 | 0.038 | 0.47 | 0.17 | 2.22 | 0.23 | 18.02 | 45.75 | |
| 140 | 1.91 | 203 | 19.9 | 9.2 | 12 | 49 | 7.4 | 0.12 | 1.45 | 0.026 | 0.55 | 0.15 | 2.56 | 0.32 | 34.06 | 41.37 | |
| 145 | 3.47 | 805 | 32.9 | 15.3 | 19.1 | 83 | 16 | 0.12 | 0.95 | 0.034 | 0.37 | 0.12 | 4.89 | 0.34 | 10.53 | 128.7 | |
| 150 | 1.39 | 112 | 14.6 | 7.1 | 8 | 37 | 4.4 | 1.12 | 1.59 | 0.048 | 0.83 | 0.14 | 2.41 | 0.16 | 6.89 | 82.59 | |
| 155 | 5.6 | 1511 | 38.8 | 16.8 | 24.5 | 101 | 22.7 | 0.08 | 0.69 | 0.027 | 0.27 | 0.1 | 3.24 | 0.24 | 3.71 | 30.84 | |
| 160 | 4.78 | 1248 | 36.2 | 15.3 | 26.3 | 92 | 20.4 | 1.73 | 1.9 | 0.125 | 0.91 | 0.17 | 7.38 | 0.07 | 5.384 | BDL | |
| 165 | 1.24 | 82 | 12.9 | 6.5 | 6.5 | 37 | 5 | 0.98 | 1.81 | 0.102 | 0.88 | 0.17 | 1.99 | 0.15 | 4.148 | 97.6 | |
| 170 | 1.58 | 146 | 14.4 | 6.5 | 7.4 | 45 | 5.3 | 0.05 | 0.69 | 0.013 | 0.25 | 0.1 | 63.46 | 3.53 | 3.415 | 106.5 | |
| 175 | 3.27 | 478 | 28.5 | 13 | 20.1 | 78 | 18.7 | 0.22 | 0.83 | 0.02 | 0.34 | 0.1 | 81.35 | 8.05 | 6.05 | 76.68 | |
| 180 | 3.08 | 422 | 30.8 | 14 | 18 | 79 | 15.7 | 0.95 | 1.5 | 0.044 | 0.8 | 0.14 | 64.42 | 4.11 | 3.191 | 57.51 | |
| 185 | 2.57 | 338 | 23 | 8.3 | 11.6 | 65 | 7 | 0.91 | 1.44 | 0.042 | 0.8 | 0.13 | 53.68 | 4.29 | 11.79 | 75.08 | |
| 190 | 4.05 | 694 | 34.4 | 15.6 | 33.5 | 102 | 21.2 | 0.75 | 1.33 | 0.038 | 0.7 | 0.15 | 2.29 | 0.40 | 105.4 | 57.42 | |
| 195 | 2.95 | 400 | 27.4 | 10.9 | 17.4 | 74 | 11 | 1.07 | 1.97 | 0.05 | 1.08 | 0.19 | 2.04 | 2.56 | 6.98 | 115.0 | |
| 200 | 2.7 | 398 | 25.8 | 10.3 | 15.7 | 73 | 10 | 0.87 | 1.46 | 0.047 | 0.77 | 0.17 | 3.47 | 0.81 | 10.65 | 63.82 | |
| Min | 0.80 | 1.04 | 69 | 12.9 | 6.5 | 6.5 | 37.0 | 4.4 | 0.05 | 0.58 | 0.013 | 0.19 | 0.10 | 0 | 0.07 | 0.184 | 0 |
| Max | 22.8 | 5.60 | 1511 | 41.1 | 18.0 | 33.5 | 102 | 28.8 | 1.73 | 2.03 | 0.125 | 1.08 | 0.21 | 81.4 | 11.3 | 189.3 | 134.2 |
| Mean | 9.93 | 2.64 | 429.1 | 25.0 | 11.0 | 14.7 | 65.5 | 12.2 | 0.63 | 1.26 | 0.042 | 0.60 | 0.14 | 9.94 | 1.96 | 29.78 | 72.6 |
| STD | 6.20 | 1.00 | 306.6 | 7.82 | 3.4 | 6.36 | 19.3 | 6.29 | 0.44 | 0.39 | 0.020 | 0.25 | 0.03 | 19.2 | 2.79 | 40.99 | 31.8 |
| Median | 8.15 | 2.40 | 372.5 | 23.2 | 9.95 | 12.0 | 61.0 | 9.50 | 0.75 | 1.22 | 0.038 | 0.55 | 0.14 | 3.30 | 0.63 | 8.76 | 75.1 |
| Sample Location | Latitude/Longitude | Depth (m) | Temp (°C) | EC (mS/cm) | TDS (mg/L) | Salinity (‰) | pH | ORP (mV) | DOC (mg/L) |
|---|---|---|---|---|---|---|---|---|---|
| Beimen 2B | N 23°17′23.4″/E 120°8′57.8″ | 60 | 24.8 | 67.9 | OFL | 46.2 | 7.1 | −128 | 23 |
| Yenshui 2 | N 23°18′4″/E 120°15′12″ | 23 | 20.9 | 1.2 | 575 | 0.4 | 7.83 | −158 | 26.4 |
| Yichu (house) | N 23°19′52.3″/E 120°13′27.2″ | 20 | 24.7 | 3.08 | 1481 | 1.5 | 7.23 | −108 | 17. |
| Lucao1B (rice field) | N 23°24′59.9″/E 120°17′50.6″ | 13 | 18.1 | 1.52 | 727 | 0.6 | 7.54 | −54 | 11.3 |
| Lucao1A (house) | N 23°24′59″/E 120°17′51″ | 30 | 24.3 | 1.79 | 857 | 0.7 | 7.54 | −118 | 19.6 |
| Liujiao2 | N 23°30′27.3″/E 120°16′19.1″ | 67 | 24.9 | 1.044 | 501 | 0.3 | 7.31 | −148 | 13.7 |
| Budai-Shinwen | N 23°20′22″/E 120°7′57.9″ | 313 | 24.7 | 1.15 | 554 | 0.4 | 7.63 | −140 | 158 |
| Budai-3 | N 23°20′29.7″/E 120°9′37″ | 233 | 32.3 | 0.97 | 463 | 0.3 | 8.25 | −96 | 129 |
| Beimen CN 9 | N 23°18′33.3″/E 120°8′45.7″ | 100 | 31.7 | 1.67 | 799 | 0.7 | 8.1 | -72 | 157 |
| Beimen-Jinhu | N 23°18′26″/E 120°9′8.2″ | 300 | 28.5 | 1.42 | 682 | 0.5 | 7.44 | −139 | 159 |
| Beimen 2A | N 23°30′27.6″/E 120°16′19″ | 277 | 24.7 | 1.86 | 893 | 0.8 | 7.33 | −144 | 163 |
| Yenshui 3 | N 23°18′6.7″/E 120°15′11.1″ | 23 | 24.7 | 1.56 | 751 | 0.6 | 7.92 | −158 | 119 |
| Yenshui 1 | N 23°18′2.4″/E 120°14′57.2″ | 23 | 24.7 | 1.37 | 657 | 0.5 | 8.12 | −133 | 124 |
| Beimen 1 | N 23°17′23.3″/E 120°5′41.2″ | 200 | 26.4 | 1.43 | 684 | 0.5 | 7.68 | −149 | 205 |
| Budai 5 | N 23°22′56.8″/E 120°9′49.6″ | 200 | 24.5 | 0.86 | 410 | 0.2 | 8.34 | 8 | 97.7 |
| Budai 4 | N 23° 19′37.8″/E 120° 9′3.2″ | 300 | 23.9 | 3.55 | 1704 | 1.8 | 7.76 | −100 | 141 |
| Hsuechia 2 | N 23°13′46.1″/E 120°10′4.3″ | 3 | 21.4 | 5.47 | 2626 | 2.9 | 7.38 | −96 | 105 |
| Siaying 3 | N 23°14′14.7″/E120°14′21.7″ | 233 | 27.5 | 0.85 | 408 | 0.2 | 8.13 | −17 | 111 |
| Siaying 1 | N 23°14′7.9″/E 120°14′40″ | 150 | 25 | 3.37 | 1619 | 1.7 | 7.05 | −27 | 103 |
| Yichu 5 | N 23°19′59.7″/E 120°13′11.6″ | 20 | 26.1 | 1.57 | 751 | 0.6 | 7.24 | −119 | 125 |
| Yichu 6 | N 23°19′29.7″/E 120°12′48.1″ | 20 | 25.1 | 2.32 | 1116 | 1 | 7.5 | −102 | 142 |
| Yichu 7 | N 23°18′55.5″/E 120°10′5.8″ | 72 | 29.1 | 1.13 | 545 | 0.4 | 8.1 | -192 | 136 |
| Yichu 8 | N 23°18′57.1″/E 120°10′25.6″ | 143 | 25.2 | 5.44 | 2750 | 2.9 | 7.9 | −10 | 138 |
| Lucao 3 | N 23°22′40.4″/E 120°16′51.8″ | 4.5 | 27.8 | 1.10 | 531 | 0.3 | 7.3 | −91 | 87 |
| Lucao 4 | N 23°23′15.1″/E 120°17′39.2″ | 83 | 26.5 | 1.49 | 716 | 0.6 | 7.84 | −148 | 101 |
| Lucao 5 | N 23°23′25.3″/E 120°17′20.4″ | 300 | 26.3 | 2.08 | 998 | 0.9 | 7.63 | −153 | 98.3 |
| Hsuechia 3 | N 23°17′7.5″/E 120°11′6.7″ | 67 | 27.8 | 0.96 | 462 | 0.2 | 7.65 | −72 | 122 |
| Hsuechia 4 | N 23°16′22.2″/E 120°9′36.2″ | 5 | 24.1 | 5.05 | 2610 | 2.7 | 7.34 | −113 | 155 |
| Hsuechia 5 | N 23°14′39.6″/E 120°9′14.7″ | 7 | 27.1 | 2.24 | 1076 | 1 | 7.59 | −118 | 115 |
| Min | 3 | 18.1 | 0.85 | 408 | 0.2 | 7.05 | −192 | 11.3 | |
| Max | 313 | 32.3 | 67.9 | 2750 | 46.2 | 8.34 | 8 | 204 | |
| Mean | 113 | 25.6 | 4.32 | 998 | 2.46 | 7.64 | −107 | 107 | |
| STD | 110 | 2.86 | 12.3 | 678 | 8.44 | 0.35 | 49.2 | 52.2 | |
| Median | 67 | 25 | 1.56 | 739 | 0.6 | 7.63 | −118 | 119 |
| Sample Location | Alkalinity (mg/L) | F (mg/L) | Cl (mg/L) | NO2 (mg/L) | NO3 (mg/L) | SO4 (mg/L) | Na (mg/L) | NH4 (mg/L) | K (mg/L) | Mg (mg/L) | Ca (mg/L) | PO4 (mg/L) | As(III) (μg/L) | As(V) (μg/L) | Fe (μg/L) | Mn (μg/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beimen 2B | 184 | 2.89 | 332 | 0.00 | 8.17 | 10.4 | 582 | 0.00 | 26.2 | 51.4 | 66 | 3.00 | 102 | 224 | 18,809 | 32.3 |
| Yenshui 2 | 182 | 2.85 | 57.0 | 0.00 | 7.70 | 29.1 | 138 | 2.3 | 17.9 | 63 | 64.7 | 3.17 | 560 | 184 | 3725 | 91.0 |
| Yichu (house) | 190 | 3.16 | 269 | 0.00 | 7.76 | 336 | 277 | 0.00 | 20.1 | 88.1 | 236 | 0.44 | 102 | 10.5 | 8338 | 160 |
| Lucao1B (rice field) | 188 | 2.88 | 89.7 | 0.00 | 8.28 | 90.7 | 1.41 | 0.00 | 3.34 | 71.2 | 144 | 1.20 | 4.95 | 3.94 | 4879 | 75.9 |
| Lucao 1A (house) | 185 | 2.86 | 108 | 0.00 | 0.00 | 111 | 1.71 | 0.00 | 3.4 | 70.7 | 176 | 2.60 | 41.1 | 5.23 | 28 | 1.19 |
| Liujiao 2 | 175 | 2.97 | 24.9 | 0.00 | 7.66 | 72.3 | 75.9 | 0.00 | 3.63 | 37.7 | 121 | 1.09 | 89.8 | 5.03 | 12,129 | 237 |
| Budai- Shinwen | 185 | 0.00 | 171 | 0.00 | 3.50 | 0.00 | 422 | 0.00 | 4.56 | 24.8 | 34.8 | 2.18 | 604 | 100 | 1271 | 30.3 |
| Budai-3 | 177 | 0.00 | 9.85 | 0.00 | 0.00 | 3.33 | 223 | 10.1 | 8.25 | 12.8 | 16.2 | 3.92 | 654 | 64.4 | 533 | 40.7 |
| Beimen CN 9 | 173 | 0.00 | 104 | 0.00 | 3.35 | 11.4 | 391 | 0.00 | 16.9 | 23 | 11.7 | 6.08 | 212 | 40.6 | 365 | 58.9 |
| Beimen-Jinhu | 180 | 0.00 | 137 | 0.00 | 3.36 | 3.11 | 362 | 28.3 | 6.88 | 31.4 | 42.3 | 1.86 | 356 | 184 | 4033 | 32.9 |
| Beimen 2A | 172 | 0.00 | 116 | 0.00 | 3.39 | 5.88 | 332 | 24.3 | 5.71 | 35.5 | 74.5 | 3.00 | 158 | 220 | 3537 | 28.0 |
| Yenshui 3 | 177 | 0.00 | 76.8 | 0.00 | 0.00 | 86.8 | 194 | 4.45 | 20 | 77.2 | 102 | 1.82 | 954 | 177 | 2994 | 27.6 |
| Yenshui 1 | 176 | 0.00 | 70.2 | 0.00 | 2.91 | 8.85 | 245 | 0.00 | 32.7 | 45.8 | 26.4 | 2.22 | 274 | 41.6 | 466 | 25.5 |
| Beimen 1 | 340 | 0.00 | 12.4 | 1.29 | 3.79 | 3.16 | 333 | 0.00 | 3.66 | 12.6 | 33.8 | 4.16 | 372 | 161 | 2326 | 28.9 |
| Budai 5 | 157 | 0.00 | 17.6 | 1.13 | 4.63 | 4.71 | 170 | 0.00 | 6.56 | 7.37 | 33.4 | 1.42 | 1.96 | 319 | 381 | 17.6 |
| Budai 4 | 166 | 0.00 | 212 | 473 | 3.89 | 11.4 | 622 | 0.00 | 13.3 | 51.5 | 98.7 | 1.72 | 24.5 | 285 | 5832 | 28.7 |
| Hsuechia 2 | 175 | 0.00 | 0.00 | 859 | 3.72 | 234 | 698 | 0.00 | 14.7 | 106 | 531 | 0.00 | 10.3 | 3.22 | 10,260 | 2609 |
| Siaying 3 | 154 | 0.00 | 5.94 | 0.00 | 3.65 | 3.55 | 191 | 5.84 | 10.7 | 13.6 | 26.8 | 1.38 | 57.9 | 10.2 | 519 | 70.1 |
| Siaying 1 | 171 | 0.00 | 0.00 | 207 | 385 | 0.95 | 531 | 0.00 | 8.55 | 32 | 112 | 2.43 | 190 | 9.15 | 12,093 | 263 |
| Yichu 5 | 183 | 0.00 | 73.2 | 0.00 | 3.71 | 92.7 | 151 | 2.66 | 16.1 | 70.4 | 161 | 0.21 | 94.2 | 12.9 | 9385 | 107 |
| Yichu 6 | 192 | 0.00 | 96.3 | 0.00 | 3.6 | 262 | 300 | 0.00 | 32.2 | 86.7 | 163 | 1.31 | 49.8 | 6.60 | 7022 | 1047 |
| Yichu 7 | 182 | 0.00 | 23.4 | 0.00 | 3.49 | 3.43 | 259 | 11.8 | 12.8 | 16.2 | 17.9 | 2.89 | 305 | 104 | 1062 | 85.8 |
| Yichu 8 | 189 | 0.00 | 258 | 539 | 4.46 | 39.8 | 816 | 0.00 | 29.9 | 77.1 | 59.9 | 1.91 | 0.62 | 96.1 | 956 | 17.5 |
| Lucao 3 | 163 | 0.00 | 14.6 | 0.00 | 0.00 | 128 | 34 | 0.00 | 6.02 | 66.2 | 203 | 0.00 | 22.9 | 1.36 | 14,508 | 1957 |
| Lucao 4 | 177 | 0.00 | 112 | 0.00 | 3.67 | 12.9 | 295 | 0.00 | 18.8 | 27.9 | 37.7 | 2.92 | 188 | 51.9 | 3428 | 87.9 |
| Lucao 5 | 177 | 0.00 | 187 | 0.00 | 3.87 | 5.45 | 273 | 12.4 | 19.4 | 58.9 | 68.6 | 1.04 | 57.8 | 18.2 | 5389 | 192 |
| Hsuechia 3 | 176 | 0.00 | 19.7 | 0.00 | 3.64 | 3.14 | 194 | 8.46 | 7.81 | 20.2 | 42 | 1.04 | 510 | 112 | 5281 | 161 |
| Hsuechia 4 | 181 | 0.00 | 226 | 965 | 4.66 | 51.9 | 872 | 0.00 | 59.9 | 103 | 130 | 0.14 | 34.1 | 3.68 | 12,104 | 359 |
| Hsuechia 5 | 179 | 0.00 | 155 | 0.00 | 3.83 | 126 | 316 | 0.00 | 18.9 | 66.2 | 157 | 0.34 | 34.8 | 0.23 | 10,965 | 339 |
| Min | 154 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.41 | 0.00 | 3.34 | 7.37 | 11.7 | 0.00 | 0.62 | 0.23 | 28.1 | 1.19 |
| Max | 340 | 3.16 | 332 | 965 | 385 | 335 | 871 | 28.2 | 59.8 | 105 | 531 | 6.08 | 954 | 318 | 18,809 | 2609 |
| Mean | 183 | 0.61 | 103 | 105 | 17.1 | 60.4 | 320 | 3.81 | 15.4 | 49.9 | 103 | 1.91 | 209 | 84.6 | 5607 | 283 |
| SD | 31.5 | 1.21 | 91.4 | 261 | 70.8 | 86.6 | 224 | 7.32 | 12.2 | 28.6 | 102 | 1.40 | 242 | 94.9 | 5046 | 595 |
| Median | 177 | 0 | 89.8 | 0 | 3.71 | 11.4 | 276 | 0 | 13.3 | 51.4 | 68.5 | 1.82 | 102 | 41.5 | 4033 | 75.9 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, L.; Jean, J.-S.; Chakraborty, S.; Lee, M.-K.; Kar, S.; Yang, H.-J.; Li, Z. Hydrogeochemistry of Groundwater and Arsenic Adsorption Characteristics of Subsurface Sediments in an Alluvial Plain, SW Taiwan. Sustainability 2016, 8, 1305. https://doi.org/10.3390/su8121305
Liao L, Jean J-S, Chakraborty S, Lee M-K, Kar S, Yang H-J, Li Z. Hydrogeochemistry of Groundwater and Arsenic Adsorption Characteristics of Subsurface Sediments in an Alluvial Plain, SW Taiwan. Sustainability. 2016; 8(12):1305. https://doi.org/10.3390/su8121305
Chicago/Turabian StyleLiao, Libing, Jiin-Shuh Jean, Sukalyan Chakraborty, Ming-Kuo Lee, Sandeep Kar, Huai-Jen Yang, and Zhaohui Li. 2016. "Hydrogeochemistry of Groundwater and Arsenic Adsorption Characteristics of Subsurface Sediments in an Alluvial Plain, SW Taiwan" Sustainability 8, no. 12: 1305. https://doi.org/10.3390/su8121305







