Protecting of Marble Stone Facades of Historic Buildings Using Multifunctional TiO2 Nanocoatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Preparation of TiO2 Nanocoating
2.1.2. Preparation of Experimental Marble Specimens
2.2. Methods
2.2.1. Application and Procedures of the Protection Nano-Coating on Stone Surface
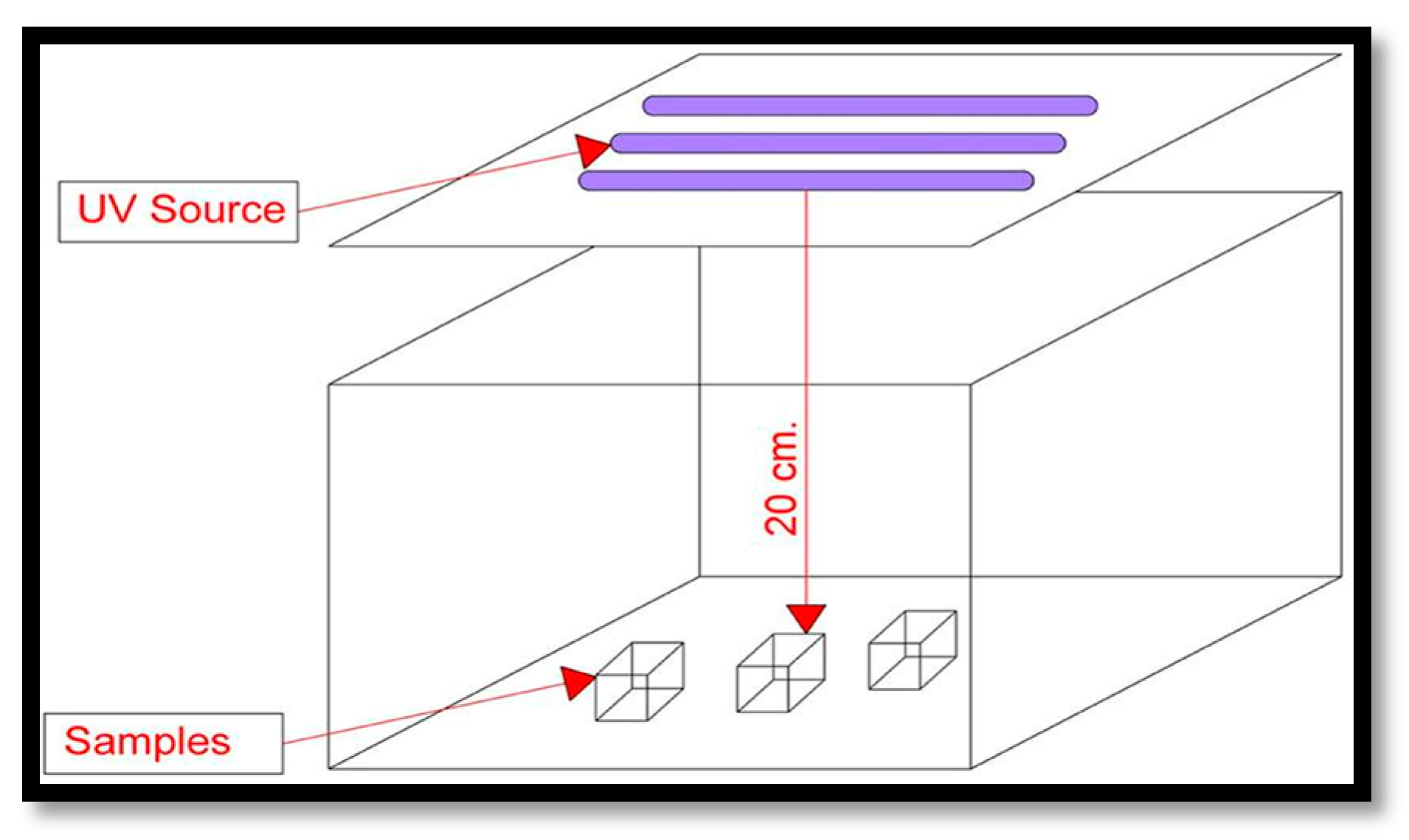
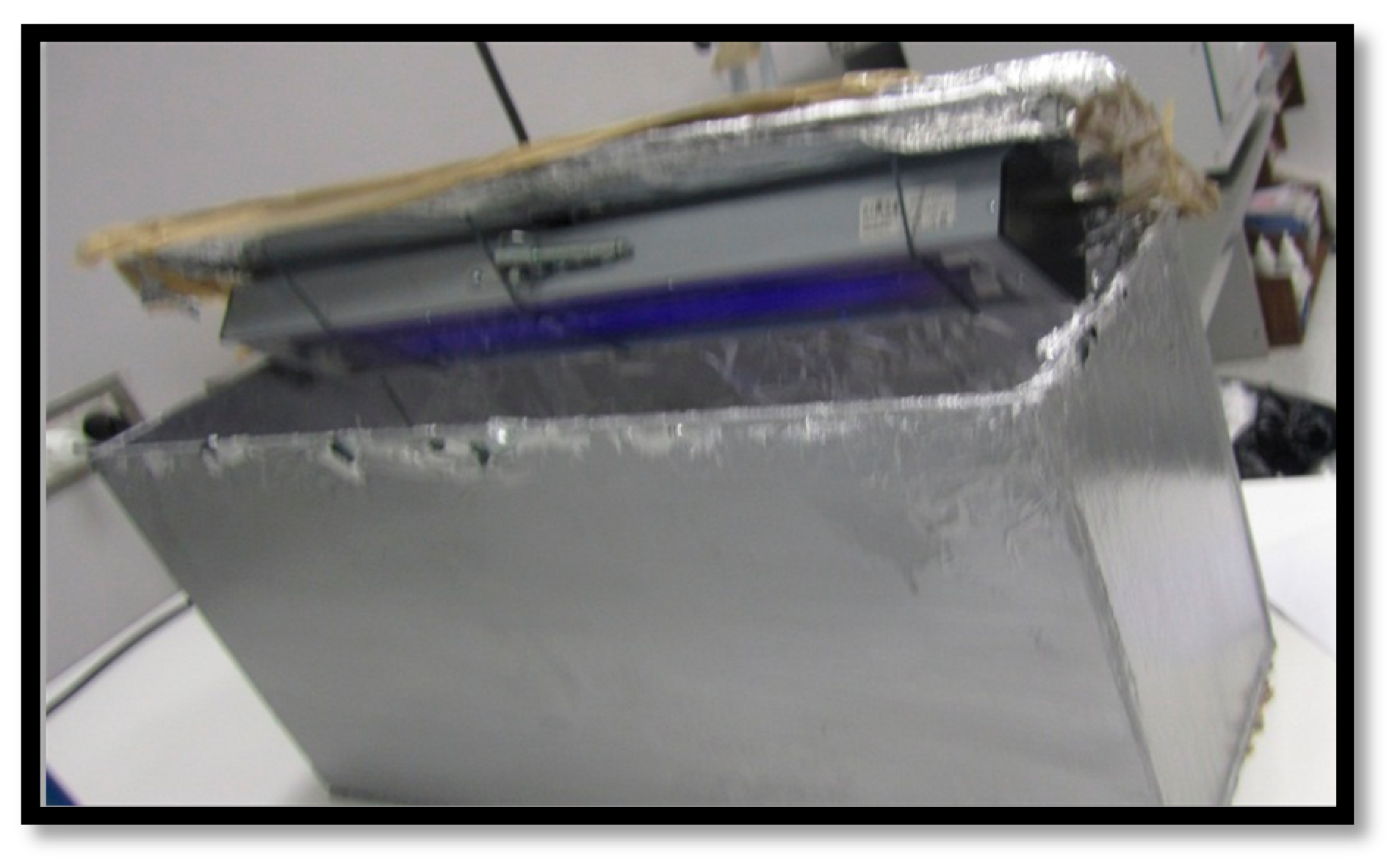
2.2.2. UV Aging Test
2.2.3. Artificial Aging Test (Wet-Dry Cycles)
2.2.4. Dirt Accumulation Test
2.2.5. Morphological Analysis of the Stone Samples
2.2.6. Colorimetric Measurements
2.2.7. Fourier Transformed Infrared Spectroscopy (ATR-FTIR)
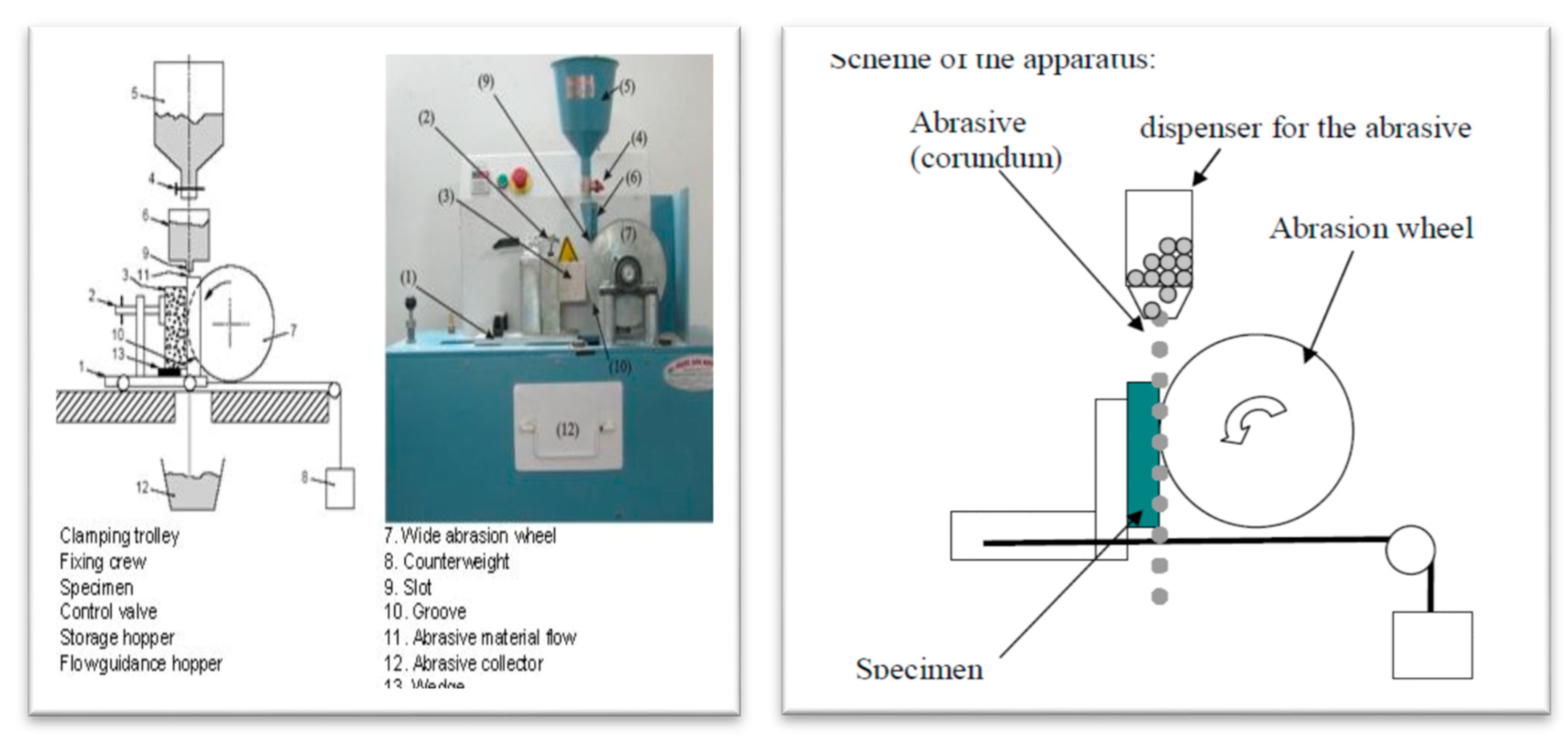
2.2.8. Mechanical Properties (Abrasion Resistance Test)
2.2.9. Water Absorption
3. Results and Discussion
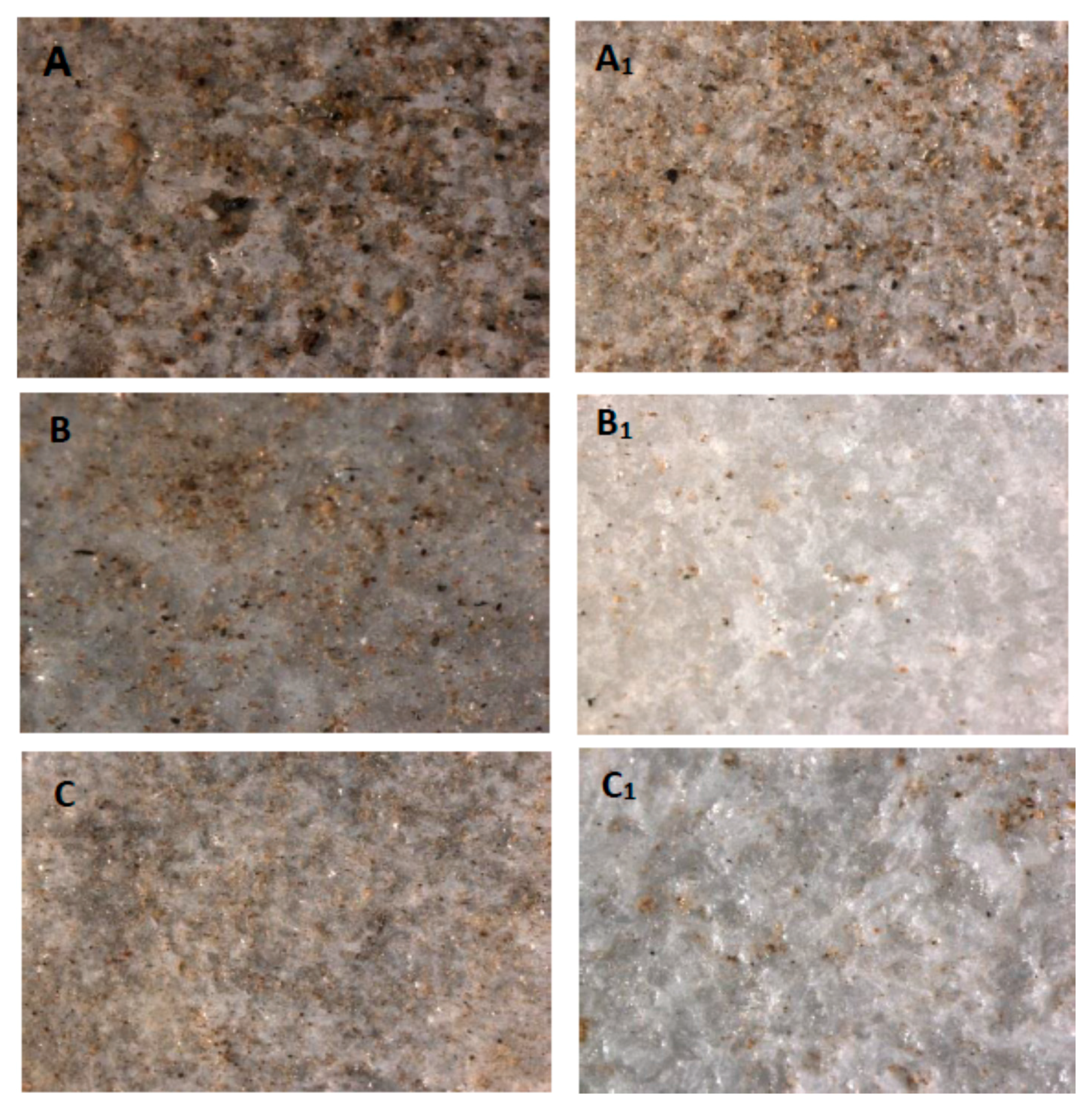
3.1. Characterization of Studied Historic Marble Samples
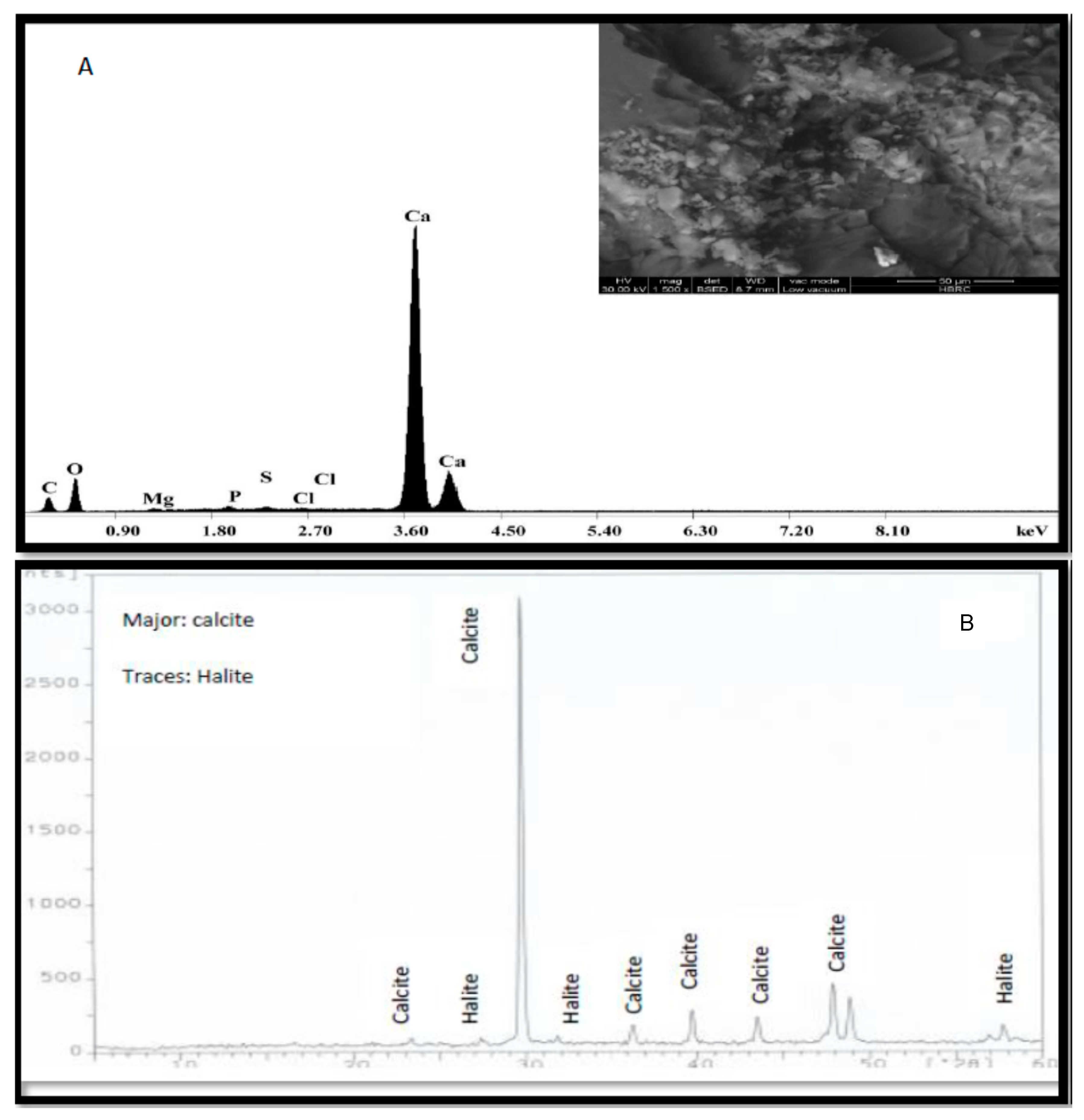
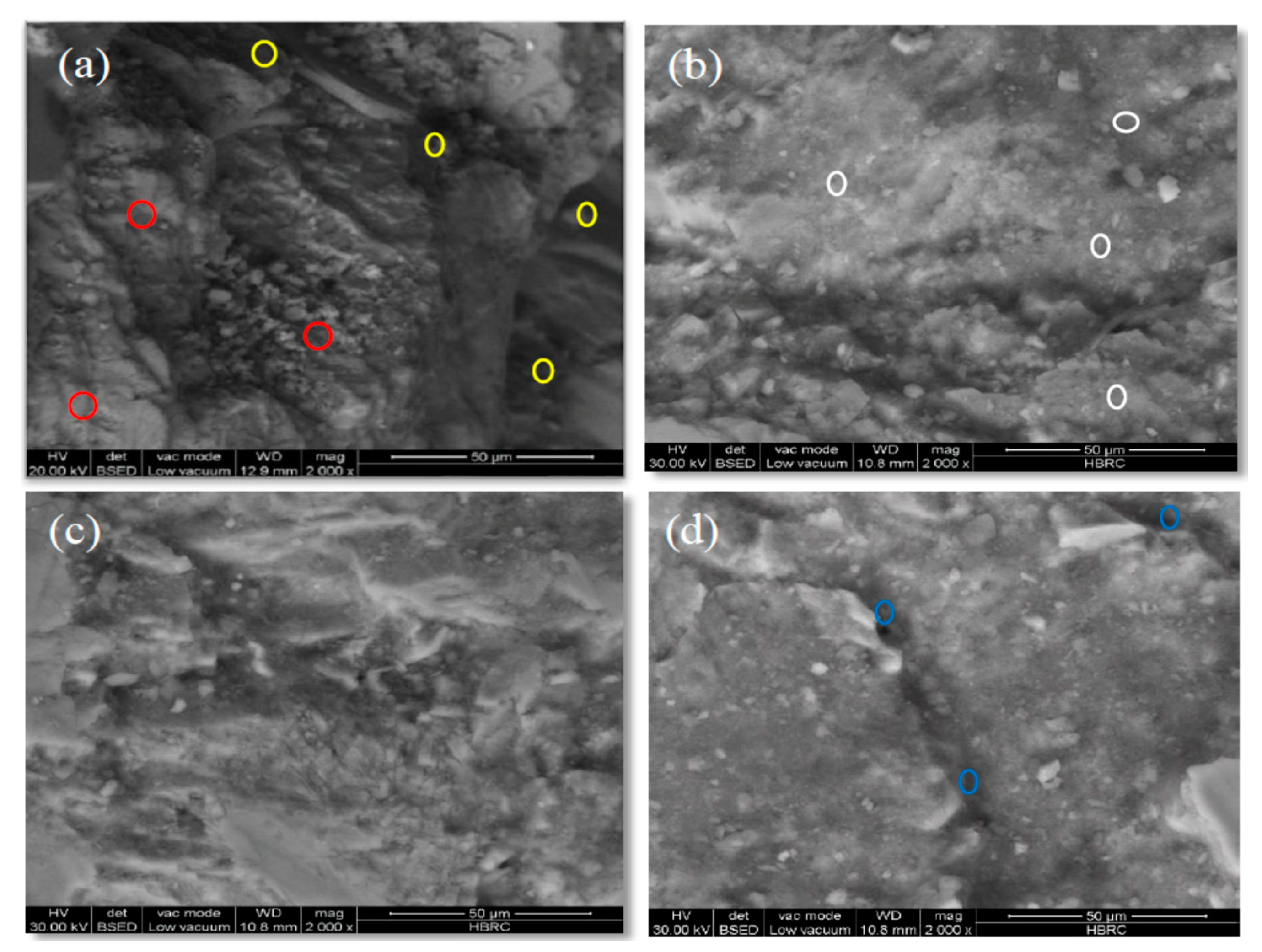
3.2. SEM/EDX Investigation
3.3. Mechanical Properties (Abrasion Resistance Test)
3.4. Effect of TiO2 Nano-Coating on Dirt Accumulation
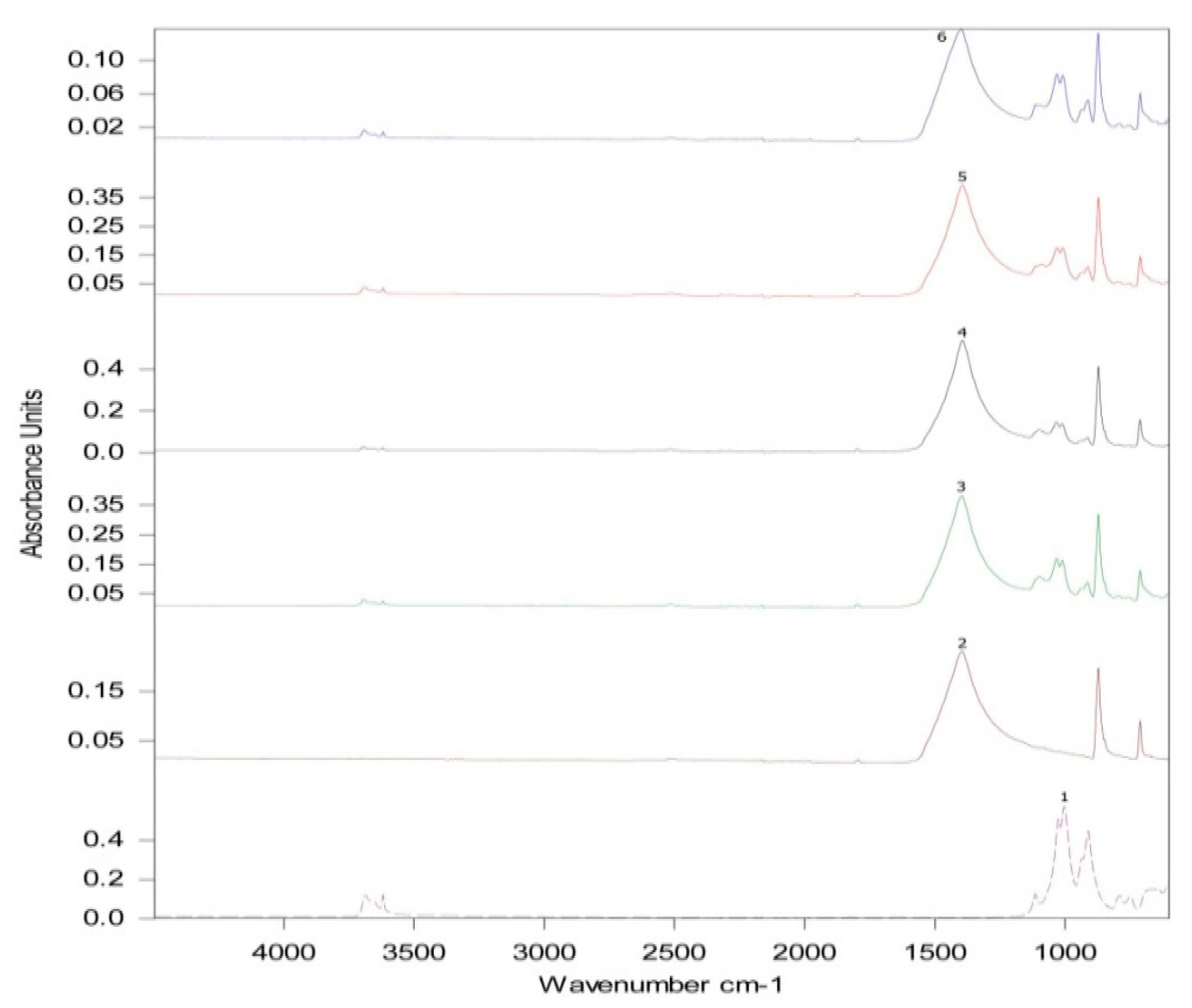
3.5. Fourier Transformed Infrared Spectroscopy (ATR-FTIR)
3.6. Colorimetric Test
- ΔE < 0.2: no perceivable difference;
- 0.2 < ΔE < 0.5: very small difference;
- 0.5 < ΔE < 2: small difference;
- 2 < ΔE < 3: fairly perceptible difference;
- 3 < ΔE < 6: perceptible difference;
- 6 < ΔE < 12: strong difference;
- ΔE > 12: different colours.
3.7. Water Absorption
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Albertano, P. Deterioration of Roman hypogea by epilythic cyanobacteria and microalgae. In Science and Technology for the Safeguard of Cultural Heritage in Mediterranean Basin; CNR Editions: Palermo, Italy, 1995; Volume 2, pp. 1303–1308. [Google Scholar]
- El-Hady, A. Ground water and the deterioration of Islamic buildings in Egypt. In The Restoration and Conservation in Islamic Monuments in Egypt; Bacharach, J.L., Ed.; American University in Cairo Press: Cairo, Egypt, 1995; pp. 115–116. [Google Scholar]
- Crisci, G.M.; la Russa, M.F.; Macchione, M.; Malagodi, M.; Palermo, A.M.; Ruffolo, S.A. Study of archaeological underwater finds: Deterioration and conservation. J. Appl. Phys. A 2010, 100, 855–863. [Google Scholar] [CrossRef]
- Nugari, M.P.; Pietrini, A.M.; Caneva, G.; Imperi, F.; Visca, P. Biodeterioration of mural paintings in a rocky habitat: The Crypt of the Original Sin (Matera, Italy). Int. Biodeterior. Biodegrad. 2009, 63, 705–711. [Google Scholar] [CrossRef]
- Poon, C.C. Photocatalytic construction and building materials: From fundamentals to applications. Build. Environ. 2009, 44, 1899–1906. [Google Scholar]
- Quagliarinia, E. Self-cleaning materials on architectural heritage: Compatibility of photo-induced hydrophilicity of TiO2 coatings on stone surfaces. Appl. Surf. Sci. 2013, 14, 1–7. [Google Scholar] [CrossRef]
- Mamalis, A.G. Recent advances in nanotechnology. J. Mater. Process. Technol. 2007, 181, 52–58. [Google Scholar] [CrossRef]
- Cessari, L.L.; Cenzia, B.; Elena, G.; Maria, G. Sustainable technologies for diagnostic analysis and restoration techniques: The challenges of the green conservation. In Proceedings of the 4th International Congress Science and Technology for the Safeguard of Cultural Heritage of the Mediterranean Basin, Cairo, Egypt, 6–8 December 2009. [Google Scholar]
- Sekhar, P.; Ramgir, N.; Joshi, R.; Bhansali, S. Selective growth of silica nanowires using an Au catalyst for optical recognition of interleukin-10. Nanotechnology 2008, 19, 245502–245507. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, T.; Namba, Y.; Nakajima, T. 751—Electrochemical sterilization of microbial cells. Bioelectrochem. Bioenerget. 1984, 13, 393. [Google Scholar] [CrossRef]
- Dong, C.; Cairney, J.; Sun, Q.; Maddan, O.L.; He, G.; Deng, Y. Investigation of Mg(OH)2 nanoparticles as an antibacterial agent. J. Nanopart. Res. 2010, 12, 2101–2109. [Google Scholar] [CrossRef]
- Tsakalof, A.; Manoudis, P.; Karapanagiotis, I.; Chryssolulakis, I.; Panayiotou, C. Assessment of synthetic polymeric coatings for the protection and preservation of stone monuments. J. Cult. Herit. 2007, 8, 69–72. [Google Scholar] [CrossRef]
- Richards, R. Surface and Nanomolecular Catalysis; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Ruffolo, S.A.; la Russa, M.F.; Malagodi, M.; Rossi, C.O.; Palermo, A.M.; Crisci, G.M. ZnO and ZnTiO3 nanopowders for antimicrobial stone coating. Appl. Phys. A 2010, 100, 829–834. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 Photocatalysis: Design and applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, Q.; Yu, J.; Liu, B. Development of multifunctional photoactive self-cleaning glasses. J. Non-Cryst. Solids 2008, 354, 1424–1430. [Google Scholar] [CrossRef]
- La Russa, M.F.; Ruffolo, S.A.; Rovella, N.; BelfioRe, C.M.; Palermo, A.M.; Guzzi, M.T.; Crisci, G.M. Multifunctional TiO2 coatings for cultural heritage. Prog. Org. Coat. 2012, 74, 1–6. [Google Scholar] [CrossRef]
- Goffredo, G.B.; Munafò, P. Preservation of historical stone surfaces by TiO2 nanocoatings. Coatings 2015, 5, 222–231. [Google Scholar] [CrossRef]
- Graziani, L.; Quagliarinia, E.; Bondioli, F.; D’Orazio, M. Durability of self-cleaning TiO2 coatings on fired clay brick façades: Effects of UV exposure and wet & dry cycles. Build. Environ. 2014, 71, 193–203. [Google Scholar]
- Munafò, P.; Goffredo, G.B.; Quagliarinia, E. TiO2 based nanocoatings for preserving architectural stone surfaces: An overview. Constr. Build. Mater. 2015, 84, 201–218. [Google Scholar] [CrossRef]
- Ferrari, A.M.; Pini, M.; Neri, P.; Bondioli, F. Nano TiO2 coatings for limestone: Which sustainability for cultural heritage. Build. Environ. 2015, 71, 193–203. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Liu, Q.; Zhu, Z.; Zhang, J.; Zhang, B. Application of TiO2 photocatalyst to the stone conservation. Coatings 2015, 53, 357–365. [Google Scholar]
- Ruot, B.; Plassais, A.; Olive, F.; Guillot, L.; Bonafous, L. TiO2-containing cement pastes and mortars: Measurements of the photocatalytic efficiency using a rhodamine B-based colorimetric test. Sol. Energy 2009, 83, 1794–1801. [Google Scholar] [CrossRef]
- Franzoni, E.; Fregni, A.; Gabrielli, R.; Graziani, G.; Sassoni, E. Compatibility of photocatalytic TiO2-based finishing for renders in architectural restoration: A preliminary study. Build. Environ. 2014, 80, 125–135. [Google Scholar] [CrossRef]
- Karatasios, I.; Katsiotis, M.S.; Likodimos, V.; Kontos, A.I.; Papavassiliou, G.; Falaras, P.; Kilikoglou, V. Photo-induced carbonation of lime-TiO2 mortars. Appl. Catal. B Environ. 2010, 95, 78–86. [Google Scholar] [CrossRef]
- Tserepi, A.D.; Vlachopoulou, M.E.; Gogolides, E. Nanotexturing of poly(dimethylsiloxane) in plasmas for creating robust super-hydrophobic surfaces. Nanotechnology 2006, 17, 3977–3983. [Google Scholar] [CrossRef]
- Manoudis, P.; Karapanagiotis, I.; Tsakalof, A.; Zuburtikudis, I.; Panayiotou, C. Super-hydrophobic polymer/nanoparticle composites for the protection of marble monuments. In Proceedings of the 9th International Conference on NDT of Art, Jerusalem, Israel, 25–30 May 2008. [Google Scholar]
- Mansour, S. Comparative Study to Evaluate Efficiency of the Conventional Composites and Nano-Composites in Cleaning and Self-Protection for Some Archaeological Stone Surfaces. Applied on Selected Objects. Master’s Thesis, Conservation Department, Faculty of Archaeology, Cairo University, Giza, Egypt, June 2014. [Google Scholar]
- Buasri, A.; Chaiyut, N.; Borvornchettanuwat, K.; Chantanachai, N.; Thonglor, K. Thermal and Mechanical Properties of Modified CaCO3/PP Nanocomposites. Int. J. Chem. Mol. Nucl. Mater. Metall. Eng. 2012, 6, 1–4. [Google Scholar]
- Eirasa, D.; Pessanb, L.A. Mechanical Properties of Polypropylene/Calcium Carbonate Nanocomposites. Mater. Res. 2009, 12, 517–522. [Google Scholar] [CrossRef]
- Graziani, L.; Quagliarini, E.; Osimani, A.; Aquilanti, L.; Clementi, F.; Yéprémian, C.; Lariccia, V.; Amoroso, S.; D’Orazio, M. Evaluation of inhibitory effect of TiO2 nanocoatings against microalgal growth on clay brick façades under weak UV exposure conditions. Build. Environ. 2013, 64, 38–45. [Google Scholar] [CrossRef]
- Licciulli, A.; Calia, A.; Lettieri, M.; Diso, D.; Masieri, M.; Franza, S.; Amadelli, R.; Casarano, G. Photocatalytic TiO2 coatings on limestone. J. Sol-Gel Sci. Technol. 2011, 60, 437–444. [Google Scholar] [CrossRef]
- Enrico, Q.; Federica, B.; Giovanni, B.G.; Antonio, L.; Placido, M. Smart surfaces for architectural heritage: Preliminary results about the application of TiO2-based coatings on travertine. J. Cult. Herit. 2011, 13, 1–6. [Google Scholar]
- Bakr, A.M. Evaluation of the reliability and durability of some chemical treatments proposed for consolidation of so called-marble decoration used in 19th century cemetery (Hosh Al Basha), Cairo, Egypt. J. Arab Archaeol. Union 2011, 12, 75–96. [Google Scholar]
- Helmi, F.M.; Hefni, Y.K. Using nanocomposites in the consolidation and protection of sandstone. Int. J. Conserv. Sci. 2016, 7, 29–40. [Google Scholar]
- Manoudis, P.N.; Karapanagiotis, I.; Tsakalof, A.; Zuburtikudis, I.; Kolinkeová, B.; Panayiotou, C. Superhydrophobic films for the protection of outdoor cultural heritage assets. Appl. Phys. A 2009, 97, 351–360. [Google Scholar] [CrossRef]
- Male, S.; Kolar, J.; Strliˇc, M.; Koˇcar, D.; Fromageotc, D.; Lemaire, J.; Haillant, O. Photo-Induced Degradation of Cellulose. Polym. Degrad. Stability 2005, 89, 64–69. [Google Scholar] [CrossRef]
- Lazzari, M.; Chiantore, O. Thermal-Ageing of Paraloid Acrylic Protective Polymers. J. Polym. 2000, 41, 6447–6455. [Google Scholar] [CrossRef]
- Khallaf, M.K.; El-Midany, A.A.; El-Mofty, S.E. Influence of acrylic coatings on the interfacial, physical, and mechanical properties of stone-based monuments. Prog. Org. Coat. 2011, 72, 592–598. [Google Scholar] [CrossRef]
- El-Feky, O.M.; Hassan, E.A.; Fadel, S.M.; Hassanb, M.L. Use of ZnO nanoparticles for protecting oil paintings on paper support against dirt, fungal attack, and UV aging. J. Cult. Herit. 2013, 15, 1–8. [Google Scholar] [CrossRef]
- The International Organization for Standardization (ISO). CIE Standard S014–4/E, Colorimetry. Part 4: CIE 1976 L*a*b* Colour Space; ISO: Genève, Switzerland, 2007. [Google Scholar]
- EN. EN 14157-2004 Natural Stones—Determination of Abrasion Resistance, European Standard—Wide Wheel Abrasion Resistance BS. Available online: https://shop.bsigroup.com/ProductDetail/?pid=000000000030049570 (accessed on 25 October 2017).
- Çobanolu, I.; Çelik, S.B.; Alkaya, D. Correlation between wide wheel abrasion (capon) and Bohme abrasion test results for some carbonate rocks. Sci. Res. Essays 2010, 5, 3398–3404. [Google Scholar]
- UNI. UNI 10859-2000 Cultural Heritage—Natural and Artificial Stones—Determination of Water Absorption by Capillarity; Unifica Zione Italian (UNI): Rome, Italy, 2000. [Google Scholar]
- Tumuluri, U.; Howe, J.D.; Mounfield, W.P.; Li, M.; Chi, M.; Hood, Z.D.; Walton, K.S.; Sholl, D.S.; Dai, S.; Wu, Z. Effect of Surface Structure of TiO2 Nanoparticles on CO2 Adsorption and SO2 Resistance. ACS Sustain. Chem. Eng. 2017, 5, 9295–9306. [Google Scholar] [CrossRef]
- Su, W.; Zhang, J.; Feng, Z.; Chen, T.; Ying, P.; Li, C. Surface Phases of TiO2 Nanoparticles Studied by UV Raman Spectroscopy and FT-IR Spectroscopy. J. Phys. Chem. C 2008, 112, 7710–7716. [Google Scholar] [CrossRef]
- Murray, M.D.; Darvell, B.W. Protocol for contact angel measurement. Phys D Appl. Phys. 1990, 23, 1150. [Google Scholar] [CrossRef]
- NorMal, R. Misure Colorimetric he Strumentali di Superfici Opache. Available online: http://www.architettiroma.it/fpdb/consultabc/File/ConsultaBC/Lessico_NorMal-Santopuoli.pdf (accessed on 2 August 2017).
- UNI. UNI 10921-2001 Evaluation of the Efficacy of Water Repellent Treatments Applied on Stone Materials of Cultural and Artistic Interest; Unifica Zione Italian (UNI): Rome, Italy, 2001. [Google Scholar]
- Self-Cleaning Test Procedure (2007). Project Self-Cleaning Glass: Nano-Structured Self-Cleaning Glasses-Modelling and Laboratory Tests for Fundamental Knowledge on Thin Film Coatings, EC Normalisation and Customer Benefits. Available online: http://cordis.europa.eu/project/rcn/74327en.html (accessed 25 October 2017).


| Month | February | March | April | May | June | July |
|---|---|---|---|---|---|---|
| Maximum temperature (°C) | 23 | 25 | 27 | 30 | 32 | 35 |
| Minimum temperature (°C) | 10 | 12 | 15 | 19 | 19 | 21 |
| Relative humidity (RH) | 40 | 36 | 43 | 47 | 51 | 55 |
| Applied Protective Materials | Wide Wheel Abrasion Values (mm) |
|---|---|
| Untreated samples | 19 |
| Treated samples with TiO2 nano-coating | 17.5 |
| Treated samples with TiO2 nano-coating after thermal aging | 18 |
| Wavenumber Range (cm−1) | Intensity (%) | ||||
|---|---|---|---|---|---|
| Stone | Standard | Thermal Aging | UV Aging | Open Air | |
| 1394.0–1398.0 | 0.231 | 0.382 | 0.137 | 0.395 | 0.537 |
| 1030.0–1033.0 | -- | 0.169 | 0.084 | 0.177 | 0.147 |
| 1008.0–1011.0 | -- | 0.163 | 0.083 | 0.175 | 0.139 |
| Relative Intensity | Standard | Thermal Aging | UV Aging | Open Air |
|---|---|---|---|---|
| 1396.3/1032.1 | 2.26 | 1.63 | 2.23 | 3.65 |
| 1396.3/1009.7 | 2.34 | 1.65 | 2.25 | 3.86 |
| Δ (Treated and Untreated Samples) | Δ (UV Aged and Untreated Samples) | Δ (Thermally Aged and Untreated Samples) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ΔL* | Δa* | Δb* | ΔE* | ΔL* | Δa* | Δb* | ΔE* | ΔL* | Δa* | Δb* | ΔE* |
| 2.03 | 0.41 | 0.66 | 2.17 | 2.89 | 0.41 | 0.92 | 3.06 | 3.82 | 0.35 | 1.27 | 4.04 |
| TiO2 Nanoparticles Consentraion (2%) | Density (gm/cm3) | Porosity (%) | Water Absorption (%) |
|---|---|---|---|
| Untreated sample | 2.656 | 0.09 | 0.11 |
| Treated sample | 2.788 | 0.07 | 0.08 |
| Treated sample after UV aging | 2.788 | 0.07 | 0.08 |
| Treated sample after thermal aging | 2.666 | 0.09 | 0.09 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aldoasri, M.A.; Darwish, S.S.; Adam, M.A.; Elmarzugi, N.A.; Ahmed, S.M. Protecting of Marble Stone Facades of Historic Buildings Using Multifunctional TiO2 Nanocoatings. Sustainability 2017, 9, 2002. https://doi.org/10.3390/su9112002
Aldoasri MA, Darwish SS, Adam MA, Elmarzugi NA, Ahmed SM. Protecting of Marble Stone Facades of Historic Buildings Using Multifunctional TiO2 Nanocoatings. Sustainability. 2017; 9(11):2002. https://doi.org/10.3390/su9112002
Chicago/Turabian StyleAldoasri, Mohammad A., Sawsan S. Darwish, Mahmoud A. Adam, Nagib A. Elmarzugi, and Sayed M. Ahmed. 2017. "Protecting of Marble Stone Facades of Historic Buildings Using Multifunctional TiO2 Nanocoatings" Sustainability 9, no. 11: 2002. https://doi.org/10.3390/su9112002





