Mass Releases of Genetically Modified Insects in Area-Wide Pest Control Programs and Their Impact on Organic Farmers
Abstract
:1. Introduction
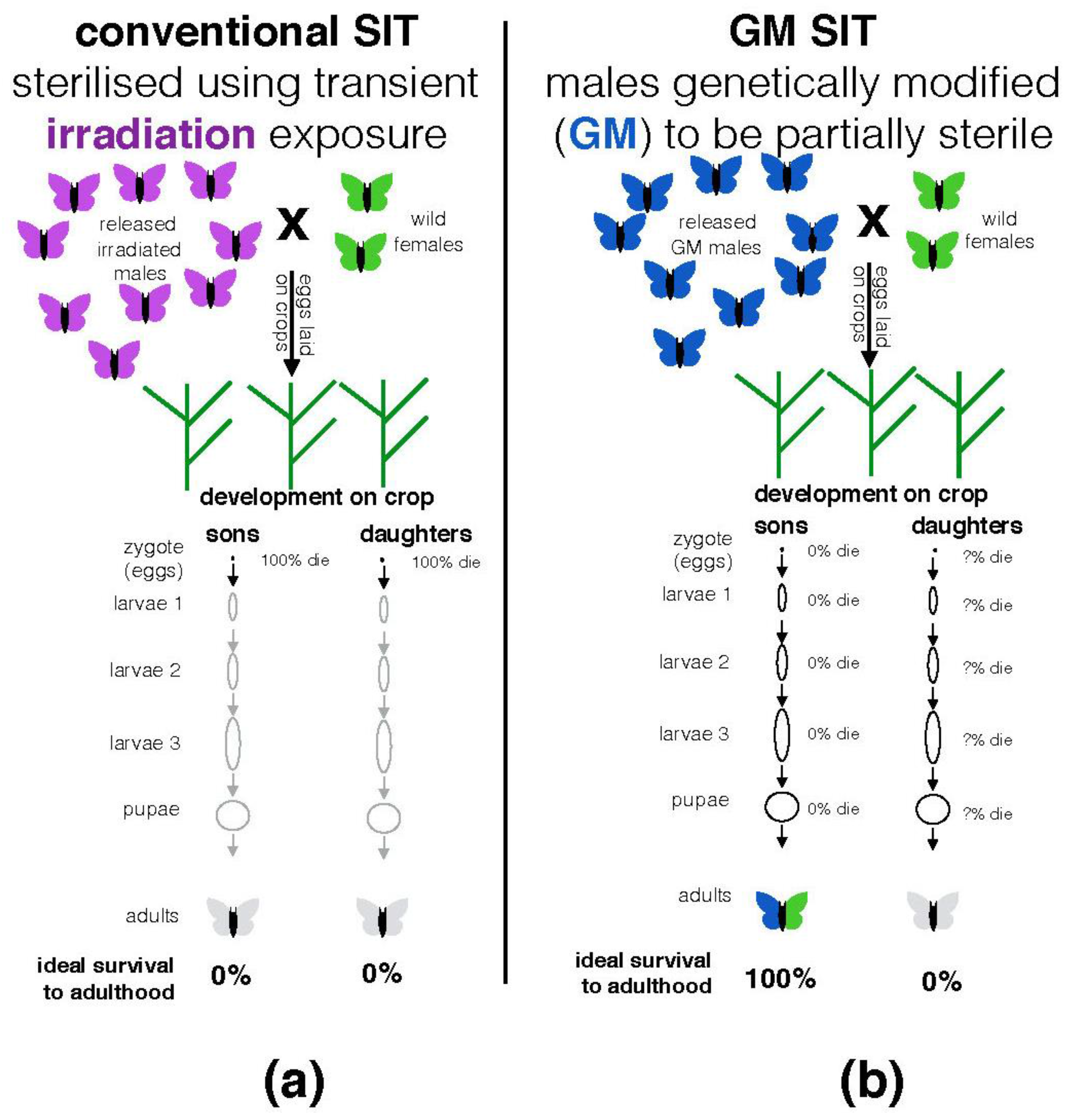
1.1. What Is Conventional (Non-GM) Sterile Insect Technique and How Is It Used?
1.2. What Is the GM Sterile Insect Technique (GM-SIT) and Why Is It Being Proposed?
There is an impending need for the development of more efficient, lower cost, and more effective control and eradication methods for the pink bollworm and invasive fruit fly species because of the continuing and increasing frequency of detection of fruit flies and other invasive and crop destructive insects. In order to achieve these objectives, the use of genetically engineered insects provides biological traits that are of value for use in sterile insect technique control methodologies. These novel biological traits are not available to present programs and could not be readily developed or adopted for program use by APHIS using other methods.[10] (Page 20)
1.3. The Experience of GM-SIT in the USA
There are organic growers found at certain locations within the [existing control] program area[s], and their needs are important program considerations.(Page 78)
Although there are risks to organic farmers from the drift of pesticides in chemical control applications, the increased use of SIT in preventive releases reduces the need for future pesticide applications. The potential mortality to predators and parasites of plant pests and to pollinators, due to pesticide use, as well as the potential loss of “pesticide-free” status, is critically important to organic farmers. The mitigation measures for pesticide applications are designed to minimize exposure to bees, through advanced notification to beekeepers, which allows them to move their hives away from exposure to pesticides. The use of nonchemical control methods, including SIT, precludes concerns of organic farmers and beekeepers.(Page 110)
Successful eradication can dramatically reduce the need to use pesticides for crop protection. Although there are risks to organic farmers from the drift of pesticides in chemical control applications, the increased use of SIT in preventive releases reduces the need for future pesticide applications.(Page 109)
Andy Fellenz grows vegetables and small fruits on five acres of Lester Road property in the town of Phelps [Upstate New York]. His farm has had organic certification from the New York Organic Farming Association since 2005. “I’m aware of the moth trials, but I’m not well informed on the topic and whether having genetically modified moths on my plants could harm my organic certification,” Fellenz said. “I do think Cornell has not been open on how they would do the trials. They did not give much public notice on the nature of their plans and did not give an opportunity for discussion.”[33]
1.4. The Hypothetical Example of an Organic Certified Spinach Farm Located near a Release Site of GM Moths
2. Results
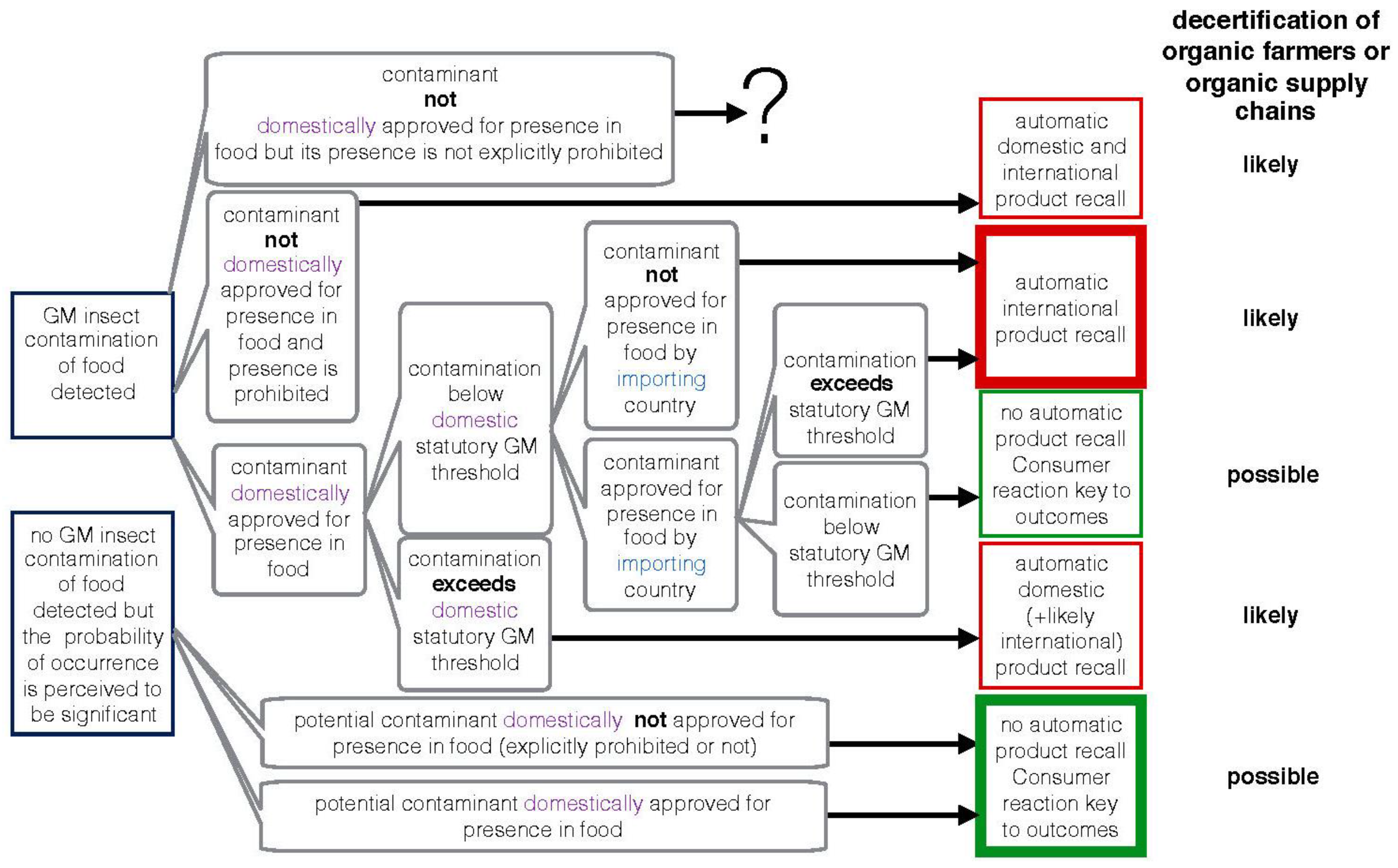
2.1. Could an Organic Farmer Lose Organic Certification?
2.1.1. Detected Contamination of GM Insects Approved for Presence in Food
Organic certification shall be withdrawn where NASAA considers there is an unacceptable risk of contamination from GMOs or their derivatives.(Clause 3.2.9)
Contamination of organic product by GMOs that results from circumstances beyond the control of the operator may alter the organic status of the operation.(Clause 3.2.11)
Liability for pure economic loss presents considerable difficulties. Most obviously, it raises the risk of indeterminate liability. Such difficulties were generally resolved on the basis of a broad rule that excluded liability for such losses.
The economic loss doctrine has grown beyond its original freedom of contract based policy justifications. Farmers’ expectations of what they will receive for their crops are just that, expectations. Absent a physical injury, plaintiffs cannot recover for drops in market prices. Nor can they recover for any additional costs, such as testing procedures imposed by the marketplace.[53]
2.1.2. No Contamination Is Reported but the Risk of Contamination Is Perceived to Be Significant by the Certification Body
Even where evidence of GMOs is not detected in finished organic product, the deliberate or negligent exposure of organic production systems or finished products to GMOs is outside organic production principles.
Operators must not knowingly permit exposure or fail to take action against the application of or exposure to GMOs.
Even if strays are found, legal experts say that national organic standards penalize only the deliberate use of a genetically modified organism. “If these moths came across into an organic field inadvertently, that would not be a problem for the farmer,” attributed to Susan Schneider, a legal expert who specializes in agriculture and food law at the University of Arkansas School of Law.[54]
2.1.3. Hoffman v. Monsanto (Saskatchewan, Canada)
2.1.4. Marsh v. Baxter (Western Australia)
2.2. Eroding Confidence in Organic Certification Bodies and or the Loss of International Export Markets
Syngenta Viptera Litigation (USA)
If you’ve arrived here, you are probably a corn farmer feeling the financial impact of Syngenta’s bioengineered corn. A recently filed class action lawsuit alleges that Switzerland-based Syngenta knowingly marketed two genetically modified strains of corn—Agrisure Viptera and Agrisure Duracade—that are illegal in China. When China detected a genetic trait found in Viptera (MIR162), they stopped accepting shipments. That caused the price of corn to plummet. That affects you, your farm and your family.[66]
We developed a superior product that helps farmers; we applied for and received government approvals from the U.S. and major export markets at the time; and we submitted an import application to the Chinese government that was timely, accurate and complete. Syngenta believes the lawsuits are without merit and strongly upholds the right of growers to have access to approved new technologies that can increase both their productivity and crop yields. The issues involved in these cases are extremely important and affect every American farmer’s right to benefit from new technologies that help grow better crops. When a U.S.-approved product like Agrisure Viptera (event MIR162) is kept out of a market for political and economic reasons, farmers—and consumers—lose.[67]
3. Discussion
Environmental considerations: soil resources; water resources; air quality; climate change; plant communities; wildlife and biological diversity.
Human Population Considerations: Farm worker health and health of general public.[30]
3.1. Taking into Account Organic Producers
3.2. Mandate Consultation and the Consideration of Impacts in Approval Regimes
When prescribing control measures, the committee shall make every effort to adhere to integrated pest management practices, to allow organic cotton producers to choose organic pest management practices that will allow them to maintain their organic certification and to adhere to the management goals of individual cotton producers consistent with the goal of complete eradication of the pink bollworm;
The pink bollworm control committee shall confer with an organic cotton producer to determine measures that might be taken to attempt to keep all or a portion of the organic cotton producer’s cotton acreage below trigger levels for required treatment. If the organic cotton producer chooses to use a nonconventional method, the committee shall pay the costs of the nonconventional method used by the organic cotton producer, provided the costs do not exceed the equivalent costs of conventional control methods. If pink bollworm trigger levels are reached on the organic cotton producer’s acres and pink bollworm migration from outside these acres has been eliminated as a cause of these levels, the organic cotton producer shall be allowed to harvest these acres but shall not be allowed to grow cotton on the acreage for one year.[70]
3.3. Ensure that the Scope of Existing Approvals Regimes Encompasses GM Material
In November 2009, Biotechnology Regulatory Services officials told us that they were working on drafting the Decision Memorandum to the Secretary setting forth three possible options for clarifying the regulations that apply to GE animals and insects: (1) arguing that these regulations in their current form give APHIS sufficient authority to regulate GE animals and insects; (2) modifying these regulations to make it clearer how they relate to GE animals and insects; or (3) formulating completely new regulations.[71]
3.4. Mandate Consultation and Engagement between the Producers of GM Insects and Potentially Affected Farmers
3.5. Clarify Where the Responsibility Lies to Consider Probable Export Market Impacts
3.6. Opportunities as the USDA Prepares a New Environmental Assessment (EA) Document for Experimental GM Diamondback Moth Release
- Will the USDA describe what if any remedial actions are likely if GM insects are reported outside release or quarantine areas authorized in approved permits?
- Will the USDA publicly clarify, prominently and in plain language, whether any agricultural products upon which unapproved GM insect parts were detected would be allowed to enter the food chain? This should include reference to all relevant regulations from the USDA, EPA, or FDA. If there are no regulations prohibiting the presence of experimental GM insects on food crops outside of authorized release zones, this should be plainly stated.
- All permits issued should proactively be made public at the earliest possible stage, including all supplementary conditions. This information is unambiguously covered under Freedom of Information Act requests but can take months or years to process through this route.
Technologies such as GMOs should only be introduced—and then under controllable circumstances only—based on democratic, transparent assessment of the technology through processes that include decision-makers from every area of society and every group of people who will be impacted by the technology.
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dyck, V.A.; Hendrichs, J.; Robinson, A.S. Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management; Springer: Berlin, Germany, 2006. [Google Scholar]
- Wimmer, E.A. Eco-friendly insect management. Nat. Biotechnol. 2005, 23, 432–433. [Google Scholar] [CrossRef] [PubMed]
- Luttikholt, L.W.M. Principles of organic agriculture as formulated by the International Federation of Organic Agriculture Movements. NJAS-Wagening. J. Life Sci. 2007, 54, 347–360. [Google Scholar] [CrossRef]
- The World of Organic Agriculture, Statistics and Emerging Trends 2016; Willer, H.; Lernoud, J. (Eds.) Research Institute of Organic Agriculture (FiBL), Frick, and IFOAM Organics International: Bonn, Germany, 2016.
- USDA Agricultural Coexistence. Available online: http://www.usda.gov/wps/portal/usda/usdahome?navid=coexistence&navtype=CO&edeployment_action=changenav (accessed on 19 October 2016).
- Wyss, J.H. USDA screw-worm eradication programs and their economic benefits. In Proceedings of the Screw-Worm Fly Emergency Preparedness Conference, Canberra, Australia, 12–15 November 2001; pp. 65–68.
- FAO Guidance for Packing, Shipping, Holding and Release of Sterile Flies in Area-Wide Fruit Fly Control Programs, Plant Production and Protection Paper 190. 2007. Available online: ftp://ftp.fao.org/docrep/fao/010/a1195e/a1195e.pdf (accessed on 10 October 2016).
- List of Sterile Insect Technique Trials. Available online: https://en.wikipedia.org/wiki/List_of_sterile_insect_technique_trials (assessed on 21 December 2016).
- Abdul Matin, A.S.M.; Wright, J.E.; Davich, T.B. Effect of low levels of gamma irradiation on longevity and sterility of the boll weevil [Anthonomus grandis]. Southwest Entomol. 1980, 5, 112–117. [Google Scholar]
- U.S. Department of Agriculture, Animal and Plant Health Inspection Service. Use of Genetically Engineered Fruit Fly and Pink Bollworm in APHIS Plant Pest Control Programs: Final Environmental Impact Statement 2008; U.S. Department of Agriculture, Animal and Plant Health Inspection Service: Raleigh, NC, USA, 2008.
- Gates Foundation Grand Challenges Develop a Biological Strategy to Deplete or Incapacitate a Disease-transmitting Insect Population. 2005. Available online: http://gcgh.grandchallenges.org/challenge/develop-biological-strategy-deplete-or-incapacitate-disease-transmitting-insect-population (accessed on 27 September 2016).
- Intrexon Corporation Intrexon Establishes Crop Protection Enterprise. 4 May 2016. Available online: http://www.prnewswire.com/news-releases/intrexon-establishes-crop-protection-enterprise-300262641.html (accessed on 15 September 2016).
- Reeves, R.G.; Denton, J.A.; Santucci, F.; Bryk, J.; Reed, F.A. Scientific Standards and the Regulation of Genetically Modified Insects. PLoS Negl. Trop. Dis. 2012, 6, e1502. [Google Scholar] [CrossRef] [PubMed]
- Intrexon Crop Protection Presentation. 4 May 2016. Available online: http://filecache.drivetheweb.com/mr5ir_intrexon/119/download/Intrexon+Investor+Day+Presentation+November+2015.pdf (accessed on 21 December 2016).
- Oxitec’s Medfly Ready for Open Field Trials. Available online: http://oxitec.com/oxitecs-medfly-ready-open-field-trials/ (accessed on 21 December 2016).
- USDA APHIS | Regulations. Available online: https://www.aphis.usda.gov/aphis/ourfocus/biotechnology/SA_Regulations (accessed on 18 November 2016).
- ECFR.io e-CFR Title Title 7 → Subtitle B → Chapter III → Part 340. Available online: https://ECFR.io/Title-07/pt7.5.340 (accessed on 18 November 2016).
- USDA-APHIS Proposal to Permit the Field Release of Genetically Engineered Diamondback Moth in New York Environmental Assessment. May 2014. Available online: https://www.aphis.usda.gov/brs/aphisdocs/13_297102r_dea.pdf (accessed on 12 September 2016).
- Nutrition, C. for F.S. and A. Food Additives & Ingredients—Determining the Regulatory Status of a Food Ingredient. Available online: http://www.fda.gov/Food/IngredientsPackagingLabeling/FoodAdditivesIngredients/ucm228269.htm (accessed on 18 November 2016).
- Nutrition, C. for F.S. and A. Food Ingredients and Packaging Terms. Available online: http://www.fda.gov/Food/IngredientsPackagingLabeling/Definitions/default.htm (accessed on 18 November 2016).
- US EPA, O. What are Biopesticides? Available online: https://www.epa.gov/ingredients-used-pesticide-products/what-are-biopesticides (accessed on 18 November 2016).
- Medicine, C. for V. CVM Updates—FDA Releases Final Environmental Assessment for Genetically Engineered Mosquito. Available online: http://www.fda.gov/AnimalVeterinary/NewsEvents/CVMUpdates/ucm490246.htm (accessed on 18 November 2016).
- CFR—Code of Federal Regulations Sec. 110.110 Natural or Unavoidable Defects in Food for Human Use that Present no Health Hazard. Available online: http://www.accessdata.fda.gov/SCRIPTs/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=110&showFR=1&subpartNode=21:2.0.1.1.10.7 (accessed on 17 November 2016).
- Amendment Application Dated Febmary 4, lOOO Regarding Modification of Term 3 of the Terms and Conditions of this Registration and Revised Labeling! StarLinkT Corn EP A Registration No. 264–669. Available online: https://www3.epa.gov/pesticides/chem_search/ppls/000264-00669-20000407.pdf (accessed on 21 December 2016).
- U.S. Department of Agriculture, Animal and Plant Health Inspection Service. Use of Genetically Engineered Fruit Fly and Pink Bollworm in APHIS Plant Pest Control Programs; Record of Decision, Docket No. APHIS-2006-0166 2009; U.S. Department of Agriculture, Animal and Plant Health Inspection Service: Raleigh, NC, USA, 2009.
- Use of Genetically Engineered Fruit Fly and Pink Bollworm in APHIS Plant Pest Control Programs; Record of Decision. Available online: https://www.federalregister.gov/documents/2009/05/07/E9-10633/use-of-genetically-engineered-fruit-fly-and-pink-bollworm-in-aphis-plant-pest-control-programs (accessed on 30 September 2016).
- NCC Pink Bollworm Technical Action Committee (2009) Meeting Minutes, Phoenix, AZ, USA. 19 March 2009. Available online: http://azcotton.org/NCC/2009/20090319_NCC_PBW_TAC_minutes.pdf (accessed on 1 January 2017).
- NCC Pink Bollworm Technical Action Committee (2010) Draft Meeting Minutes, Tempe, AZ, USA. 25 October 2010. Available online: http://azcotton.org/NCC/2010/1b%202010%2010%2025%20NCC%20PBW%20TAC%20minutes.pdf (accessed on 1 January 2017).
- NCC Pink Bollworm Technical Action Committee (2009) Meeting Minutes, Tempe, AZ, USA. 27 October 2009. Available online: http://azcotton.org/NCC/2009/20091027_NCC_PBW_TAC_MINUTES.pdf (accessed on 1 January 2017).
- U.S. Department of Agriculture, Animal and Plant Health Inspection Service. FONSI-Field Release of Genetically Engineered Diamondback Moth Strains, 13-297-102r; U.S. Department of Agriculture, Animal and Plant Health Inspection Service: Raleigh, NC, USA, 2014; p. 28.
- Letter of Questions to Cornell from the Northeast Organic Farming Association—New York. 13 September 2016. Available online: https://www.nofany.org/files/Letter_of_questions_to_Cornell_Sep_13_2016.pdf (accessed on 28 December 2016).
- NOFA-NY Why Should Farmers Be Concerned about Open Field Genetically Engineered (GE) Diamondback Moth Trials in Geneva NY? 2014. Available online: https://www.nofany.org/files/FARMERS_GDMoth_Info_SheetREVISED_copy.pdf (accessed on 5 October 2016).
- Shaw, D.L. Experiment Station Defends Moth Trials. Available online: http://www.fltimes.com/news/experiment-station-defends-moth-trials/article_cae7ecf2-1ffd-11e5-9d1b-6b73566a4ed8.html (accessed on 27 September 2016).
- USDA Regional IPM Centers Information Network Crop Profiles-Spinach. 2001. Available online: http://www.ipmcenters.org/cropprofiles/docs/AZspinach.pdf (accessed on 27 September 2016).
- Shirai, Y.; Nakamura, A. Dispersal Movement of Male Adults of the Diamondback Moth, Plutella xylostella (Lepidoptera: Yponomeutidae), on Cruciferous Vegetable Fields, Studied Using the Mark-Recapture Method. Appl. Entomol. Zool. 1994, 29, 339–348. [Google Scholar]
- Mo, J.; Baker, G.; Keller, M.; Roush, R. Local Dispersal of the Diamondback Moth (Plutella xylostella (L.)) (Lepidoptera: Plutellidae). Environ. Entomol. 2003, 32, 71–79. [Google Scholar] [CrossRef]
- Chu, Y.-I. The Migration of Diamondback Moth. Available online: http://ntur.lib.ntu.edu.tw/handle/246246/109830#.V-qSbsfHN2k (accessed on 27 September 2016).
- Nofa New York. Organic Management of Diamondback Moth and Similar Insects. Available online: https://www.nofany.org/blog/organic-management-of-diamondback-moth-and-similar-insects (assessed on 21 December 2016).
- Genewatch Oxitec’s Genetically Modified Moths: Summary of Concerns. Available online: http://www.genewatch.org/uploads/f03c6d66a9b354535738483c1c3d49e4/DBMbrief_fin.pdf (accessed on 21 December 2016).
- NOFA-NY Organic and Local Food Directory. Available online: https://www.nofany.org/directory/ (accessed on 27 September 2016).
- Withdrawal of an Environmental Assessment for the Field Release of Genetically Engineered Diamondback Moths. Available online: https://www.federalregister.gov/documents/2016/11/08/2016-26935/withdrawal-of-an-environmental-assessment-for-the-field-release-of-genetically-engineered (accessed on 15 November 2016).
- Wallace, H. Oxitec’s Genetically Engineered Diamondback Moths GeneWatch Presentation to NOFA-NY. Available online: https://www.nofany.org/files/Wallace_2016_WC_Oxitecs_GE_Diamondback_Moths-fin.pdf (accessed on 27 September 2016).
- Center for Food Safety (USA) Comments on APHIS’s Environmental Assessment for Field Release of Genetically Engineered Diamondback Moth (Docket No. APHIS-2014-0056). Available online: http://www.centerforfoodsafety.org/files/2014-09-29-ge-moth-comments-final_96458.pdf (accessed on 5 October 2016).
- 7 CFR Part 205—National Organic Program. Available online: https://www.law.cornell.edu/cfr/text/7/part-205 (assessed on 21 December 2016).
- Accreditation of Organic Certification Bodies—NOP-PM-11-13-GMOClarification.pdf. Available online: https://www.ams.usda.gov/sites/default/files/media/NOP-PM-11-13-GMOClarification.pdf (accessed on 21 November 2016).
- Fraiture, M.-A.; Herman, P.; Taverniers, I.; De Loose, M.; Deforce, D.; Roosens, N.H. Current and New Approaches in GMO Detection: Challenges and Solutions. BioMed Res. Int. 2015, 2015, e392872. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration (US) Sanitation & Transportation—Defect Levels Handbook. Available online: http://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/SanitationTransportation/ucm056174.htm (accessed on 15 September 2016).
- NASAA Organic Standard 06-02-2012.doc—AAAA-NASAA-Organic-Standard-06-02-2012.pdf. Available online: http://www.organicdairyfarmers.com.au/downloads/AAAA-NASAA-Organic-Standard-06-02-2012.pdf (accessed on 21 November 2016).
- National Sustainable Agriculture Information Service Forms, Documents, and Sample Letters for Organic Producers. Available online: https://attra.ncat.org/attra-pub/summaries/summary.php?pub=170 (accessed on 5 October 2016).
- Marsh -v- Baxter [2015] Supreme Court of Western Australia 169. Available online: http://www.austlii.edu.au/cgi-bin/sinodisp/au/cases/wa/WASCA/2015/169.html?stem=0&synonyms=0&query=Marsh (accessed on 21 December 2016).
- Ultramares Corp v Touche 174 N.E. 441 (1932). Available online: http://www.eejlaw.com/materials/Ultramares_v_Touche_vT08.pdf (accessed on 21 December 2016).
- Solomon, R.M.; McInnes, M.; Chamberlain, E.; Pitel, S.G.A. Cases and Materials on the Law of Torts; Carswell: Toronto, Canada, 2015. [Google Scholar]
- tarLink Corn Products Liability Litigation, 212 F. Supp. 2d 828 (N.D. Ill. 2002). Available online: http://law.justia.com/cases/federal/district-courts/FSupp2/212/828/2343014/ (accessed on 10 October 2016).
- Powell, D. Replacing Pesticides with Genetics. Available online: http://www.nytimes.com/2015/09/01/science/replacing-pesticides-with-genetics.html (accessed on 27 September 2016).
- 2010 CPHST LABORATORY REPORT Fort Collins and Phoenix. Available online: https://www.aphis.usda.gov/plant_health/cphst/downloads/2010FortCollins-PhoenixAnnualReport.pdf (assessed on 21 December 2016).
- Hoffman v. Monsanto Canada Inc., 2005 SKQB 225 (CanLII). Available online: http://canliiconnects.org/en/summaries/13978 (assessed on 21 December 2016).
- The Class Actions Act, SS 2001, c-12.01. Available online: https://www.canlii.org/en/sk/laws/stat/ss-2001-c-c-12.01/latest/ss-2001-c-c-12.01.html (assessed on 21 December 2016).
- Hoffman v. Monsanto Canada Inc., 2007 SKCA 47. Available online: http://www.ecelaw.ca/wildlife-and-biodiversity/case-law/hoffman-v-monsanto-canada-inc.html (assessed on 21 December 2016).
- Canadian Organic Growers—Consumers & the Standards. Available online: https://www.cog.ca/index.php?page=consumers-and-standards (accessed on 21 November 2016).
- Koch, B.A. Liability and Redress Options for Damage Caused by GMOs. In Genetically Modified and Non-Genetically Modified Food Supply Chains: Co-Existence and Traceability; Blackwell Publishing Ltd.: Oxford, UK, 2013; pp. 405–413. [Google Scholar]
- The results of the FAO Survey on Low Levels of Genetically Modified (GM) Crops in International Food and Feed Trade. Available online: http://www.fao.org/fileadmin/user_upload/agns/topics/LLP/AGD803_4_Final_En.pdf (accessed on 17 November 2016).
- GMOs—EU Science Hub—European Commission. Available online: https://ec.europa.eu/jrc/en/research-topic/gmos (accessed on 17 October 2016).
- Milavec, M.; Dobnik, D.; Yang, L.; Zhang, D.; Gruden, K.; Žel, J. GMO quantification: Valuable experience and insights for the future. Anal. Bioanal. Chem. 2014, 406, 6485–6497. [Google Scholar] [CrossRef] [PubMed]
- Pew Initiative on Food and Biotechnology 2004 Bugs in the System? Issues in the Science and Regulation of Genetically Modified Insects. Available online: http://www.pewtrusts.org/en/research-and-analysis/reports/2004/01/22/bugs-in-the-system-issues-in-the-science-and-regulation-of-genetically-modified-insects (accessed on 27 September 2016).
- Syngenta Agrisure Viptera—Corn Insect Control. Available online: http://www.syngenta-us.com/agrisure/agrisure-viptera.aspx (accessed on 4 October 2016).
- Syngenta Corn Litigation Homepage. Available online: http://www.syngentacornlitigation.com/ (accessed on 4 October 2016).
- Gray Reed & McGraw Ruling Means Syngenta Corn Multidistrict Litigation May Proceed Forward towards Trial, Press Release. 2015. Available online: http://www.prnewswire.com/news-releases/ruling-means-syngenta-corn-multidistrict-litigation-may-proceed-forward-towards-trial-300147081.html (accessed on 4 October 2016).
- Carter, C.A.; Smith, A. Estimating the Market Effect of a Food Scare: The Case of Genetically Modified Starlink Corn. Rev. Econ. Stat. 2007, 89, 522–533. [Google Scholar] [CrossRef]
- USDA-APHIS Notice of Availability for an Environmental Assessment Associated with a Permit Request for Field Release of Genetically Engineered Diamondback Moths within the Grounds of the Cornell University New York State Agricultural Experiment Station, Docket ID: APHIS-2014-0056. Available online: https://www.regulations.gov/docket?D=APHIS-2014-0056 (accessed on 1 October 2016).
- New Mexico Statutes 6B New Mexico Pink Bollworm Control Act. Available online: http://www.nmda.nmsu.edu/wp-content/uploads/2012/04/Pink-Bollworm-Control-Act.pdf (accessed on 21 December 2016).
- USDA-Office of Inspector General Controls over Genetically Engineered Animal and Insect Research, Audit Report 50601-16-Te. 2011. Available online: https://www.usda.gov/oig/webdocs/50601-16-TE.pdf (accessed on 5 October 2016).
- Beattie, R.B. Everything you already know about EIA (but don’t often admit). Environ. Impact Assess. Rev. 1995, 15, 109–114. [Google Scholar] [CrossRef]
- Phillipson, M. Legal impediments to the surivval of organic production? Available online: http://www.inter-disciplinary.net/ptb/ejgc/ejgc5/phillipson%20paper.pdf (assessed on 21 December 2016).
- Wickson, F.; Binimelis, R.; Herrero, A. Should Organic Agriculture Maintain Its Opposition to GM? New Techniques Writing the Same Old Story. Sustainability 2016, 8, 1105. [Google Scholar] [CrossRef]
- Public Consultation on the Position of IFOAM—Organics International on Genetic Engineering and Genetically Modified Organisms | IFOAM. Available online: http://www.ifoam.bio/en/news/2016/02/26/public-consultation-position-ifoam-organics-international-genetic-engineering-and (accessed on 1 November 2016).
- Monsanto Co. v. Geertson Seed Farms (Majority) U.S. Supreme Court 2010 June 21. Available online: https://www.law.cornell.edu/supct/html/09-475.ZS.html (accessed on 22 October 2016).
- Justice Stevens Monsanto Co. v. Geertson Seed Farms (Stevens, J., dissenting) U.S. Supreme Court 2010 June 21. Available online: https://www.law.cornell.edu/supct/html/09-475.ZD.html (accessed on 22 October 2016).
- Alphey, L.; Bourtzis, K.; Miller, T. Genetically Modified Insects as a Tool for Biorational Control. In Biorational Control of Arthropod Pests; Ishaaya, I., Horowitz, A.R., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 189–206. [Google Scholar]
- FAO/IAEA Technical Co-operation Projects, Insect Pest Control—NAFA. Available online: http://www-naweb.iaea.org/nafa/ipc/field-projects-ipc.html (accessed on 10 October 2016).
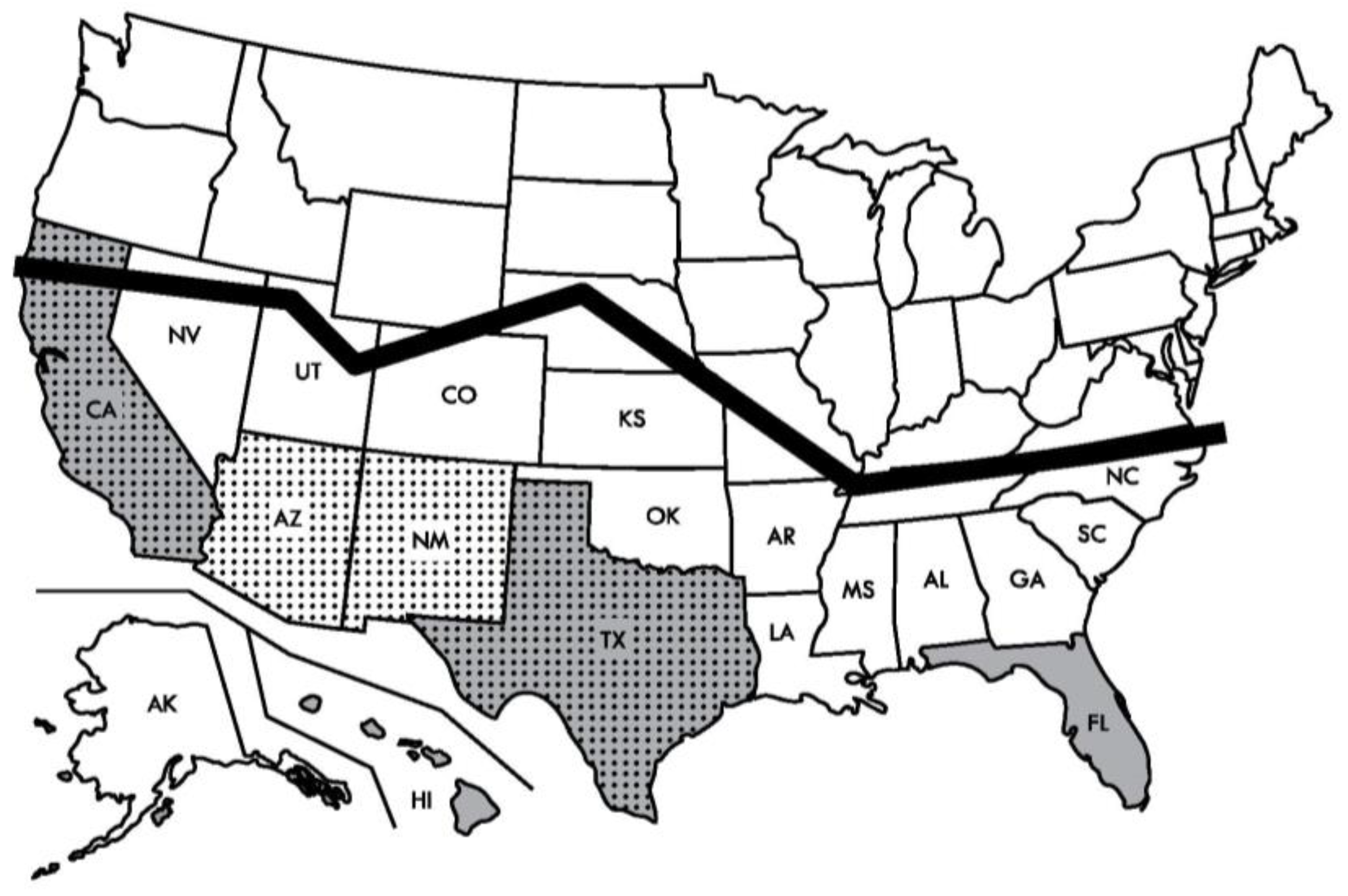
| Diamondback moth | Features |
|---|---|
| Host range | Very broad, most Brassicas—including broccoli, Brussels sprouts, cabbage, Chinese cabbage, canola, cauliflower, collard, kale, kohlrabi, mustard, radish, turnip, and watercress. Also occasionally found on other plants, including spinach [34]. |
| Physical characteristics of life stages | Larvae (caterpillars) 1.7, 3.5, 7.0, and 11.2 mm, respectively, for each instars, pupa is 7 to 9 mm. Both caterpillars and pupa are generally found on leaves. |
| Number of eggs laid by mated female | 100–300 Generation time of approximately 1 month (but can be shorter). |
| Dispersion capacity | Not considered a particularly strong flier and the only two published studies using release-and-recapture experiments with this species indicate that within growing crops 99.5% of released individuals probably disperse much less than an average of 300 m [35,36]. However, in one of the studies 8% of all recaptured individuals in their summer release dispersed at least 800 m (7/86, [35]). While in the second study the longest reported dispersion was restricted up to 300 m [36]. Dispersion from harvested areas remains to be explored as noted by these studies authors. Diamondback moths are considered a migratory species and can cover hundreds of kilometers per day [37] in suitable winds, though it remains unclear what triggers migratory behavior. |
| Diamondback moth control methods | Diamondback moths exhibit increasing resistance to some chemical insecticides. Organic control methods do exist [38]. |
| New York State | Diamondback moths are not native to the USA. It is unlikely that this species represents a significant pollinator in NY. Any wild populations are likely initially established each year by long-range migrants after harsh winter conditions in NY. Not known what the frequency of accidentally released GM females will be (1% reported in [39] page 11). Not clear at what developmental stage (zygote, larval instars, or as pupae) female progeny of the OX4319L-Pxy stock described in the NY permit die due to the action of the genetic construct integrated in to their chromosomes (see Figure 2b). Organic farms growing cabbage, cauliflower and broccoli are located within 10 km of probable release sites [40]. |
| Product | Action Level |
|---|---|
| Spinach, Canned or Frozen | Average of 50 or more aphids, thrips and/or mites per 100 g
OR 2 or more 3 mm or longer larvae and/or larval fragments or spinach worms (caterpillars) whose aggregate length exceeds 12 mm are present in 24 pounds OR Leaf miners of any size average 8 or more per 100 grams or leaf miners 3 mm or longer average 4 or more per 100 g |
| Defect Source: Pre-harvest infestation Significance: Aesthetic |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reeves, R.G.; Phillipson, M. Mass Releases of Genetically Modified Insects in Area-Wide Pest Control Programs and Their Impact on Organic Farmers. Sustainability 2017, 9, 59. https://doi.org/10.3390/su9010059
Reeves RG, Phillipson M. Mass Releases of Genetically Modified Insects in Area-Wide Pest Control Programs and Their Impact on Organic Farmers. Sustainability. 2017; 9(1):59. https://doi.org/10.3390/su9010059
Chicago/Turabian StyleReeves, R. Guy, and Martin Phillipson. 2017. "Mass Releases of Genetically Modified Insects in Area-Wide Pest Control Programs and Their Impact on Organic Farmers" Sustainability 9, no. 1: 59. https://doi.org/10.3390/su9010059





