Evaluation of Energy Consumption in the Mercury Treatment of Phosphor Powder from Spent Fluorescent Lamps Using a Thermal Process
Abstract
:1. Introduction
2. Theoretical Background
3. Material and Methods
3.1. Materials
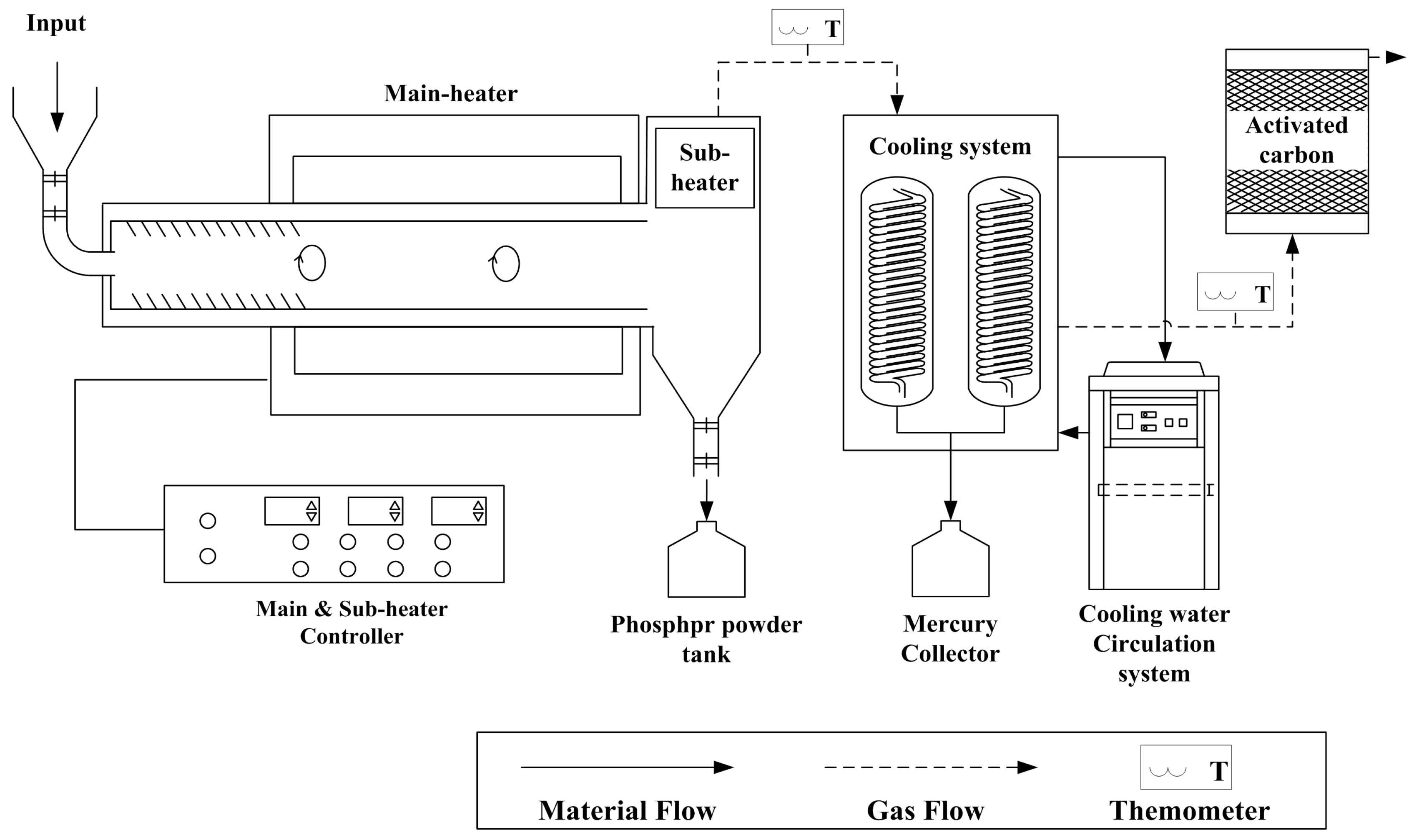
3.2. Experimental Apparatus and Method
4. Results and Discussion
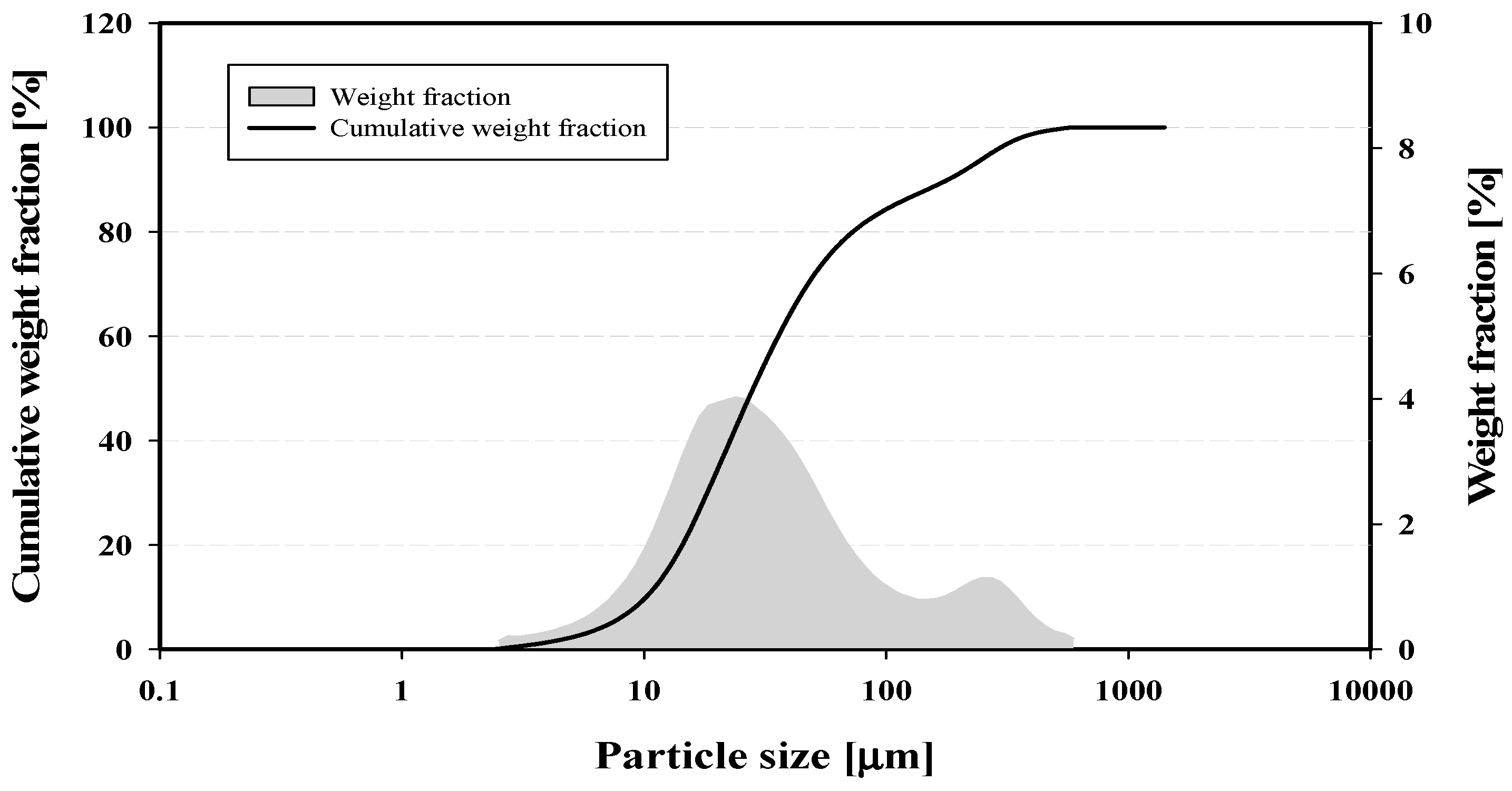
4.1. Characteristics of Phosphor Powder
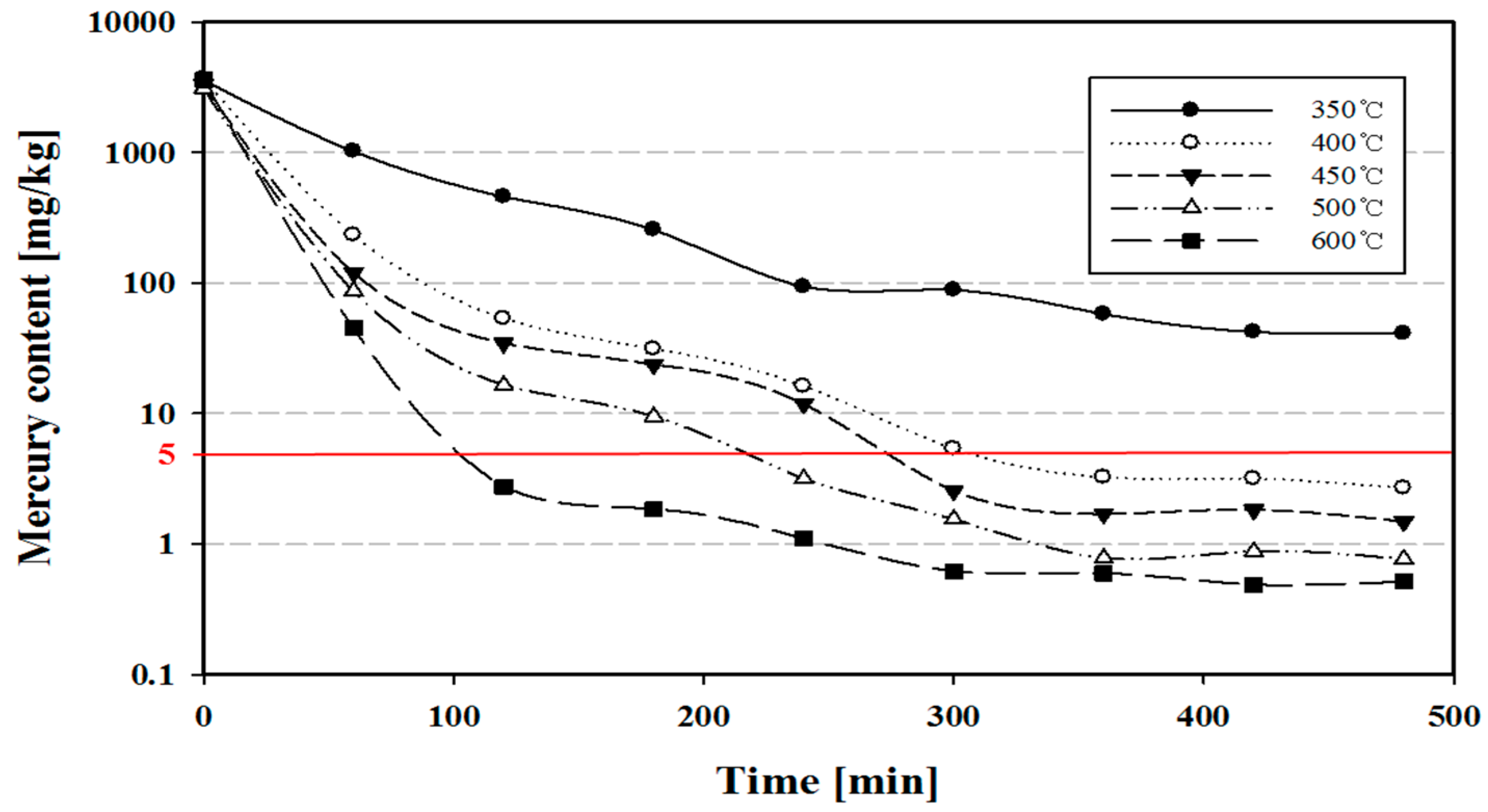
4.2. Mercury Content Variations with Temperature and Time
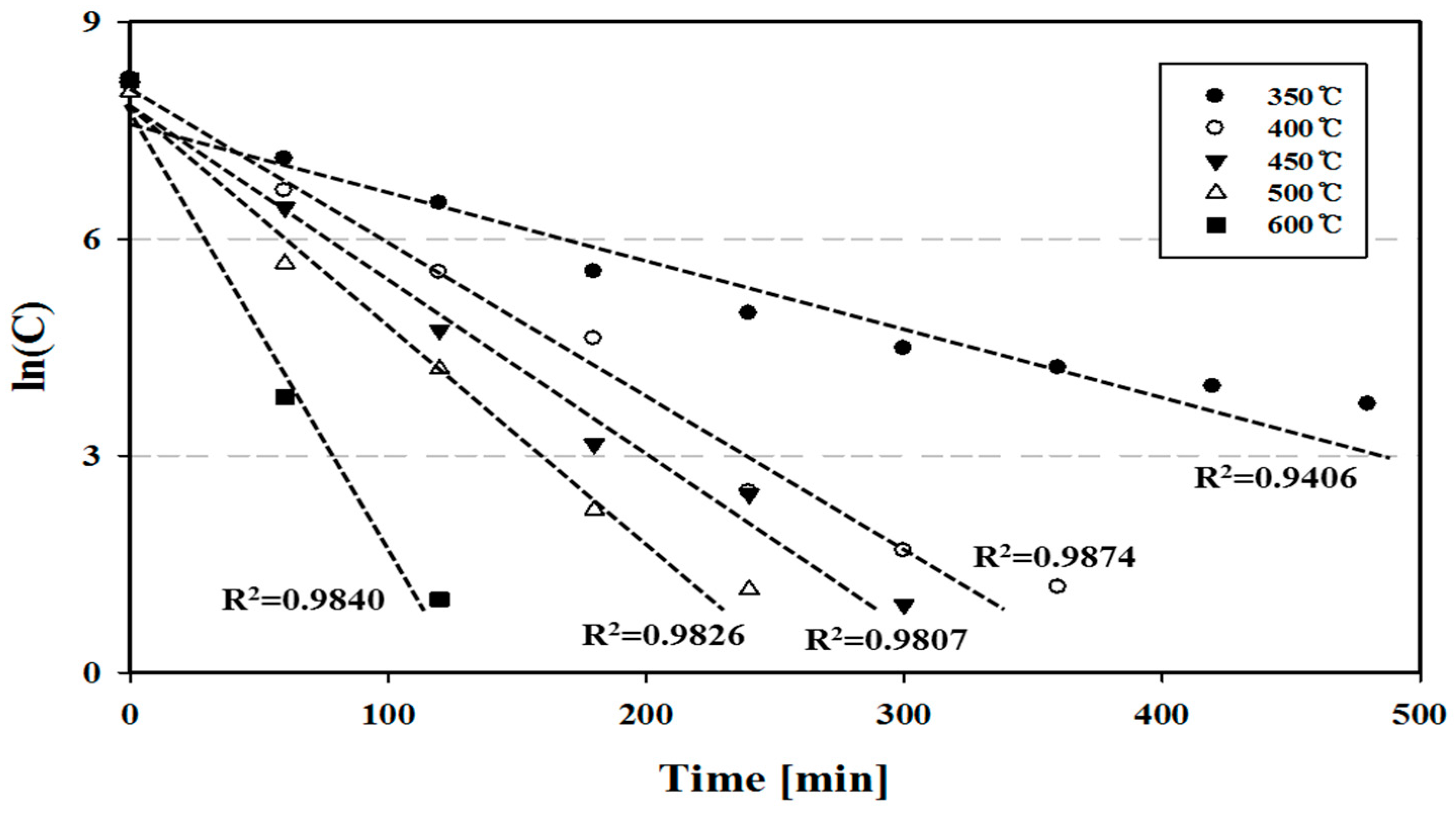
4.3. Reaction Rate in a Pilot-Plant-Scale Thermal Process
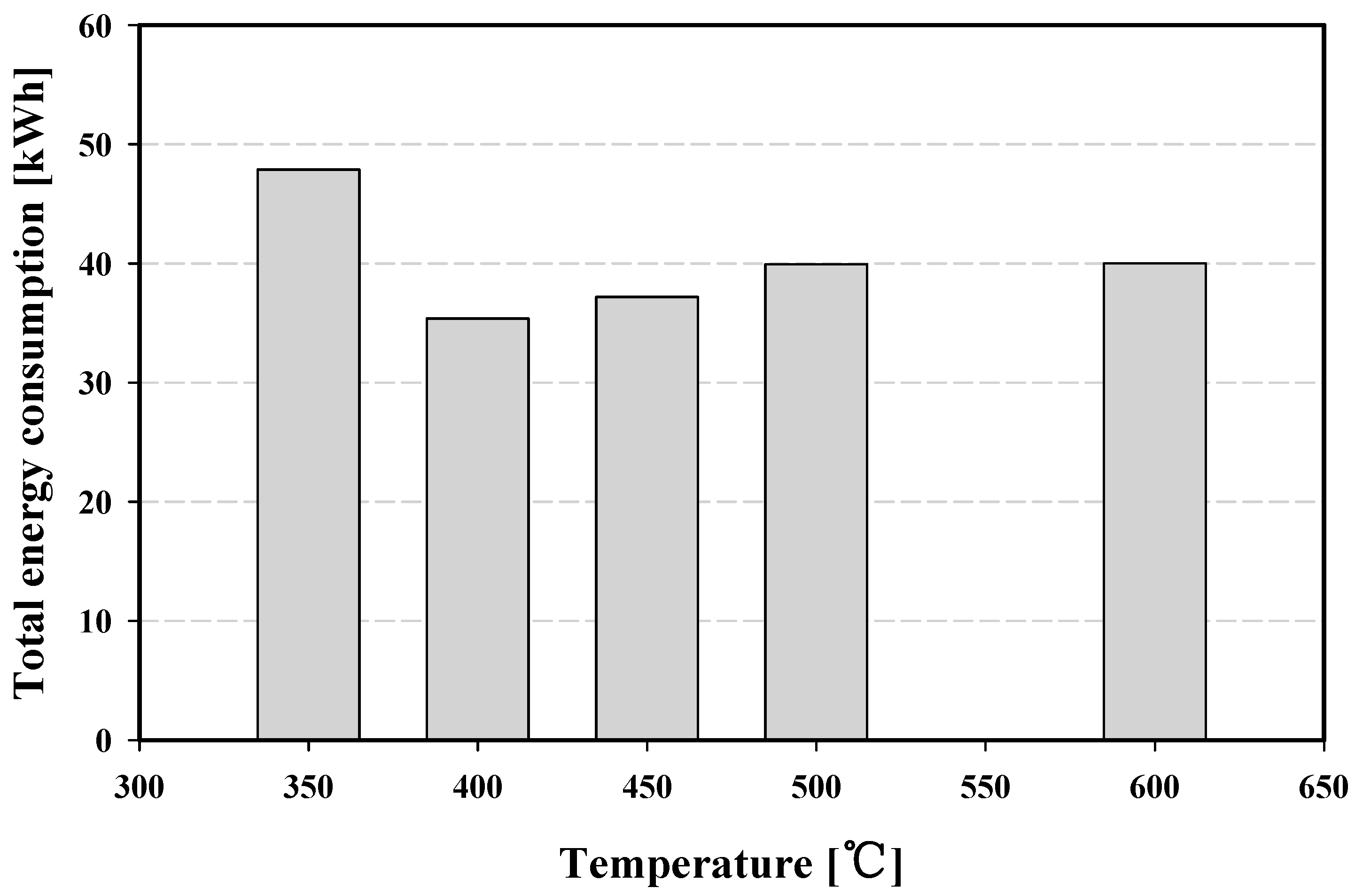
4.4. Energy Consumption in a Pilot-Plant-Scale Thermal Process
5. Conclusions
- From the characteristics of phosphor powder, the median particle size was 29.00 ± 1.96 μm, and the characteristic particle size was 38.28 ± 2.14 μm.
- The initial mercury content in phosphor powder was very high, 3418.34 ± 269.26 mg/kg. In the thermal process, mercury content decreased to less than 5.00 mg/kg at 400 °C or higher.
- The required treatment time for controlling mercury in phosphor powder was estimated from the 1st reaction equation, and it decreased exponentially from 9.79 h at 350 °C to 1.96 h at 600 °C. At 400 °C, the operating time in the thermal process for safe mercury treatment was determined to be 360 min.
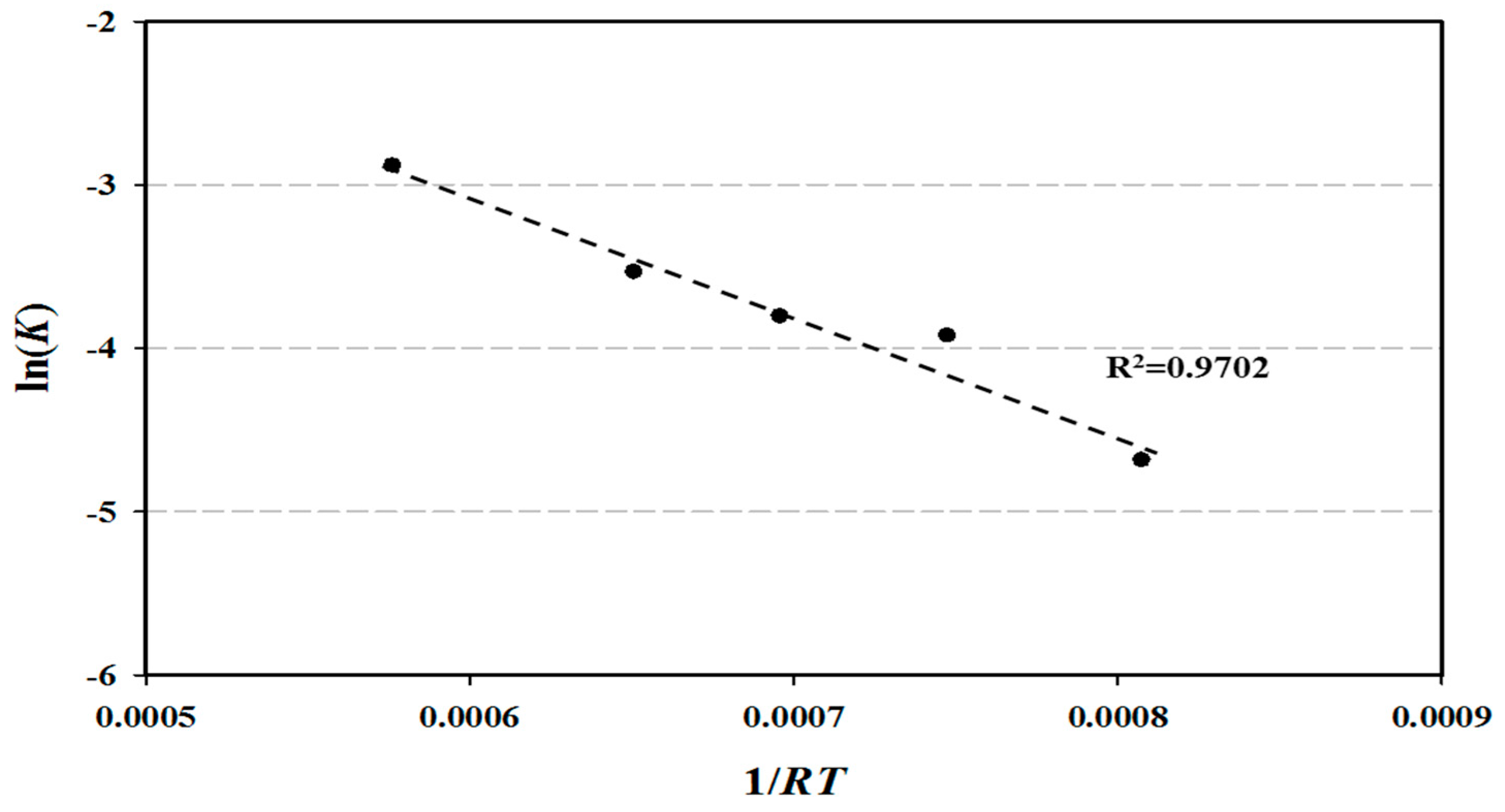
- The reaction rate constant increased as temperature increased from 1.87 × 10−4 s−1 at 350 °C to 9.30 × 10−4 s−1 at 600 °C. The frequency factor was 0.042 s−1, and the activation energy was 6509.11 kcal/kmol in the 1st order reaction.
- The total energy consumption of the pilot-plant-scale thermal process was the lowest at 400 °C at 35.37 kWh. Hence, the optimum conditions for treating phosphor powder was 400 °C in temperature and 360 min in operation time.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Park, H.S.; Rhee, S.W. Estimation of retorted phosphor powder from spent fluorescent lamps by thermal process. Waste Manag. 2016, 50, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.W. Control of mercury emissions: Policies, technologies, and future trends. Energy Emiss. Control Technol. 2015, 4, 1–15. [Google Scholar] [CrossRef]
- Rhee, S.W.; Park, H.S.; Yoo, H.S. Efficient Management System for Mercury-containing Waste according to the Current Status of Spent Fluorescent Lamps. J. Environ. Policy 2015, 14, 135–158. [Google Scholar] [CrossRef]
- Herborn GMBH. Lamp Recycling “System Herborn”. Available online: http://www.system-herborn.de/en/recyclingsystem-herborn/lamp-recycling-unit (accessed on 3 August 2017).
- MRT System. Lamp Processing. Available online: http://www.mrtsystem.com/products/lamp-recycling/ (accessed on 3 August 2017).
- Massacci, P.; Piga, L.; Ferrini, M. Technical note application of physical and thermal treatment for the removal of mercury from contaminated materials. Miner. Eng. 2000, 13, 963–967. [Google Scholar] [CrossRef]
- Wang, J.; Feng, X.; Anderson, C.W.N.; Xing, Y.; Shang, L. Remediation of mercury contaminated sites—A review. J. Hazard. Mater. 2012, 221, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Korea Environment Corporation (KECO). Expended Producer Responsibility Enforced 13 Years (Assessment on Result of Operation); Korea Environment Corporation (KECO): Incheon, Korea, 2017. [Google Scholar]
- Rhee, S.W.; Choi, H.H.; Park, H.S. Performance evaluation of material separation from spent fluorescent lamps using the thermal end-cutting method. J. Mater. Cycles Waste Manag. 2013, 15, 503–509. [Google Scholar] [CrossRef]
- Rhee, S.W.; Park, H.S. Mercury distribution of major components from 3-banded and general spent fluorescent lamp. Korea Soc. Waste Manag. 2013, 30, 265–271. [Google Scholar] [CrossRef]
- Rhee, S.W.; Park, H.S.; Choi, H.H. Comparison of mercury distribution between the types of spent fluorescent lamp. Arch. Metall. Mater. 2015, 60, 1297–1299. [Google Scholar] [CrossRef]
- Choi, H.J.; Rhee, S.W. Material flow analysis of the recycling process for linear type spent fluorescent lamp. J. Korea Soc. Waste Manag. 2016, 33, 537–546. [Google Scholar] [CrossRef]
- Rhee, S.W.; Choi, H.H.; Park, H.S. Characteristics of mercury emission from linear type of spent fluorescent lamp. Waste Manag. 2014, 34, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yin, X.; Zhang, Q.; Wang, W.; Mu, X. The Recycling of rare earths from waste tricolor phosphors in fluorescent lamps: A review of processes and technologies. Resour. Conserv. Recycl. 2014, 8, 21–31. [Google Scholar] [CrossRef]
- Binnermans, K.; Jones, P.T.; Blanpain, B.; Gerven, T.V.; Yang, Y.; Walton, A.W.; Buchert, M. Recycling of Rare earths: A critical review. J. Clear Prot. 2013, 51, 1–22. [Google Scholar] [CrossRef]
- Innocenzi, V.; Michelis, I.D.; Kopacek, B.; Veglio, F. Yttrium recovery from primary and secondary sources: A review of main hydrometallurgical processes. Waste Manag. 2014, 34, 1237–1250. [Google Scholar] [CrossRef] [PubMed]
- Belardi, G.; Ippolito, N.; Piga, L.; Serracino, M. Investigation on the status of rare earth elements contained in the powder of spent fluorescent lamps. Thermochim. Acta 2014, 591, 22–23. [Google Scholar] [CrossRef]
- Ippolito, N.M.; Innocenzi, V.; Michelis, I.D.; Medici, F. Rare earth elements recovery from fluorescent lamps: A new thermal pretreatment to improve the efficiency of the hydrometallurgical process. J. Clear Prot. 2017, 153, 287–298. [Google Scholar] [CrossRef]
- Raposo, C.; Windmoller, C.C.; Durao Junior, W.A. Mercury speciation in fluorescent lamps by thermal release analysis. Waste Manag. 2003, 23, 879–886. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (US EPA). Treatment Technologies for Mercury in Soil, Waste and Water. Section 5 Thermal Treatment; United States Environmental Protection Agency (US EPA): Washington, DC, USA, 2007.
- United States Environmental Protection Agency (US EPA). Method 7473. Mercury in Solids and Solutions by Thermal Decomposition, Amalgamation, and Atomic Absorption Spectrophotometry. Available online: https://www.epa.gov/sites/production/files/2015-12/documents/7473.pdf (accessed on 16 October 2017).
- Michael, D.; LaGrega·Phillip, L.; Buckingham·Jeffrey, C.E. Hazardous Waste Management, 2nd ed.; Mc Graw Hill: New York, NY, USA, 2001; pp. 137–138. ISBN 0-07-039365-6. [Google Scholar]
- Maddock, R.J.; Calcutt, D.M. Electronics for Engineers, 2nd ed.; Longman Scientific & Technical: Harlow, Essex, UK, 1994; pp. 28–75. ISBN 0-582-21583-8. [Google Scholar]
- Klein, S.; Nellis, G. Thermodynamics; Cambridge University Press: New York, NY, USA, 2012; pp. 92–137. ISBN 978-0-521-19570-6. [Google Scholar]
- Hirajima, T.; Bissombolo, A.; Sasaki, K.; Nakayama, K.; Hirai, H.; Tsunekawa, M. Floatability of rare earth phosphors from waste fluorescent lamps. Int. J. Miner. Process. 2005, 77, 187–198. [Google Scholar] [CrossRef]
- Minamata Convention on Mercury. Compilation of Additional Information on the Use of Mercury Waste Tresholds. Available online: http://www.mercuryconvention.org/Portals/11/documents/meetings/COP1/English/1_26_e_waste.pdf (accessed on 16 August 2017).
- Busto, Y.; Cabrera, X.; Tack, F.M.G.; Verloo, M.G. Potential of thermal treatment for decontamination of mercury containing wastes from chlor-alkali industry. J. Hazard. Mater. 2011, 186, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.C.; You, S.J.; Yu, B.S.; Chen, C.M.; Chiu, Y.C. Treating high-mercury-containing lamps using full-scale thermal desorption technology. J. Hazard. Mater. 2009, 162, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Back, S.K.; Seo, Y.C.; Sung, J.H.; Jang, H.N.; Kim, J.H.; Kim, K.H.; Kim, Y.L.; Kwon, M.H. Thermal Degradation Characteristics of Mercury in Waste Sludge Containing High Concentrated Mercury. Journal of Korea Society of Waste Management. J. Korea Soc. Waste Manag. 2014, 31, 300–306. [Google Scholar] [CrossRef]
| Type | Phosphor Powder |
|---|---|
| Median particle size (μm) | 29.00 ± 1.96 |
| Characteristic particle size (μm) | 38.28 ± 2.14 |
| Mercury content (mg/kg) 1 | 3418.34 ± 269.26 |
| Temperature (°C) | Reaction Rate Constant (s−1) | Frequency Factor (s−1) | Activation Energy (kcal/kmol) |
|---|---|---|---|
| 350 | 1.87 × 10−4 | 0.042 | 6509.11 |
| 400 | 3.28 × 10−4 | ||
| 450 | 3.70 × 10−4 | ||
| 500 | 4.85 × 10−4 | ||
| 600 | 9.30 × 10−4 |
| Temperature (°C) | Heat-Up Time (h) | Maintaining Time (h) | Operation Time (h) |
|---|---|---|---|
| 350 | 0.33 | 9.79 | 10.12 |
| 400 | 0.50 | 5.58 | 6.08 |
| 450 | 0.67 | 4.88 | 5.55 |
| 500 | 1.00 | 3.68 | 4.68 |
| 600 | 1.33 | 1.96 | 3.29 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.; Rhee, S.-W. Evaluation of Energy Consumption in the Mercury Treatment of Phosphor Powder from Spent Fluorescent Lamps Using a Thermal Process. Sustainability 2017, 9, 2013. https://doi.org/10.3390/su9112013
Choi Y, Rhee S-W. Evaluation of Energy Consumption in the Mercury Treatment of Phosphor Powder from Spent Fluorescent Lamps Using a Thermal Process. Sustainability. 2017; 9(11):2013. https://doi.org/10.3390/su9112013
Chicago/Turabian StyleChoi, Yong, and Seung-Whee Rhee. 2017. "Evaluation of Energy Consumption in the Mercury Treatment of Phosphor Powder from Spent Fluorescent Lamps Using a Thermal Process" Sustainability 9, no. 11: 2013. https://doi.org/10.3390/su9112013




