Land Use Alters the Plant-Derived Carbon and Nitrogen Pools in Terraced Rice Paddies in a Mountain Village
Abstract
:1. Introduction
2. Materials and Methods
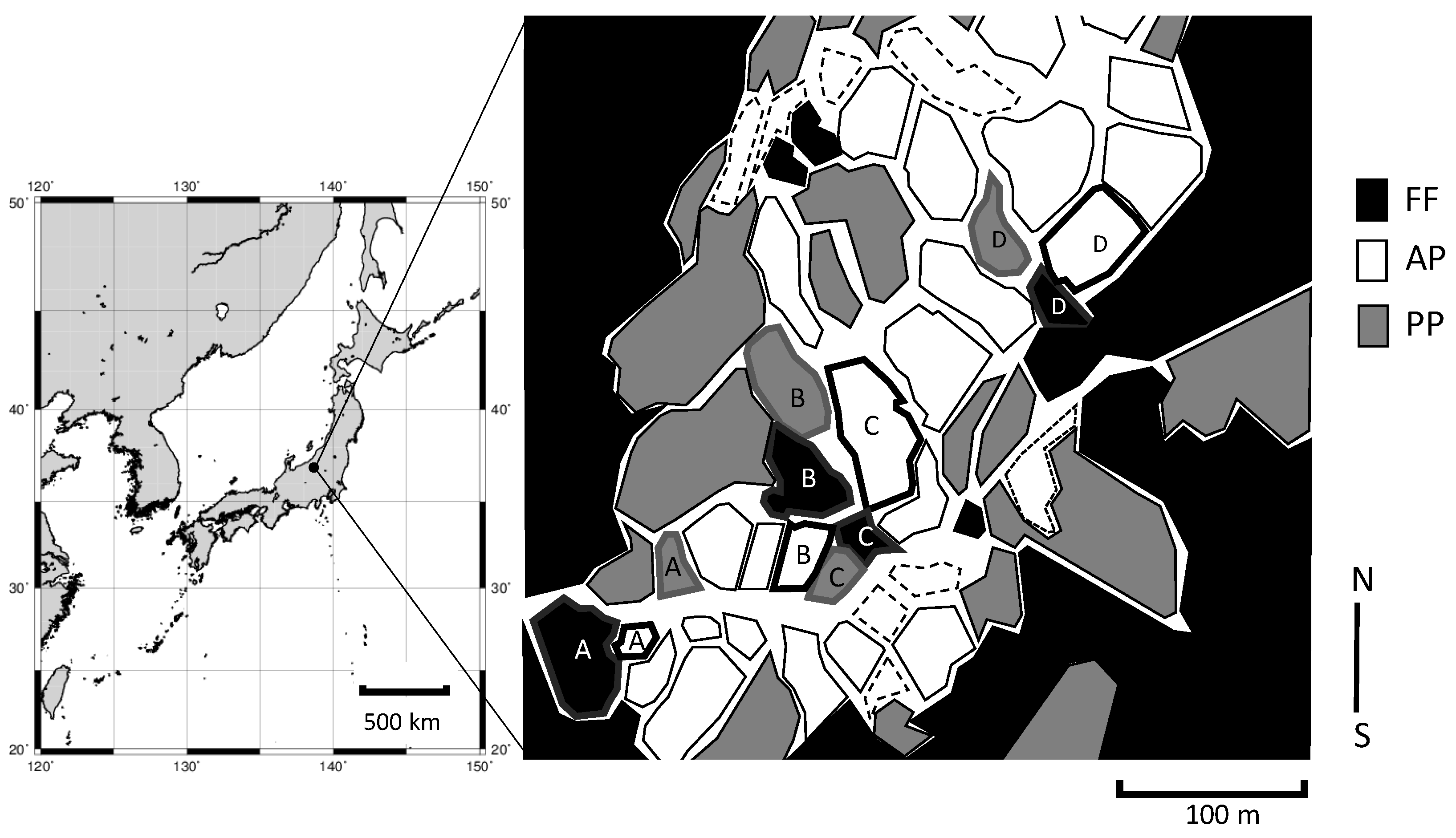
2.1. Site Description
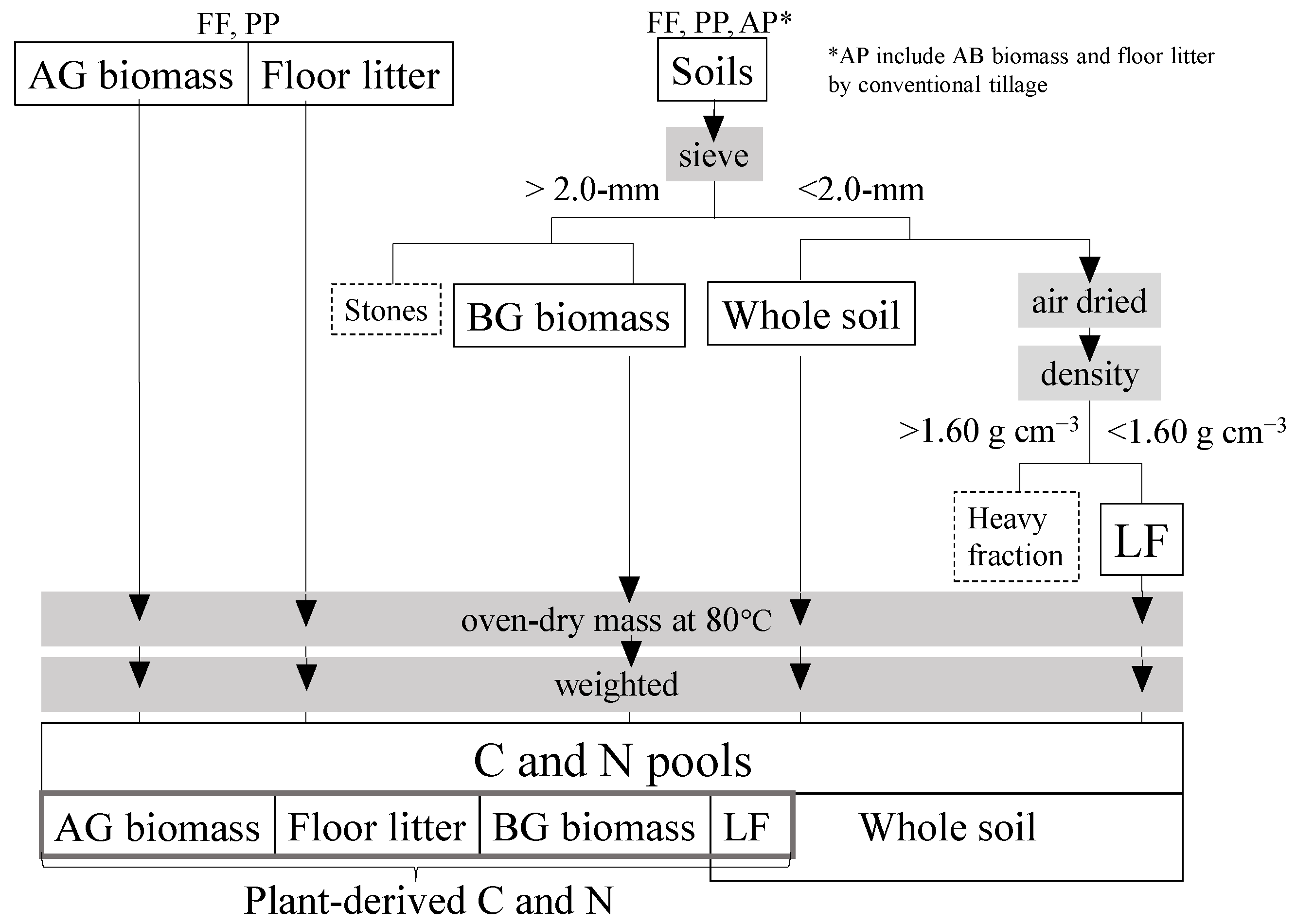
2.2. Estimation of Soil and Plant C and N
2.3. Statistical Analysis
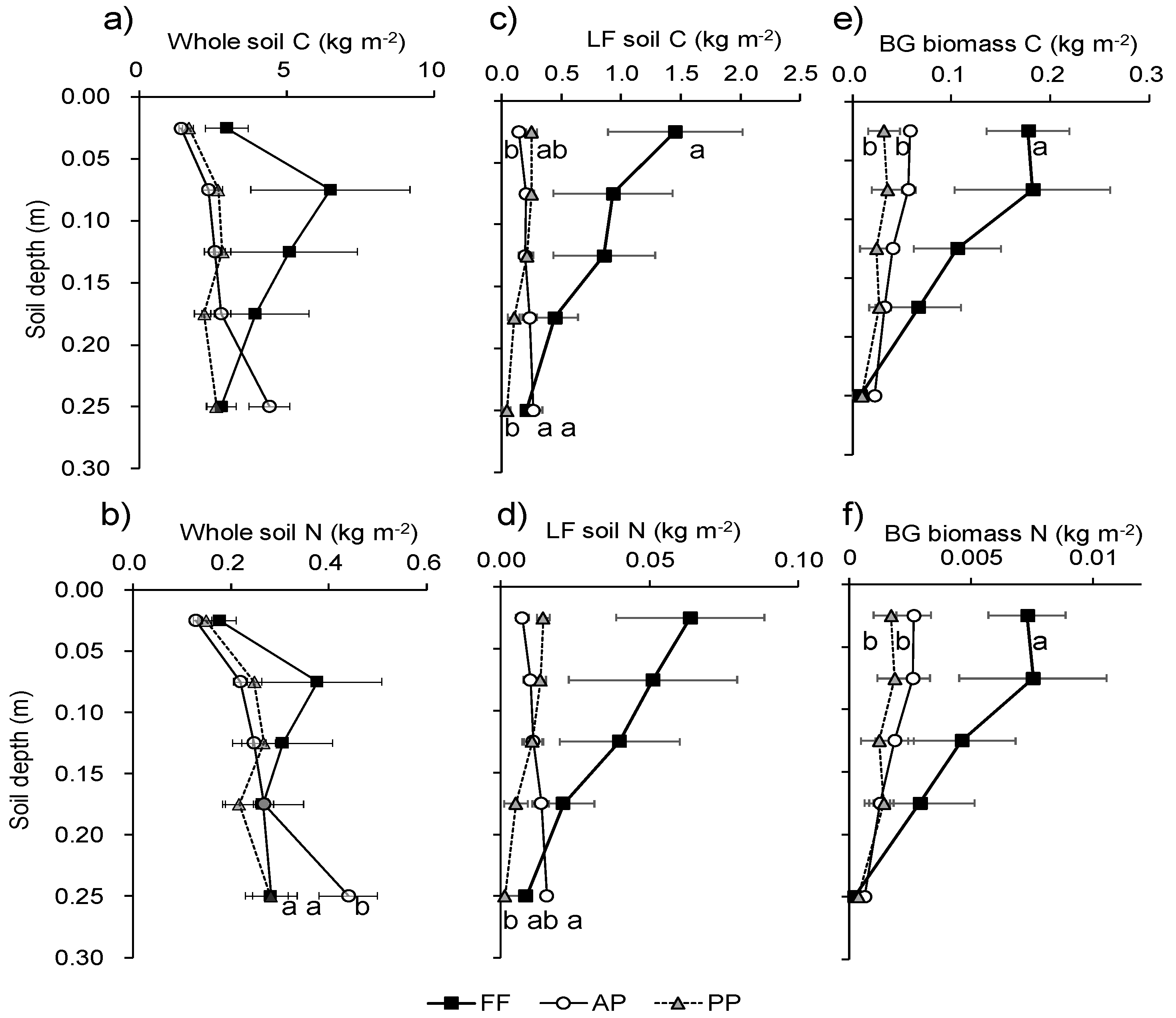
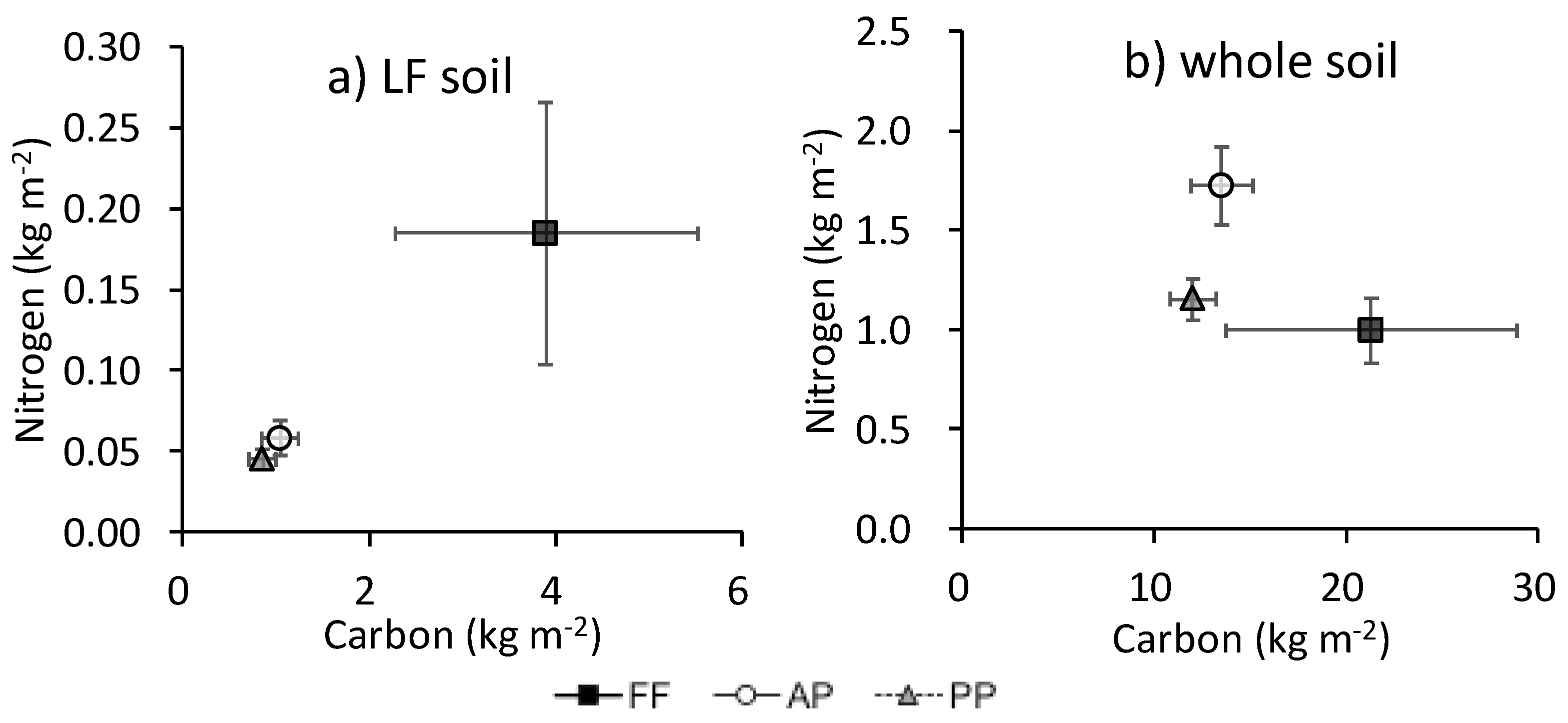
3. Results
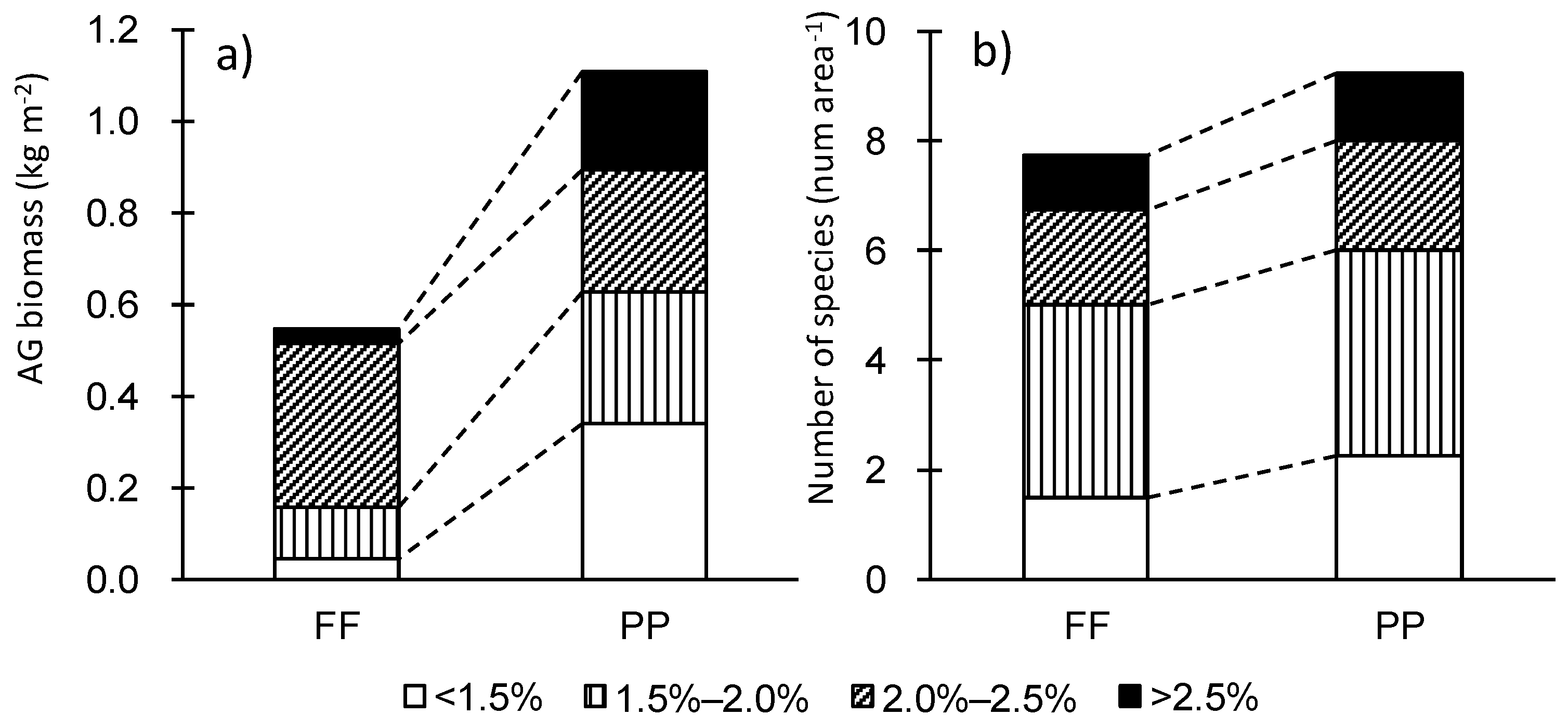
3.1. Plant Species Composition and Biomass
3.2. Soil C, N, and Belowground Biomass at Each Soil Depth
3.3. Total C and N
4. Discussion
4.1. Plant-Derived C and N in Forest Floor Litter and Abandoned Paddies
4.2. Crop-Derived C and N
4.3. Possible Effect of Agricultural Abandonment on Nutrient Cycling
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AG | aboveground |
| AP | agricultural paddy field |
| BG | belowground |
| C | carbon |
| FF | forest floor |
| LF | low-density fraction |
| N | nitrogen |
| OM | organic matter |
| PP | post-agricultural paddy field |
Appendix A
| Area | A | B | C | D | A | B | C | D | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Land Use | FF | FF | FF | FF | PP | PP | PP | PP | ||
| Life | Family | Species | ||||||||
| Annuals | Asteraceae | Bidens frondosa | 100 | 42 | ||||||
| Annuals | Balsaminaceae | Impatiens noli-tangere | 77 | |||||||
| Annuals | Commelinaceae | Commelina communis | 4 | |||||||
| Annuals | Fabaceae | Amphicarpaea bracteata subsp. edgeworthii | 42 | 12 | 5 | 7 | ||||
| Annuals | Fabaceae | Desmodium paniculatum | 10 | |||||||
| Annuals | Fabaceae | Vigna angularis var. nipponensis | 31 | 85 | ||||||
| Annuals | Poaceae | Digitaria ciliaris | 17 | 7 | ||||||
| Annuals | Poaceae | Oplismenus undulatifolius | 15 | |||||||
| Annuals | Polygonaceae | Persicaria longiseta | 3 | |||||||
| Annuals | Polygonaceae | Polygonum thunbergii | 12 | 58 | 506 | 4 | 266 | |||
| Perennials | Apiaceae | Hydrocotyle ramiflora | 2 | 3 | ||||||
| Perennials | Apiaceae | Oenanthe javanica | 1 | |||||||
| Perennials | Asteraceae | Artemisia indica | 89 | 20 | 544 | |||||
| Perennials | Asteraceae | Petasites japonicus | 86 | 2 | ||||||
| Perennials | Houttuynia | Houttuynia cordata | 97 | 110 | 2 | 17 | ||||
| Perennials | Fabaceae | Pueraria lobata | ||||||||
| Perennials | Onagraceae | Oenothera odorata | 1 | 1 | ||||||
| Perennials | Plantaginaceae | Plantago asiatica | 1 | |||||||
| Perennials | Poaceae | Eragrostis ferruginea | 11 | 5 | ||||||
| Perennials | Poaceae | Miscanthus sinensis | 8 | 35 | 268 | 34 | 325 | 713 | ||
| Perennials | Poaceae | Sasa nipponica | 734 | 4 | ||||||
| Perennials | Polygonaceae | Reynoutria japonica | 44 | 6 | ||||||
| Perennials | Equisetaceae | Equisetum arvense | 18 | |||||||
| Perennials | Thelypteridaceae | Thelypteris palustris | 17 | 9 | 213 | 286 | ||||
| Perennials | Vitaceae | Cayratia japonica | 2 | 37 | 649 | 24 | 325 | 482 | 29 | |
| Perennials | Vitaceae | Vitis ficifolia var. lobata | 42 |
Appendix B
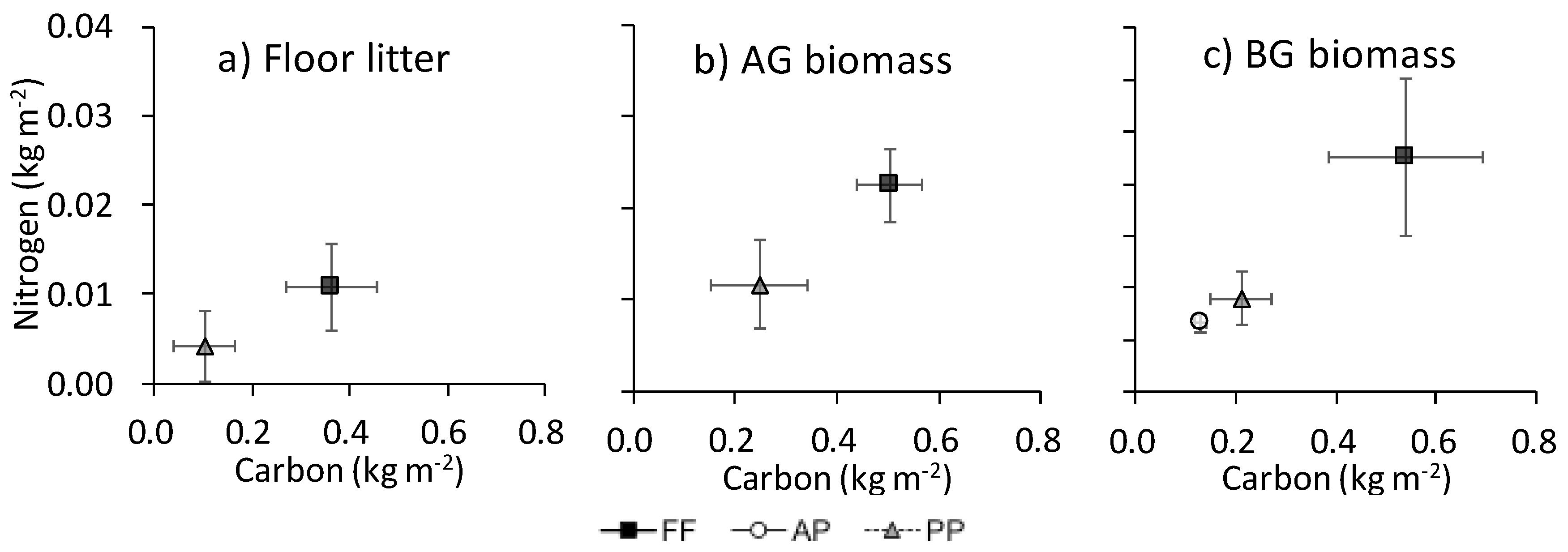
References
- Matsumura, T.; Takeda, Y. Relationship between species richness and spatial and temporal distance from seed source in semi-natural grassland. Appl. Veg. Sci. 2010, 13, 336–645. [Google Scholar] [CrossRef]
- Koyanagi, T.F.; Yamada, S.; Yonezawa, K.I.; Kitagawa, Y.; Ichikawa, K. Plant species richness and composition under different disturbance regimes in marginal grasslands of a Japanese terraced paddy field landscape. Appl. Veg. Sci. 2014, 17, 636–644. [Google Scholar] [CrossRef]
- Normile, D. Nature from nurture. Science 2016, 351, 908–910. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, M.; Inagaki, H. Abandonment process of terraced paddy fields, and restoration of their landscapes and floras in recent years—Case studies in Shizuoka Prefecture, Central Japan. Wetl. Res. 2012, 2, 15–26. [Google Scholar]
- Nakajima, M.; Cheng, W.; Hanayama, S.; Okada, M. Shallow autumn tillage does not reduce CH4 emission from an Andisol paddy field in Morioka, a cold region in Japan. J. Agric. Meteorol. 2017, 73, 92–99. [Google Scholar] [CrossRef]
- Weaver, J.E. Summary and interpretation of underground development in natural grassland communities. Ecol. Monogr. 1958, 28, 55–78. [Google Scholar] [CrossRef]
- Wagai, R.; Mayer, L.M.; Kitayama, K. Nature of the “occluded” low-density fraction in soil organic matter studies: A critical review. Soil Sci. Plant Nutr. 2009, 55, 13–25. [Google Scholar] [CrossRef]
- Kalbitz, K.; Schmerwitz, J.; Schwesig, D.; Matzner, E. Biodegradation of soil-derived dissolved organic matter as related to its properties. Geoderma 2003, 113, 273–291. [Google Scholar] [CrossRef]
- Faucon, M.P.; Houben, D.; Lambers, H. Plant functional traits: Soil and ecosystem services. Trends Plant Sci. 2017, 22, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Natuhara, Y. Ecosystem services by paddy fields as substitutes of natural wetlands in Japan. Ecol. Eng. 2013, 56, 97–106. [Google Scholar] [CrossRef]
- Homma, K.; Horie, T.; Shiraiwa, T.; Supapoj, N.; Matsumoto, N.; Kabaki, N. Toposequential variation in soil fertility and rice productivity of rainfed lowland paddy fields in mini-watershed (Nong) in Northeast Thailand. Plant Prod. Sci. 2003, 6, 147–153. [Google Scholar] [CrossRef]
- Boling, A.A.; Tuong, T.P.; Suganda, H.; Konboon, Y.; Harnpichitvitaya, D.; Bouman, B.A.M.; Franco, D.T. The effect of toposequence position on soil properties, hydrology, and yield of rainfed lowland rice in Southeast Asia. Field Crops Res. 2008, 106, 22–33. [Google Scholar] [CrossRef]
- Yonemura, S.; Ono, K.; Ikawa, H.; Kim, W.; Mano, M.; Miyata, A. Comparison of fallow season CO2 efflux from paddy soil estimated using laboratory incubation with eddy covariance-based flux. J. Agric. Meteorol. 2017, 73, 140–145. [Google Scholar] [CrossRef]
- Descloux, S.; Chanudet, V.; Poilvé, H.; Grégoire, A. Co-assessment of biomass and soil organic carbon stocks in a future reservoir area located in Southeast Asia. Environ. Monit. Assess. 2011, 173, 723–741. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, S. Plant-derived carbon and nitrogen addition due to mowing in the early stages of post-agricultural succession. Ecol. Eng. 2017, 98, 24–31. [Google Scholar] [CrossRef]
- Shimoda, S.; Koga, N. Rapid change in soil C storage associated with vegetation recovery after cessation of cultivation. Soil Sci. Plant Nutr. 2013, 59, 27–34. [Google Scholar] [CrossRef]
- Ellert, B.H.; Bettany, J.R. Calculation of organic matter and nutrients stored in soils under contrasting management regimes. Can. J. Soil Sci. 1995, 75, 529–538. [Google Scholar] [CrossRef]
- Yashiro, Y.; Lee, N.Y.M.; Ohtsuka, T.; Shizu, Y.; Saitoh, T.M.; Koizumi, H. Biometric-based estimation of net ecosystem production in a mature Japanese cedar (Cryptomeria japonica) plantation beneath a flux tower. J. Plant Res. 2010, 123, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Dirnböck, T.; Grandin, U.; Bernhardt-Römermann, M.; Beudert, B.; Canullo, R.; Forsius, M.; Grabner, M.T.; Holmberg, M.; Kleemola, S.; Lundin, L.; et al. Forest floor vegetation response to nitrogen deposition in Europe. Glob. Chang. Biol. 2014, 20, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Schrumpf, M.; Kaiser, K.; Guggenberger, G.; Persson, T.; Kögel-Knabner, I.; Schulze, E.D. Storage and stability of organic carbon in soils as related to depth, occlusion within aggregates, and attachment to minerals. Biogeosciences 2013, 10, 1675–1691. [Google Scholar] [CrossRef]
- Arai, M.; Minamiya, Y.; Tsuzura, H.; Watanabe, Y.; Yagioka, A.; Kaneko, N. Changes in water stable aggregate and soil carbon accumulation in a no-tillage with weed mulch management site after conversion from conventional management practices. Geoderma 2014, 221, 50–60. [Google Scholar] [CrossRef]
- Poeplau, C.; Don, A. Carbon sequestration in agricultural soils via cultivation of cover crops – a meta-analysis. Agric. Ecosyst. Environ. 2015, 200, 33–41. [Google Scholar] [CrossRef]
- Anzai, T.; Matsumoto, N. Emergence of weeds and changes in soil properties in fallow paddy fields. Bull. Chiba-Ken Agric. Exp. Stn. 1989, 29, 93–104. (In Japanese) [Google Scholar]
- Zhang, J.B.; Song, C.C.; Wang, S.N. Dynamics of soil organic carbon and its fractions after abandonment of cultivated wetlands in northeast China. Soil Tillage Res. 2007, 96, 350–360. [Google Scholar]
- Ohta, K.; Taniyama, I.; Kusaba, T.; Mori, A.; Araya, H. Changes in the soil properties of terrace paddy fields with the years after abandoned. Soil Phys. Cond. Plant Growth Jpn. 1996, 73, 3–10. (In Japanese) [Google Scholar]
- Shimoda, S.; Sakurai, Y. Effect of flooding management on carbon content of soil and plant after cessation of paddy rice cultivation. Appl. Ecol. Environ. Res. 2013, 11, 513–524. [Google Scholar] [CrossRef]
- Oyanagi, N.; Nakata, M. Dynamics of dissolved ions in the soil of abandoned terraced paddy fields in Sado Island, Japan. Paddy Water Environ. 2010, 8, 121–129. [Google Scholar] [CrossRef]
- Pampolino, M.F.; Laureles, E.V.; Gines, H.C.; Buresh, R.J. Soil carbon and nitrogen changes in long-term continuous lowland rice cropping. Soil Sci. Soc. Am. J. 2008, 72, 798–807. [Google Scholar] [CrossRef]
- Iwata, F.; Narioka, H. Change of soil structure and plant succession of renounced rice field in hilled rural area. J. Agric. Eng. Soc. Jpn. 2002, 70, 207–210. (In Japanese) [Google Scholar]
- Stewart, J.; Toma, Y.; Fernandez, F.G.; Nishiwaki, A.; Yamada, T.; Bollero, G. The ecology and agronomy of Miscanthus sinensis, a species important to bioenergy crop development, in its native range in Japan: A review. Glob. Chang. Biol. Bioenergy 2009, 1, 126–153. [Google Scholar] [CrossRef]
- Shimoda, S.; Lee, G.; Yokoyama, T.; Liu, J.; Saito, M.; Oikawa, T. Response of ecosystem CO2 exchange to biomass productivity in a high yield grassland. Environ. Exp. Bot. 2009, 65, 425–431. [Google Scholar] [CrossRef]
- Knops, J.M.; Tilman, D. Dynamics of soil nitrogen and carbon accumulation for 61 years after agricultural abandonment. Ecology 2000, 81, 88–98. [Google Scholar] [CrossRef]
- Orwin, K.H.; Buckland, S.M.; Johnson, D.; Turner, B.L.; Smart, S.; Oakley, S.; Bardgett, R.D. Linkages of plant traits to soil properties and the functioning of temperate grassland. J. Ecol. 2010, 98, 1074–1083. [Google Scholar] [CrossRef]
- Kusumoto, Y.; Ohkuro, T.; Ide, M. The relationships between the management history and vegetation types of fallow paddy field and abandoned paddy fields—Case study of Sakuragawa and Kokaigawa river basin in Ibaraki prefecture. J. Rural Plan. Assoc. 2005, 7, 7–12. (In Japanese) [Google Scholar] [CrossRef]
| Floor Litter | AG Biomass | BG Biomass | Whole Soil | Soil LF | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | |||||||||||||||
| FF | 0.36 | a | ±0.09 | 0.25 | a | ±0.09 | 0.54 | a | ±0.15 | 21.3 | a | ±7.59 | 3.90 | a | ±1.63 |
| AP | – | – | 0.13 | b | ±0.01 | 13.5 | a | ±1.59 | 1.05 | ab | ±0.19 | ||||
| PP | 0.10 | b | ±0.06 | 0.50 | a | ±0.06 | 0.21 | b | ±0.06 | 12.0 | a | ±1.20 | 0.86 | b | ±0.14 |
| N | |||||||||||||||
| FF | 0.011 | a | ±0.005 | 0.012 | a | ±0.005 | 0.023 | a | ±0.008 | 1.0 | a | ±0.16 | 0.18 | a | ±0.08 |
| AP | – | – | 0.007 | a | ±0.001 | 1.7 | a | ±0.19 | 0.06 | a | ±0.01 | ||||
| PP | 0.004 | a | ±0.004 | 0.022 | a | ±0.004 | 0.009 | a | ±0.003 | 1.2 | a | ±0.11 | 0.04 | a | ±0.01 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimoda, S.; Koyanagi, T.F. Land Use Alters the Plant-Derived Carbon and Nitrogen Pools in Terraced Rice Paddies in a Mountain Village. Sustainability 2017, 9, 1973. https://doi.org/10.3390/su9111973
Shimoda S, Koyanagi TF. Land Use Alters the Plant-Derived Carbon and Nitrogen Pools in Terraced Rice Paddies in a Mountain Village. Sustainability. 2017; 9(11):1973. https://doi.org/10.3390/su9111973
Chicago/Turabian StyleShimoda, Seiji, and Tomoyo F. Koyanagi. 2017. "Land Use Alters the Plant-Derived Carbon and Nitrogen Pools in Terraced Rice Paddies in a Mountain Village" Sustainability 9, no. 11: 1973. https://doi.org/10.3390/su9111973





