Disinfection in Wastewater Treatment Plants: Evaluation of Effectiveness and Acute Toxicity Effects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of the WWTPs and Characteristics of the Influent
2.2. Experimental Tests
2.3. Analytical Methods
3. Results and Discussion
3.1. WWTPs Performance Evaluation
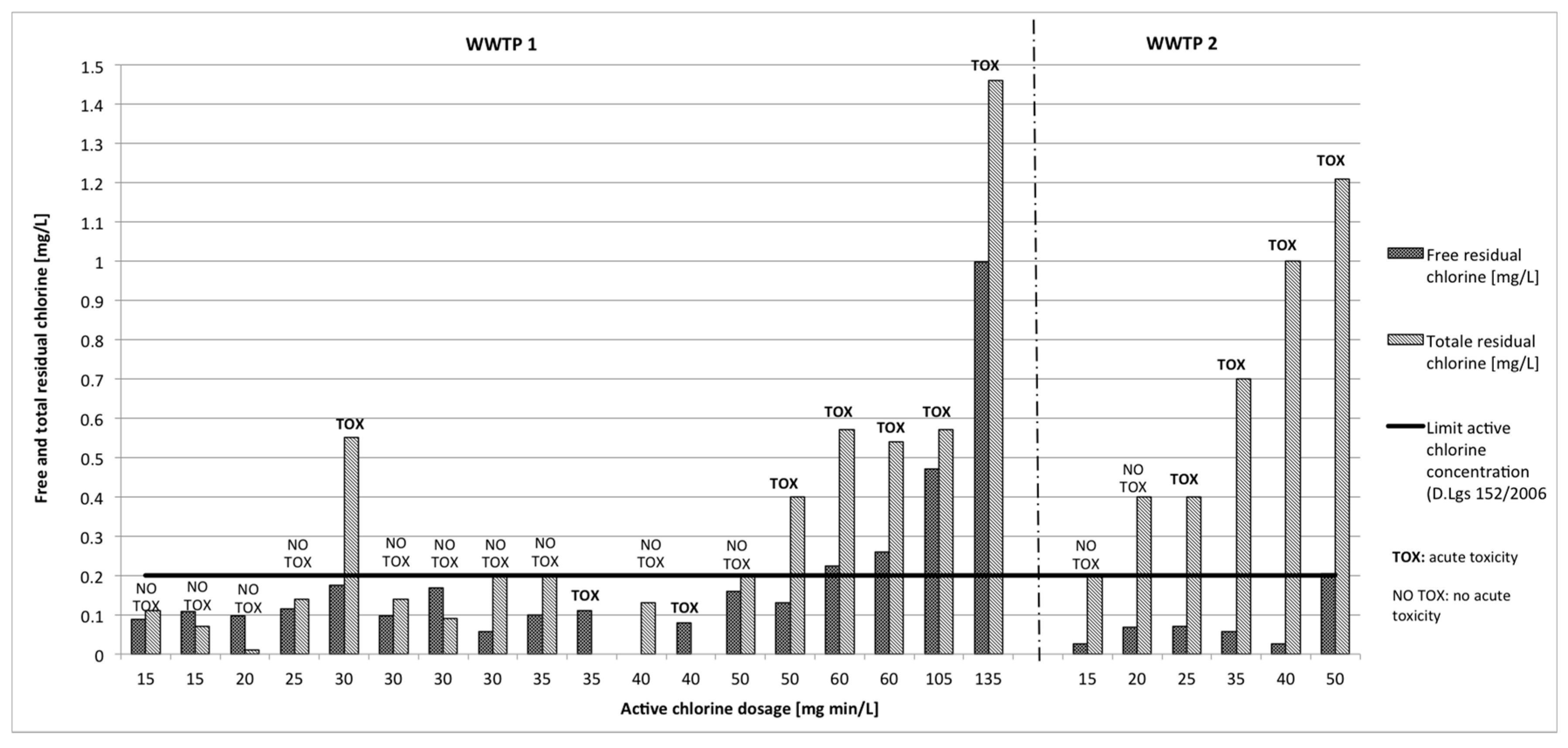
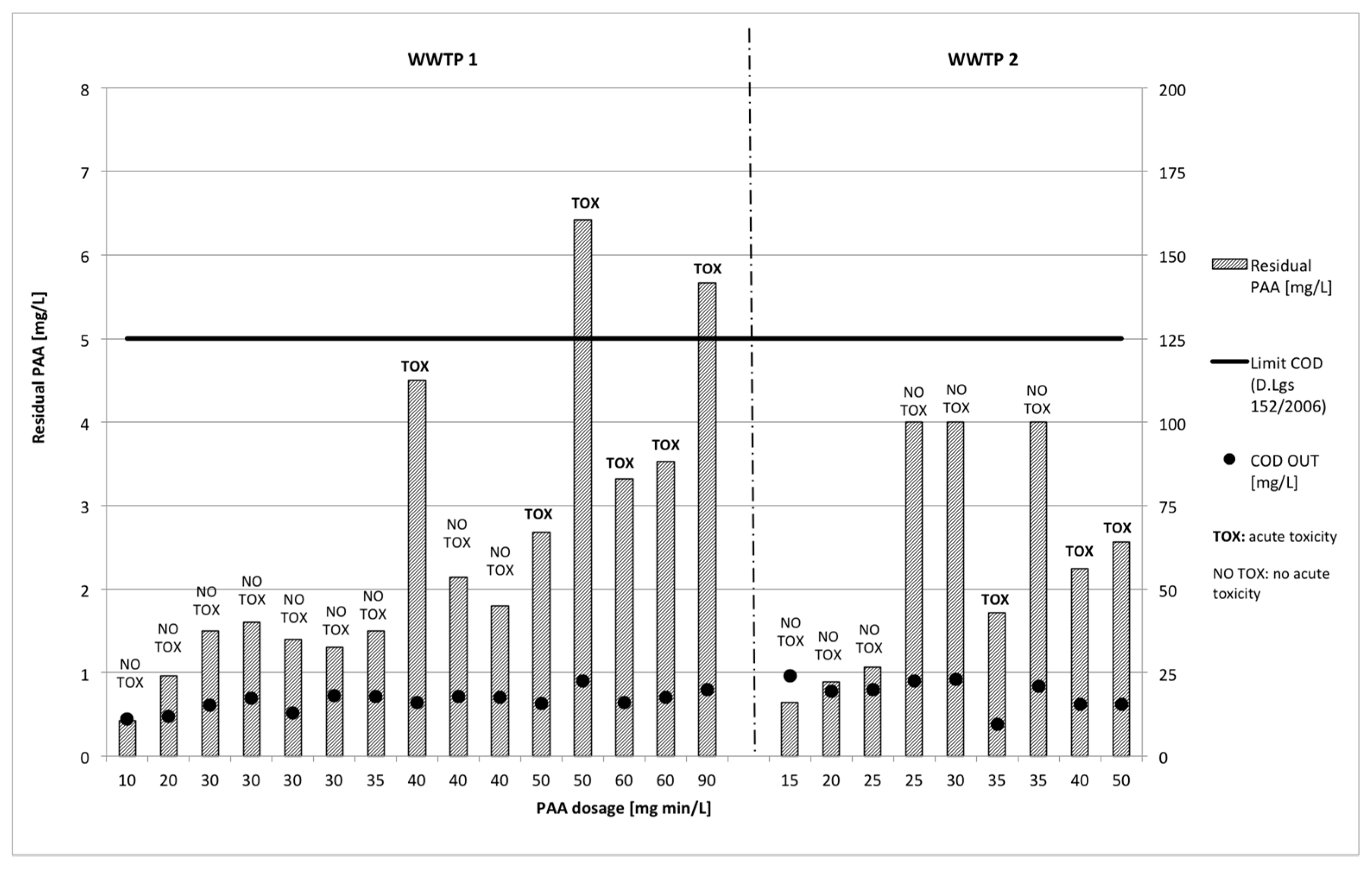
3.2. Disinfectant Residual
3.2.1. Sodium Hypochlorite
3.2.2. Peracetic Acid
3.3. Acute Toxicity Assessment
- in the case of Daphnia magna, the percentage of immobilisation is greater or equal to 50%;
- in the case of Vibrio fischeri, the inhibition of luminescence is greater or equal to 50%;
- in the case of Pseudokirchneriella subcapitata, inhibition of algal growth is greater or equal to 50%.
3.3.1. Sodium Hypochlorite
3.3.2. Peracetic Acid
3.4. OUR Tests
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Monarca, S.; Feretti, D.; Collivignarelli, C.; Guzzella, L.; Zerbini, I.; Bertanza, G.; Pedrazzani, R. The influence of different disinfectants on mutagenicity and toxicity of urban wastewater. Water Res. 2000, 34, 4261–4269. [Google Scholar] [CrossRef]
- Blatchley, E.R., III; Hunt, B.A.; Duggirala, R.; Thompson, J.E.; Zhao, J.; Halaby, T.; Cowger, R.L.; Straub, C.M.; Alleman, J.E. Effects of disinfectants on wastewater effluent toxicity. Water Res. 1997, 31, 1581–1588. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, X. Comparative Developmental Toxicity of New Aromatic Halogenated DBPs in a Chlorinated Saline Sewage Effluent to the Marine Polychaete Platynereis dumerilii. Environ. Sci. Technol. 2013, 47, 10868–10876. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Liu, J.; Zhang, X.; Richardson, S.D. Comparative Toxicity of Chlorinated Saline and Freshwater Wastewater Effluents to Marine Organisms. Environ. Sci. Technol. 2015, 49, 14475–14483. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, X. Comparative toxicity of new halophenolic DBPs in chlorinated saline wastewater effluents against a marine alga: Halophenolic DBPs are generally more toxic than haloaliphatic ones. Water Res. 2014, 65, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-X.; Wu, Q.-Y.; Hu, H.-Y.; Tian, J. Effect of ammonia on the formation of THMs and HAAs in secondary effluent chlorination. Chemosphere 2009, 76, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.N.; Wang, X.C.; Ma, X.Y. Characteristics of THMFP increase in secondary effluent and its potential toxicity. J. Hazard. Mater. 2013, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Sorlini, S.; Biasibetti, M.; Gialdini, F.; Collivignarelli, M.C. How can drinking water treatments influence chlorine dioxide consumption and by-product formation in final disinfection? Water Sci. Technol. Water Supply 2016, 16. [Google Scholar] [CrossRef]
- Sorlini, S.; Collivignarelli, M.C.; Canato, M. Effectiveness in chlorite removal by two activated carbons under different working conditions: A laboratory study. J. Water Supply Res. Technol. AQUA 2015, 64, 450–461. [Google Scholar] [CrossRef]
- Watson, K.; Shaw, G.; Leusch, F.D.L.; Knight, N.L. Chlorine disinfection by-products in wastewater effluent: Bioassay-based assessment of toxicological impact. Water Res. 2012, 46, 6069–6083. [Google Scholar] [CrossRef] [PubMed]
- De Souza, J.B.; Queiroz Valdez, F.; Jeranoski, R.F.; de Sousa Vidal, C.M.; Cavallini, G.S. Water and Wastewater Disinfection with Peracetic Acid and UV Radiation and Using Advanced Oxidative Process PAA/UV. Int. J. Photoenergy 2014. [Google Scholar] [CrossRef]
- Kitis, M. Disinfection of wastewater with peracetic acid: A review. Environ. Int. 2004, 30, 47–55. [Google Scholar] [CrossRef]
- Antonelli, M.; Rossi, S.; Mezzanotte, V.; Nurizzo, C. Secondary effluent disinfection: PAA long term efficiency. Environ. Sci. Technol. 2006, 40, 4771–4775. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Antonelli, M.; Mezzanotte, V.; Nurizzo, C. Peracetic acid disinfection: A feasible alternative to wastewater chlorination. Water Environ. Res. 2007, 79, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Mezzanotte, V.; Antonelli, M.; Citterio, S.; Nurizzo, C. Wastewater Disinfection Alternatives: Chlorine, Ozone, Peracetic Acid, and UV Light. Water Environ. Res. 2007, 79, 2373–2379. [Google Scholar] [CrossRef] [PubMed]
- Luukkonen, T.; Teeriniemi, J.; Prokkola, H.; Rämö, J.; Lassi, U. Chemical aspects of peracetic acid based wastewater disinfection. Water SA 2014, 40. [Google Scholar] [CrossRef]
- Dell’Erba, A.; Falsanisi, D.; Liberti, L.; Notarnicola, M.; Santoro, D. Disinfection by-products formation during wastewater disinfection with peracetic acid. Desalination 2007, 215, 177–186. [Google Scholar] [CrossRef]
- Regulations on Environmental Matters; Decree Italian Law 152/2006. 2006. Available online: http://www.gazzettaufficiale.it/atto/serie_generale/caricaDettaglioAtto/originario?atto.dataPubblicazioneGazzetta=2006-04-14&atto.codiceRedazionale=006G0171 (accessed on 10 July 2017).
- Directive 2000/60/EC, 23 October 2000. Establishing a Framework for Community Actions in the Field of Water Policy. Available online: http://ec.europa.eu/environment/water/water-framework/index_en.html (accessed on 10 July 2017).
- Farré, M.L.; García, M.-J.; Tirapu, L.; Ginebreda, A.; Barceló, D. Wastewater toxicity screening of non-ionic surfactants by Toxalert® and Microtox® bioluminescence inhibition assays. Anal. Chim. Acta 2001, 427, 181–189. [Google Scholar] [CrossRef]
- Pignata, C.; Fea, E.; Rovere, R.; Degan, R.; Lorenzi, E.; de Ceglia, M.; Schilirò, T.; Gilli, G. Chlorination in a wastewater treatment plant: Acute toxicity effects of the effluent and of the recipient water body. Environ. Monit. Assess. 2012, 184, 2091–2103. [Google Scholar] [CrossRef] [PubMed]
- Papa, M.; Bertanza, G.; Abbà, A. Reuse of wastewater: A feasible option, or not? A decision support system can solve the doubt. Desalination Water Treat. 2015. [Google Scholar] [CrossRef]
- Raboni, M.; Gavasci, R.; Torretta, V. Assessment of the Fate of Escherichia coli in Different Stages of Wastewater Treatment Plants. Water Air Soil Pollut. 2016, 227, 455. [Google Scholar] [CrossRef]
- Da Costa, J.B.; Rodgher, S.; Daniel, L.A.; Espındola, E.L.G. Toxicity on aquatic organisms exposed to secondary effluent disinfected with chlorine, peracetic acid, ozone and UV radiation. Ecotoxicology 2014, 23, 1803–1813. [Google Scholar] [CrossRef] [PubMed]
- Farré, M.; Arranz, F.; Ribó, J.; Barceló, D. Interlaboratory study of the bioluminescence inhibition tests for rapid wastewater toxicity assessment. Talanta 2004, 62, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Choi, S.; Lee, S.; Kweon, J.; Song, J. Comparison of formation of disinfection by-products by chlorination and ozonation of wastewater effluents and their toxicity to Daphnia magna. Environ. Pollut. 2016, 215, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Amodei, M.; Azzoni, R.; Marina Pocar, M. Daphnia magna nel monitoraggio ambientale. Biol. Ambient. 2000, 14, 13–20. [Google Scholar]
- Antonelli, M.; Mezzanotte, V.; Panouillères, M. Assessment of peracetic acid disinfected effluents by microbiotests. Environ. Sci. Technol. 2009, 43, 6579–6584. [Google Scholar] [CrossRef] [PubMed]
- Tisler, T.; Zagorc-Koncan, J.; Cotman, M.; Drolc, A. Toxicity potential of disinfection agent in tannery wastewater. Water Res. 2004, 38, 3503–3510. [Google Scholar] [CrossRef] [PubMed]
- APAT-CNR-IRSA. Metodi Analitici per le Acque. Manuali e Linee Guida; 2003. Available online: http://www.isprambiente.gov.it/it/pubblicazioni/manuali-e-linee-guida/metodi-analitici-per-le-acque (accessed on 10 July 2017).
- Qualità Dell’acqua—Determinazione Dell’azoto Totale. 1992. Available online: http://www.unichim.it/index.php?n=Pubblicazioni.MetodiAcque (accessed on 10 July 2017).
- Qualità Dell’acqua—Determinazione Dell’azoto Ammoniacale in Acque di Diversa Natura Mediante Prova in Cuvette. UNICHIM 2363/2009. 2017. Available online: http://www.unichim.it/index.php?n=Pubblicazioni.MetodiAcque (accessed on 10 July 2017).
- Qualità Dell’acqua—Determinazione del Fosfato Solubile e del Fosforo Totale; International Organization for Standardization: Geneva, Switzerland, 2008; Available online: http://www.unichim.it/index.php?n=Pubblicazioni.MetodiAcque (accessed on 10 July 2017).
- Hach 10070 Pillows Powder. Available online: https://www.hach.com/dpd-total-chlorine-reagent-powder-pillows-25-ml-pk-100/product-downloads?id=7640188396 (accessed on 10 July 2017).
- Water Quality—Test for Inhibition of Oxygen Consumption by Activated Sludge for Carbonaceous and Ammonium Oxidation; International Organization for Standardization: Geneva, Switzerland, 2007; Available online: https://www.iso.org/standard/37369.html (accessed on 10 July 2017).
- Water Quality—Enumeration of Escherichia coli and Coliform Bacteria; International Organization for Standardization: Geneva, Switzerland, 2012; Available online: https://www.iso.org/standard/52246.html (accessed on 10 July 2017).
- Water Quality—Determination of the Inhibition of the Mobility of Daphnia Magna Straus (Cladocera, Crustacea). Acute Toxicity Test; International Organization for Standardization: Geneva, Switzerland, 2013; Available online: https://www.iso.org/standard/54614.html (accessed on 10 July 2017).
- Water Quality—Determination of the Inhibitory Effect of Water Samples on the Light Emission of Vibrio Fischeri (Luminescent bacteria Test)—Part 3: Method Using Freeze-Dried Bacteria; International Organization for Standardization: Geneva, Switzerland, 2009; Available online: https://www.iso.org/standard/40518.html (accessed on 10 July 2017).
- Water Quality—Fresh Water Algal Growth Inhibition Test with Unicellular Green Algae; International Organization for Standardization: Geneva, Switzerland, 2012; Available online: https://www.iso.org/standard/54150.html (accessed on 10 July 2017).
- Lombardy Regional Regulation No. 3/2006. Available online: http://www.atocittametropolitanadimilano.it/documenti/Regolamenti%20Regionali%2028%20marzo%202006.pdf (accessed on 10 July 2017).
- Aragno, M.; Cometto, P.; Beccaria, T.; Ghigo, M.; Vincenzi, M. Batterie di saggi ecotossicologici: Sintesi e prospettive dopo 13 anni di controlli ambientali in provincia di Cuneo. Atti 6° Edizione Giornate di Studio. 2014, pp. 147–151. Available online: http://www.artaabruzzo.it/download/pubblicazioni/20170523_atti_ecotossicologia_livorno_2016.pdf (accessed on 21 September 2017).
- Arizzi Novelli, A.; Melchiorri, M.; Mastrangioli, L.; Di Deo, N.; Sergiacomo, G.; Scamosci, E.; Surricchio, G.; Spatola Mayo, C. Valutazione degli effetti della disinfezione con acido peracetico sulle acque di scarico urbane. In Atti Delle Giornate di Studio su: L’ecotossicologia Come Strumento di Gestione; ISPRA: Livorno, Italy, 2016. [Google Scholar]
- Petala, M.; Samaras, P.; Zouboulis, A.; Koungolos, A.; Sakellaropoulos, G.P. Influence of ozonation on the in vitro mutagenic and toxic potential of secondary effluents. Water Res. 2008, 42, 4929–4940. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, A.; Samaras, P.; Ntampou, X.; Petala, M. Potential ozone applications for water/wastewater treatment. Sep. Sci. Technol. 2007, 42, 1433–1466. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Yang, M.; Liu, J.; Li, W.; Graham, N.J.D.; Li, X.; Yang, B. Three-step effluent chlorination increases disinfection efficiency and reduces DBP formation and toxicity. Chemosphere 2017, 168, 1302–1308. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, M.; Zhang, X.; Jiang, J.; Liu, J.; Yau, C.F.; Graham, N.J.D.; Li, X. Two-step chlorination: A new approach to disinfection of a primary sewage effluent. Water Res. 2017, 108, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Young, J.C. Waste strength and water pollution parameters. In Water Analysis—Organic Species; Minear, R.A., Keith, L.H., Eds.; Academic Press Inc.: Cambridge, MA, USA, 1984; ISBN 9780124983038. [Google Scholar]


| Type of Treatment |  | ||
|---|---|---|---|
| WWTP 1 | WWTP 2 | ||
| Capacity (Population Equivalent) | 12,000 | 10,000 | |
| Influent | Flow rate (m3 d−1) | 2980 | 2000 |
| COD (mg L−1) | 260 ± 158 | 433 ± 508.5 | |
| BOD5 (mg L−1) | 119 ± 90 | 199 ± 167.6 | |
| TN (mg L−1) | 35 ± 25 | 51 ± 43.6 | |
| TP (mg L−1) | 4 ± 5.7 | 6 ± 8.3 | |
| TSS (mg L−1) | 170 ± 230.3 | 243 ± 468.6 | |
| E. coli (CFU 100 mL−1) | 4800 ÷ 26,000 | 4500 ÷ 7600 | |
| Type of Disinfectant Agents | Dosage of Disinfectant (mg min L−1) | Contact Time (min) | Analysis/Tests | |||||
|---|---|---|---|---|---|---|---|---|
| Parameters Measured on Site | Physical-Chemical Analysis | Microbiological Analysis | Ecotoxicological Tests | Respirometric Tests | ||||
| WWTP 1 | NaClO | 15 ÷ 135 | 30 | Free and total residual chlorine; pH | COD, BOD5, TSS, TN, N-NH4+, N-NO3−, N-NO2−, TP | E. coli | Daphnia magna, Vibrio fischeri, Pseudokirchneriella subcapitata | endogenous OUR; exogenous OUR |
| CH3CO3H | 10 ÷ 90 | 30 | residual PAA; pH | |||||
| WWTP 2 | NaClO | 15 ÷ 50 | 30 | Free and total residual chlorine; pH | COD, BOD5, TSS, TN, N-NH4+, N-NO3−, N-NO2−, TP | E. coli | Daphnia magna, Vibrio fischeri, Pseudokirchneriella subcapitata | endogenous OUR; exogenous OUR |
| CH3CO3H | 15 ÷ 50 | 30 | residual PAA; pH | |||||
| COD (mg L−1) | BOD5 (mg L−1) | SST (mg L−1) | TN (mg L−1) | N-NH4+ (mg L−1) | N-NO3− (mg L−1) | N-NO2− (mg L−1) | TP (mg L−1) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| WWTP 1 | DAY #1 | IN WWTP | 381 | 203 | 196 | 45.7 | 36.0 | 1.2 | 0.75 | 5.6 | |
| IN test | 13.4 | <5 | 5 | 10.9 | <0.2 | 11 | 0.04 | 1 | |||
| OUT | NaClO | 13.4 ÷ 14 | <5 | 5 ÷ 6 | 10.9 ÷ 11.1 | <0.2 | 11 ÷ 11.3 | 0.04 ÷ 0.05 | 1 ÷ 1.1 | ||
| PAA | 15.2 ÷ 19.9 | <5 ÷ 7.6 | <5 | 11.2 ÷11.6 | <0.1 ÷ 0.1 | 11.4 ÷ 12.3 | 0.04 ÷ 0.09 | 1 ÷ 1.1 | |||
| DAY #2 | IN WWTP | 270 | 142 | 150 | 40.9 | 35.0 | 1.0 | 0.67 | 4.8 | ||
| IN test | 13.9 | <5 | 5 | 10.8 | 0.1 | 8.6 | <0.01 | 1 | |||
| OUT | NaClO | 13.9 ÷ 14.7 | <5 | 5 ÷ 5.2 | 10.8 ÷ 10.9 | 0.1 ÷ 0.2 | 8.6 ÷ 8.8 | <0.01 | 1 ÷ 1.1 | ||
| PAA | 15.8 ÷ 17.5 | <5 ÷ 7.6 | 5 ÷ 5.1 | 10.8 ÷ 11.2 | 0.1 ÷ 0.2 | 8.6 ÷ 9.2 | <0.01 ÷ 0.01 | 1 ÷ 1.2 | |||
| DAY #3 | IN WWTP | 290 | 117 | 138 | 21.9 | 11.8 | 1.5 | 0.53 | 21.9 | ||
| IN test | 15.7 | 11.5 | <5 | 10.5 | 0.11 | 9.8 | 0.06 | 1.3 | |||
| OUT | NaClO | 15.7 ÷ 16.3 | 11.5 ÷ 12 | <5 | 10.5 ÷ 10.6 | 0.11 ÷ 0.13 | 9.8 ÷ 9.9 | 0.06 ÷ 0.07 | 1.3 ÷ 1.4 | ||
| PAA | 17.3 ÷ 22.4 | 12 ÷ 15.3 | <5 | 10.4 ÷ 10.6 | 0.14 ÷ 0.15 | 10 ÷ 10.5 | 0.05 ÷ 0.07 | 1.3 ÷ 1.4 | |||
| DAY #4 | IN WWTP | 69 | 29 | 54 | 17.3 | 2.7 | 1 | 0.67 | 2.4 | ||
| IN test | 12 | 14 | <5 | 12.7 | <0.1 | 12.6 | 0.04 | 0.7 | |||
| OUT | NaClO | 12 ÷ 12.2 | 14 ÷ 14.6 | <5 | 12.7 ÷ 12.9 | <0.1 | 12.6 ÷ 12.7 | 0.04 ÷ 0.05 | 0.7 ÷ 0.8 | ||
| PAA | 11 ÷ 13 | 15 ÷ 19 | <5 | 12.3 ÷ 13.5 | <0.1 | 12.6 ÷ 13.1 | 0.03 ÷ 0.04 | 0.7 ÷ 0.9 | |||
| DAY #5 | IN WWTP | 266 | 148 | 216 | 41.3 | 34 | 1.1 | 0.55 | 4.4 | ||
| IN test | 22.6 | 13.1 | 6 | 15 | <0.1 | 14.9 | 0.03 | 1.2 | |||
| OUT | NaClO | 22.6 ÷ 22.7 | 13.1 ÷ 14 | 6 ÷ 6.1 | 15 ÷ 15.1 | <0.1 | 14.9 ÷ 15.1 | 0.03 ÷ 0.04 | 1.2 ÷ 1.3 | ||
| PAA | 17.5 ÷ 18.1 | 18.6 ÷ 20.8 | <5 ÷ 5 | 14.9 ÷ 15 | <0.1 | 14.7 ÷ 14.9 | 0.02 ÷ 0.05 | 1.2 ÷ 1.3 | |||
| DAY #6 | IN WWTP | 172 | 79 | 60 | 32 | 29 | 1.2 | 0.76 | 3.2 | ||
| IN test | 31.4 | 14.1 | 13 | 13.8 | 1.8 | 11.6 | 0.4 | 1.3 | |||
| OUT | NaClO | 31.4 ÷ 32 | 14.1 ÷ 14.7 | 13 ÷ 13.1 | 13.8 ÷ 13.9 | 1.8 ÷ 1.9 | 11.6 ÷ 11.8 | 0.40 ÷ 0.41 | 1.3 ÷ 1.5 | ||
| PAA | 30.4 ÷ 33 | 15.8 ÷ 18 | 12 ÷ 13 | 13.9 ÷ 14.7 | 1.8 ÷ 2.1 | 11.3 ÷ 11.5 | 0.35 ÷ 0.36 | 1.3 ÷ 1.6 | |||
| WWTP 2 | DAY #1 | IN WWTP | 304 | 153 | 156 | 33.4 | 32.8 | 0.98 | 0.15 | 3.6 | |
| IN test | 9.4 | <5 | <5 | 2 | <0.2 | 1.8 | n.a. | 0.3 | |||
| OUT | NaClO | 9.4 ÷ 9.6 | <5 | <5 | 2 ÷ 2.3 | <0.2 | 1.8 ÷ 2 | n.a. | 0.3 ÷ 0.5 | ||
| PAA | 9.6 ÷ 15.6 | <5 | <5 | 2.3 ÷ 3.8 | <0.1 | 2.2 ÷ 2.3 | n.a. | 0.3 ÷ 0.6 | |||
| DAY #2 | IN WWTP | 413 | 155 | 152 | 39.3 | 37.5 | 1.09 | 0.18 | 4.5 | ||
| IN test | 25.2 | 8.2 | 12 | 5.4 | <0.1 | 4.2 | 0.08 | 0.7 | |||
| OUT | NaClO | 25.2 ÷ 25.4 | 8.2 ÷ 8.3 | 12 ÷ 12.3 | 5.4 ÷ 5.5 | <0.1 | 4.2 ÷ 4.3 | 0.08 ÷ 0.09 | 0.7 ÷ 0.8 | ||
| PAA | 25 ÷ 35 | 6 ÷ 6.5 | 8 ÷ 10 | 5.3 ÷ 5.4 | <0.1 ÷ 0.1 | 4.4 ÷ 4.5 | 0.08 ÷ 0.09 | 0.6 ÷ 0.7 | |||
| DAY #3 | IN WWTP | 452 | 251 | 280 | 44.6 | 38.2 | 1.1 | 0.21 | 5.9 | ||
| IN test | 20.1 | <5 | 5 | 3.9 | <0.1 | 2.7 | 0.01 | 0.3 | |||
| OUT | NaClO | 20.1 ÷ 20.4 | <5 | 5 ÷ 5.6 | 3.9 ÷ 4.1 | <0.1 | 2.7 ÷ 2.8 | 0.01 ÷ 0.02 | 0.3 ÷ 0.4 | ||
| PAA | 19.5 ÷ 24 | <5 | 5 ÷ 6 | 3.3 ÷ 3.8 | <0.1 | 2.8 ÷ 2.9 | 0.01 ÷ 0.03 | 0.3 ÷ 0.4 | |||
| Limit values (mg L−1) | 125 | 25 | 35 | 15 | - | - | - | 2 | |||
| Active Chlorine Dosage (mg min L−1) | Free Residual Chlorine (mg L−1) | Acute Toxicity | E. coli (CFU 100 mL−1) | Conformity | |||||
|---|---|---|---|---|---|---|---|---|---|
| Daphnia magna 24 h | Vibrio fischeri | P. subcapitata 72 h | IN | OUT | |||||
| 15 min | 30 min | ||||||||
| WWTP 1 | 60 | 0.26 | 100/100 | 81/100 | 81/100 | 27/100 | 4800 | 5 | NO |
| 105 | 0.47 | 0/100 | 88/100 | 88/100 | 37/100 | 4800 | <1 | NO | |
| 135 | 0.998 | 0/100 | 87/100 | 87/100 | 59/100 | 4800 | <1 | NO | |
| 15 | 0.088 | 0/100 | 0/100 | 0/100 | 0/100 | 7000 | 5400 | NO | |
| 30 | 0.175 | 90/100 | 83/100 | 82/100 | 90/100 | 7000 | <1 | NO | |
| 60 | 0.224 | 100/100 | 82/100 | 82/100 | 100/100 | 7000 | <1 | NO | |
| 15 | 0.109 | 0/100 | 0/100 | 1/100 | 5/100 | 26,000 | 21,000 | NO | |
| 25 | 0.114 | 0/100 | 23/100 | 31/100 | 1/100 | 26,000 | 9500 | NO | |
| 30 | 0.096 | 0/100 | 18/100 | 25/100 | 8/100 | 26,000 | 9300 | NO | |
| 20 | 0.097 | 5/100 | 0/100 | 5/100 | 1/100 | 8100 | 3800 | YES | |
| 30 | 0.169 | 0/100 | 0/100 | 5/100 | 3/100 | 8100 | 320 | YES | |
| 40 | 0.129 | 45/100 | 9/100 | 13/100 | 16/100 | 8100 | 74 | YES | |
| 30 | 0.058 | 0/100 | 11/100 | 16/100 | 2/100 | 6100 | 3700 | YES | |
| 35 | 0.1 | 0/100 | 14/100 | 20/100 | 1/100 | 6100 | 290 | YES | |
| 50 | 0.13 | 0/100 | 40/100 | 42/100 | 3/100 | 6100 | 17 | YES | |
| WWTP 2 | 35 | 0.057 | 0/100 | 80/100 | 78/100 | 70/100 | 7100 | 58 | NO |
| 40 | 0.05 | 0/100 | 86/100 | 88/100 | 47/100 | 7100 | 37 | NO | |
| 50 | 0.203 | 100/100 | 91/100 | 93/100 | 65/100 | 7100 | 21 | NO | |
| 25 | n.a. | 100/100 | 91/100 | 93/100 | 65/100 | 4500 | 38 | NO | |
| 30 | n.a. | 100/100 | 0/100 | 5/100 | 8/100 | 4500 | 33 | NO | |
| 35 | n.a. | 100/100 | 8/100 | 6/100 | 1/100 | 4500 | 36 | NO | |
| 15 | 0.05 | 5/100 | 0/100 | 0/100 | 4/100 | 7600 | 5000 | YES | |
| 20 | 0.069 | 0/100 | 31/100 | 25/100 | 0/100 | 7600 | 100 | YES | |
| 25 | 0.07 | 0/100 | 6/100 | 6/100 | 0/100 | 7600 | 230 | YES | |
| PAA Dosage (mg min L−1) | Residual PAA (mg L−1) | Acute toxicity | E. coli (CFU 100 mL−1) | Conformity | |||||
|---|---|---|---|---|---|---|---|---|---|
| Daphnia magna 24 h | Vibrio fischeri | P. subcapitata 72 h | IN | OUT | |||||
| 15 min | 30 min | ||||||||
| WWTP 1 | 30 | 1.50 | 0/100 | 13/100 | 5/100 | 5/100 | 4800 | 1 | YES |
| 60 | 3.32 | 100/100 | 100/100 | 96/100 | 100/100 | 4800 | <1 | NO | |
| 90 | 5.67 | 100/100 | 100/100 | 100/100 | 54/100 | 4800 | <1 | NO | |
| 40 | 4.49 | 100/100 | 99/100 | 99/100 | 100/100 | 7000 | <1 | NO | |
| 50 | 2.68 | 20/100 | 71/100 | 37/100 | 20/100 | 7000 | <1 | NO | |
| 60 | 3.53 | 100/100 | 100/100 | 96/100 | 100/100 | 7000 | <1 | NO | |
| 30 | 1.61 | 0/100 | 13/100 | 20/100 | 6/100 | 26,000 | 38 | YES | |
| 40 | 2.14 | 0/100 | 5/100 | 10/100 | 4/100 | 26,000 | 29 | YES | |
| 50 | 6.42 | 100/100 | 86/100 | 51/100 | 73/100 | 26,000 | 8 | NO | |
| 20 | 0.43 | 0/100 | 0/100 | 0/100 | 0/100 | 8100 | 5000 | YES | |
| 30 | 0.96 | 0/100 | 0/100 | 3/100 | 0/100 | 8100 | 180 | YES | |
| 40 | 1.39 | 0/100 | 23/100 | 16/100 | 3/100 | 8100 | 15 | YES | |
| 30 | 1.30 | 0/100 | 19/100 | 13/100 | 0/100 | 6100 | 16 | YES | |
| 35 | 1.50 | 0/100 | 33/100 | 22/100 | 1/100 | 6100 | 45 | YES | |
| 40 | 1.80 | 0/100 | 45/100 | 32/100 | 3/100 | 6100 | 110 | YES | |
| WWTP 2 | 35 | 1.71 | 5/100 | 82/100 | 44/100 | 1/100 | 7100 | 50 | NO |
| 40 | 2.25 | 5/100 | 100/100 | 80/100 | 1/100 | 7100 | 22 | NO | |
| 50 | 2.57 | 0/100 | 100/100 | 94/100 | 1/100 | 7100 | 37 | NO | |
| 25 | 4.00 | 0/100 | 0/100 | 0/100 | 0/100 | 4500 | 75 | YES | |
| 30 | 4.00 | 0/100 | 0/100 | 0/100 | 0/100 | 4500 | 54 | YES | |
| 35 | 4.00 | 0/100 | 0/100 | 0/100 | 0/100 | 4500 | 21 | YES | |
| 15 | 0.64 | 0/100 | 0/100 | 0/100 | 0/100 | 7600 | 7500 | NO | |
| 20 | 0.07 | 0/100 | 0/100 | 0/100 | 0/100 | 7600 | 3800 | YES | |
| 25 | 0.08 | 0/100 | 3/100 | 0/100 | 1/100 | 7600 | 2600 | YES | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Collivignarelli, M.C.; Abbà, A.; Alloisio, G.; Gozio, E.; Benigna, I. Disinfection in Wastewater Treatment Plants: Evaluation of Effectiveness and Acute Toxicity Effects. Sustainability 2017, 9, 1704. https://doi.org/10.3390/su9101704
Collivignarelli MC, Abbà A, Alloisio G, Gozio E, Benigna I. Disinfection in Wastewater Treatment Plants: Evaluation of Effectiveness and Acute Toxicity Effects. Sustainability. 2017; 9(10):1704. https://doi.org/10.3390/su9101704
Chicago/Turabian StyleCollivignarelli, Maria Cristina, Alessandro Abbà, Gianpaolo Alloisio, Eleonora Gozio, and Ilaria Benigna. 2017. "Disinfection in Wastewater Treatment Plants: Evaluation of Effectiveness and Acute Toxicity Effects" Sustainability 9, no. 10: 1704. https://doi.org/10.3390/su9101704





