Synergistic Effects of n-Hexane Fraction of Parkia biglobosa (Jacq.) Bark Extract and Selected Antibiotics on Bacterial Isolates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Sample
2.2. Drying and Extraction of Crude Extract
2.3. Solvent Partitioning of the Crude Extract
2.4. Sensitivity Testing of n-Hexane Fraction from Crude Bark Extract of Parkia biglobosa and Standard Antibiotics on Bacterial Isolates
2.5. Determination of Minimum Inhibitory Concentration (MIC) of n-Hexane Fraction and Standard Antibiotics
2.6. Antibiotic-Extract Combination Experiment
2.7. Determination of Protein Leakage
2.8. Fourier Transform Infrared Spectroscopy (FTIR)
3. Results
3.1. Determination of Antibacterial Activities and Minimum Inhibitory Concentrations (MICs) of the n-Hexane Fraction and Standard Antibiotics on Bacterial Isolates
3.2. Synergy Experiment (The Time-Kill Assay)
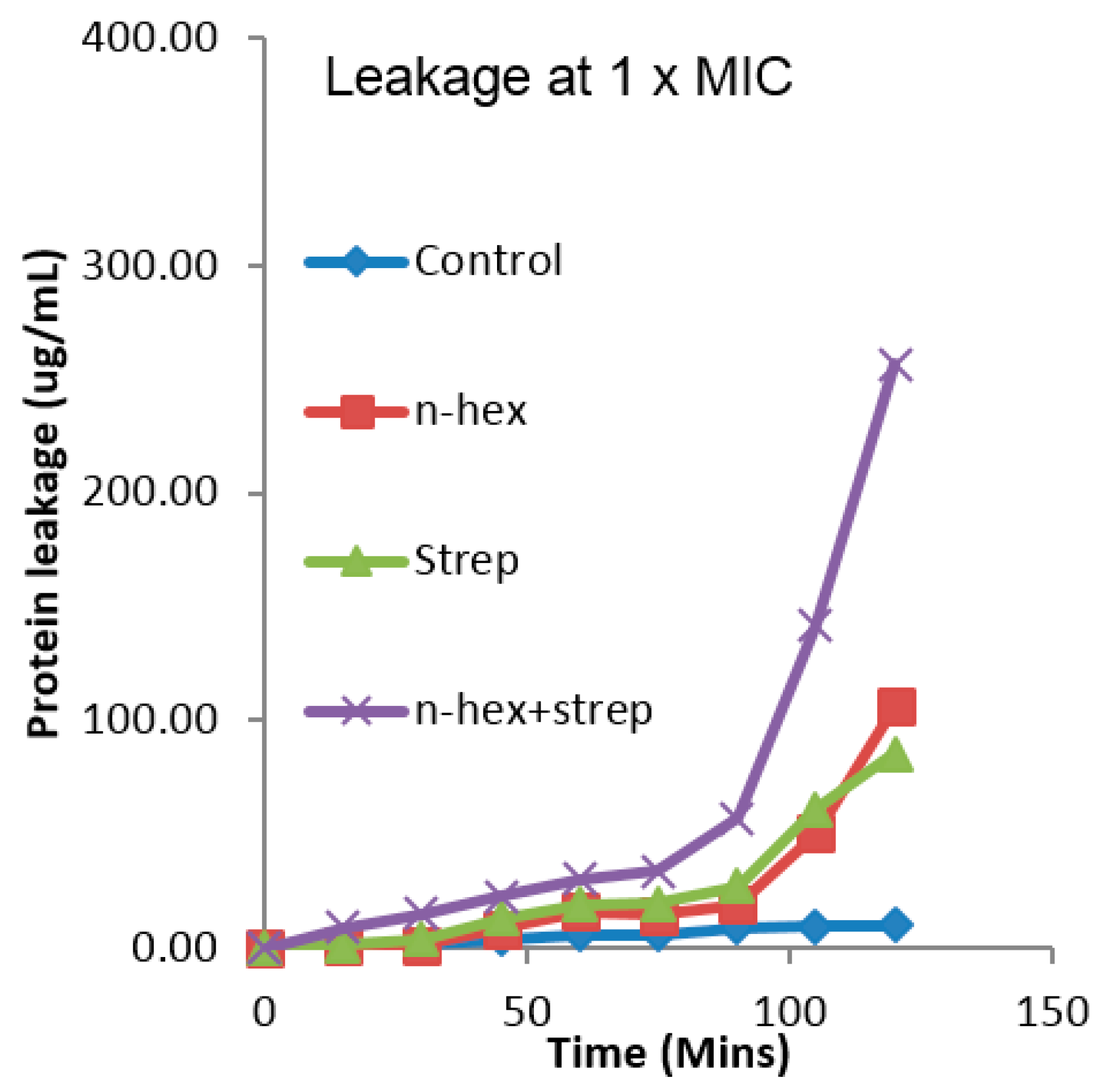
3.3. The Effect of the Combination of n-Hexane Fraction and Streptomycin on Protein Leakage from E. faecalis Cells
3.4. The Effect of the Combination of n-Hexane Fraction and Streptomycin on Protein Leakage from Pseudomonas aureginosa Cells
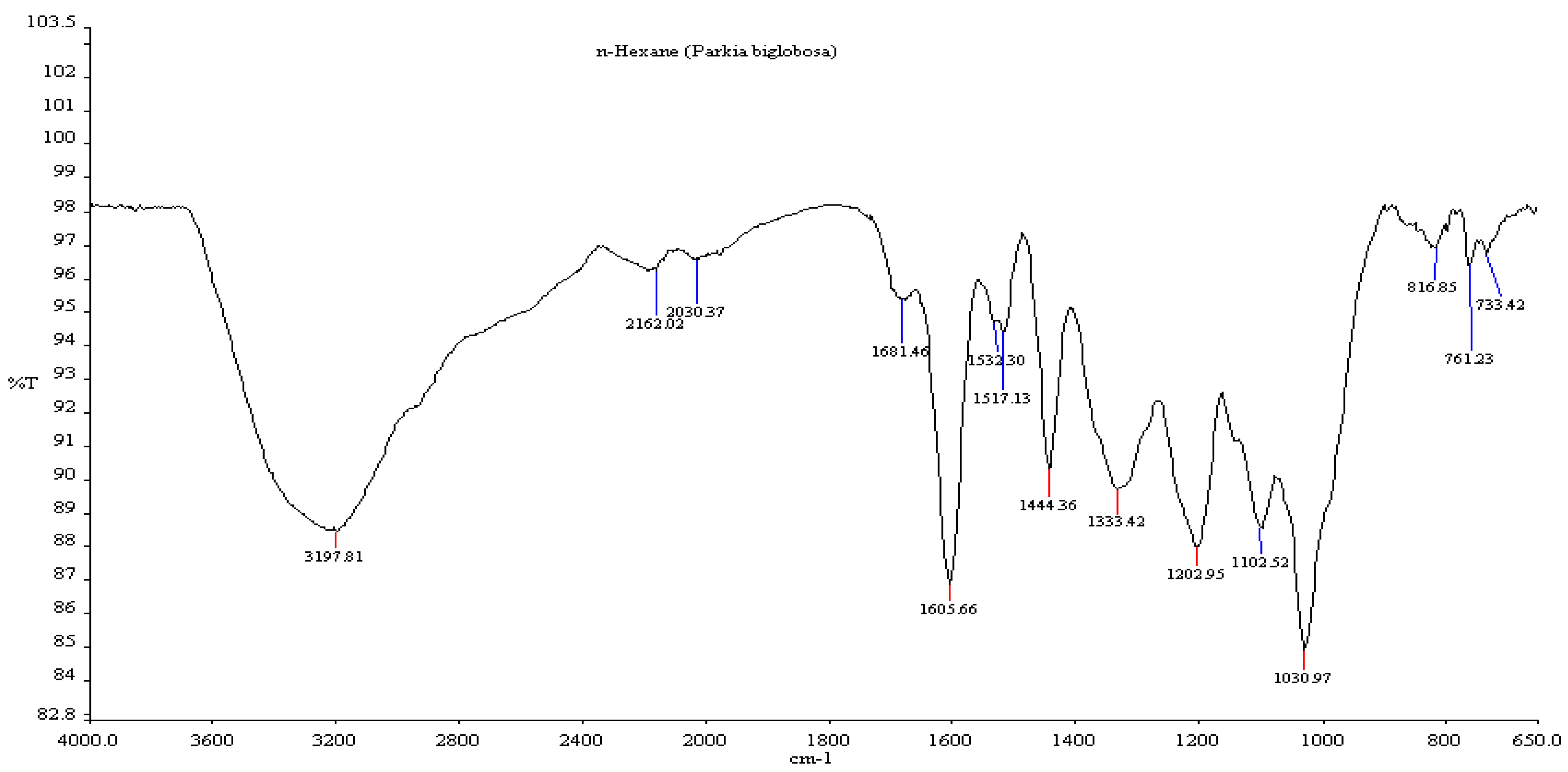
3.5. Fourier Transform Infrared Spectroscopy (FTIR)
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Authors Contributions
Conflicts of Interests
References
- Adwan, G.M.; Abu-Shanab, B.A.; Adwan, K.M. In vitro activity of certain drugs in Combination with plant extracts against Staphylococcus aureus infections. Pak. J. Med. Sci. 2008, 24, 541–544. [Google Scholar]
- Aiyegoro, O.A.; Afolayan, A.J.; Okoh, A.I. Synergistic interaction of Helichrysum pedunculatum leaf extracts with antibiotics against wound infection associated bacteria. Biol. Res. 2009, 42, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Hemaiswarya, S.; Kruthiventi, A.K.; Doble, M. Synergism between natural products and antibiotics against infectious diseases. Phytomedicine 2008, 15, 639–652. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.H.; Kazim, S.U.; Faizi, S. Activity of synergistic combination amoxy-cassia against salmonella. Pak. J. Pharma. Sci. 2007, 20, 140–145. [Google Scholar]
- Nascimento, G.G.F.; Juliana, L.; Paulo, C.F.; Giuliana, L.S. Antibacterial activity of plant extracts and phytochemicals on antibiotic-resistant bacteria. Braz. J. Microbiol. 2000, 31, 247–256. [Google Scholar] [CrossRef]
- Yang, Z.C.; Wang, B.C.; Yang, X.S.; Wang, Q.; Ran, L. The synergistic activity of antibiotics combined with eight traditional Chinese medicines against two different strains of Staphyloccus aureus. Coll. Surf. B Biointerfaces 2005, 41, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Aqil, F. In vitro efficacy of bioactive extract of 15 medicinal plants against ESβL-producing multidrug-resistant enteric bacteria. Microbiol. Res. 2007, 162, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Center for Disease Dynamics, Economics & Policy (CDDEP). State of the World’s Antibiotics; CDDEP: Washington, DC, USA, 2015; Available online: https://cddep.org/sites/default/files/swa_2015_final.pdf (accessed on 9 November 2016).
- Sibanda, T.; Okoh, A.I. In vitro evaluation of the interactions between acetone extracts of Garcinia kola seeds and some antibiotics. Afr. J. Biotechnol. 2008, 7, 1672–1678. [Google Scholar]
- Levy, S.B.; Marshal, B. Antibacterial resistance worldwide: Causes, challenges and responses. Nat. Med. 2004, 10 (Suppl. 12), 122–129. [Google Scholar] [CrossRef] [PubMed]
- Udobi, C.E.; Onaolapo, J.A.; Agunu, A. Antibacterial activities and bioactive components of the aqueous fraction of the stem bark of Parkia biglobosa (Jacq) (mimosaceae). Niger. J. Pharm. Sci. 2008, 7, 49–55. [Google Scholar]
- Baylor College of Medicine. Emerging Infectious Diseases. Available online: https://www.bcm.edu/departments/molecular-virology-and-microbiology/emerging-infections-and-biodefense/emerging-infectious-diseases (accessed on 20 December 2016).
- Ghaleb, A.; Mohammad, M. Synergistic effects of plant extracts and antibiotics on Staphylococcus aureus strains isolated from clinical specimens. Middle-East J. Sci. Res. 2008, 3, 134–139. [Google Scholar]
- Salvat, A.; Antonnacci, L.; Fortunato, R.H.; Suarez, E.Y.; Godoy, H.M. Screening of some plants from Northern Argentina for their antimicrobial activity. Lett. Appl. Microbiol. 2001, 32, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Aiyegoro, O.A.; Okoh, A.I. Use of bioactive plant products in combination with standard antibiotics: Implications in antimicrobial chemotherapy. J. Med. Plant. Res. 2009, 3, 1147–1152. [Google Scholar]
- El-Mahmood, A.M.; Ameh, J.M. In vitro antibacterial activity of Parkia biglobosa (Jacq.) root bark extract against some microorganisms associated with urinary tract infections. Afr. J. Biotechnol. 2007, 6, 1272–1275. [Google Scholar]
- World Health Organisation (WHO) Traditional Medicine Strategy: 2014–2023. Available online: http://apps.who.int/iris/bitstream/10665/92455/1/9789241506090_eng.pdf (accessed on 28 October 2016).
- Veloira, W.G.; Domenico, P.; Lipuma, J.J.; Davis, J.M.; Gurzenda, E.; Kazzaz, J. In vitro activity and synergy of bismuth thiols and tobramycin against Burkholderia cepacia complex. J. Antimicrob. Chemother. 2003, 52, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Dawis, M.A.; Isenberg, H.D.; France, K.A.; Jenkinsin, S.G. In vitro activity of gatifloxacin alone and in combination with cefepime, meropenem, piperacillin and gentamicin against multi-drug resistant organisms. J. Antimicrob. Chemother. 2003, 51, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Mandal, M.D.; Pal, N.K. Combination effects of ciprofloxacine and gentamicin against clinical isolates of Salmonella enterica Serover typhi with reduced susceptibility to ciproflocin. Jpn. J. Infect. Dis. 2003, 56, 156–157. [Google Scholar] [PubMed]
- Hugo, W.B.; Russel, A.D. Pharmaceutical Microbiology, 5th ed.; Blackwell Scientific Publishing: New York, NY, USA, 1993; pp. 119–120. [Google Scholar]
- Levinson, W.; Jawetz, E. Medical Microbiology and Immunology: Examination and Board Review, 7th ed.; Lange Medical Books; McGraw-Hill: New York, NY, USA, 2002; pp. 73–83. [Google Scholar]
- Gilbert, B.; Alves, L.F. Synergy in plant medicines. Curr. Med. Chem. 2003, 10, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Young, L.S. Synergy between antimicrobial agents: Review of clinical significance of synergy in gram-negative infections at the University of California Los Angeles hospital. Infection 1978, 6 (Suppl. 1), S47. [Google Scholar] [CrossRef]
- Cunha, B.A. Antibiotic side effects. Medical Clinics of North America. Med. Clin. N. Am. 2001, 85, 149–185. [Google Scholar] [CrossRef]
- Wijsman, J.A.; Rieder, M.J.; Dekaban, G.A. Differential toxicity of reactive metabolites of clindamycin and sulphonmide in HIV-Infected cells. J. Clin. Pharmacol. 2005, 45, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Williamson, E.M. Synergy and other interactions in phytomedicines. Phytomedicines 2001, 8, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Ginsburg, H.; Deharo, E. A call for using natural compounds in the development of new antimalarial treatments an introduction. Malar. J. 2011, 10 (Suppl. S1). [Google Scholar] [CrossRef] [PubMed]
- Odunbaku, O.A.; Ilusanya, O.A.F. Synergistic effect of ethanol leaf extract of Senna alata and antimicrobial drugs on some pathogenic microbes. Adv. Environ. Biol. 2011, 5, 2162–2165. [Google Scholar]
- Abioye, O.E.; Akinpelu, D.A.; Aiyegoro, O.A.; Adegboye, M.F.; Oni, M.O.; Okoh, I.O. Preliminary Phytochemical Screening and Antibacterial Properties of Crude Stem Bark Extracts and Fractions of Parkia biglobosa (Jacq.). Molecules 2013, 18, 8485–8499. [Google Scholar] [CrossRef] [PubMed]
- Edeoga, H.O.; Okwu, D.E.; Mbaebie, B.O. Phytochemical constituents of some Nigeria medicinal plants. Afr. J. Biotechnol 2005, 4, 685–688. [Google Scholar] [CrossRef]
- Betoni, J.E.C.; Mantovani, R.P.; Barbosa, L.N.; Di Stasi, L.C.; Fernandes, A., Jr. Synergism between plant extract and antimicrobial drugs used on Staphylococcus aureus diseases. Mem. Inst. Oswaldo Cruz 2006, 101, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Akinpelu, D.A.; Kolawole, D.O. Phytochemistry and antimicrobial activity of leaf extract of Piliostigma thonningii (Schum). Sci. Focus 2004, 7, 64–70. [Google Scholar]
- White, R.L.; Burgess, D.S.; Manduru, M.; Bosso, J.A. Comparison of three different In Vitro Methods of Detecting Synergy: Time-Kill, Checkerboard, and E test. Antimicrob. Agents Chemother. 1996, 40, 1914–1918. [Google Scholar] [PubMed]
- Pankey, G.; Ashcraft, D. In vitro synergy of ciprofloxacin and gatifloxacin against ciprofloxacin-resistant Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2005, 49, 2959–2964. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Oh, W.S.; Ko, K.S.; Heo, S.T.; Moon, C.S.; Ki, H.K.; Kiem, S.; Peck, K.R.; Song, J.H. Synergy of arbekacin-based combinations against vancomycin hetero-intermediate Staphylococcus aureus. J. Korean Med. Sci. 2006, 21, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Tanaka, H.; Yamaguchi, R.; Kato, K.; Etoh, H. Synergistic effects of mupirocin and an isoflavanone isolated from Erythrina variegata on growth and recovery of methicillin-resistant Staphylococcus aureus. Int. J. Antimicrob. Agents 2004, 24, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1976, 72, 245–254. [Google Scholar]
- Starlin, T.; Gopalakrishnan, V.K.; Arul, R.C.; Ragavendran, P. Phytochemical screening, functional groups and element analysis of Tylophora pauciflora Wight and Arn. IRJP 2012, 3, 180–183. [Google Scholar]
- Ashokkumar, R.; Ramaswamy, M. Phytochemical screening by FTIR spectroscopicanalysis of leaf extracts of selected Indian Medicinal plants. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 395–406. [Google Scholar]
- European Committee for Antimicrobial Susceptibity Testing (EUCAST). Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by agar dilution. Clin. Microbiol. Inf. 2000, 6, 509–515. [Google Scholar]
- Comite de L’antibiogramme de la Societe Francaise de Microbiologie Recommandations 2012. Available online: http://www.sfm-microbiologie.org/UserFiles/files/casfm/CASFM_2012.pdf (accessed on 20 December 2016). (In French)
- Frank, R.S.; Peter, L.; Jeanne, N.T.; Lauren, A.Z.; Kim, L. Synergy in a medicinal plant: Antimicrobial action of berberine potentiated by 5′-methoxyhydnocarpin, a multidrug pump inhibitor. Proc. Natl. Acad. Sci. USA 2000, 15, 1433–1437. [Google Scholar]
- Basri, D.F.; Zin, N.M.; Bakar, N.S.; Rahmat, F.; Mohtar, M. Synergistic effects of phytochemicals and oxacillin on laboratory passage-derived vancomycin-intermediate Staphylococcus aureus strain. J. Med. Sci. 2008, 8, 131–136. [Google Scholar]
- Wisatre, K.; Chintana, C.; Thitiporn, D.; Wipaphorn, J.; Sungwan, H.; Benjamas, S.F.; Patra, R.; Kaikwan, J. The potential co-treatment effects of three plant extracts and three antibiotics on multidrug-resistant bacteria. J. Sci. Technol. Humanit. 2007, 5, 17–27. [Google Scholar]
- Esimone, C.O.; Iroha, I.R.; Ibezim, E.C.; Okeh, C.O.; Okpana, E.M. In vitro evaluation of the interaction between tea extracts and penicillin G against Staphylococcus aureus. Afr. J. Biotechnol. 2006, 5, 1082–1086. [Google Scholar]
- Savoia, D. Plant-derived antimicrobial compounds. Future Microbiol. 2012, 7, 979–990. [Google Scholar] [CrossRef] [PubMed]
- Seasotiya, L.; Dalal, S. Screening of Indian medicinal plants as efflux pump inhibitors of fluoroquinolones. J. Pharmacogn. Phytochem. 2014, 3, 235–241. [Google Scholar]
- Fiamegos, Y.C.; Kastritis, P.L.; Exarchou, V.; Han, H.; Bonvin, A.M.J.J.; Vervoort, J.; Lewis, K.; Hamblin, M.R.; Tegos, G.P. Antimicrobial and Efflux Pump Inhibitory Activity of Caffeoylquinic Acids from Artemisia absinthium against GramPositive Pathogenic Bacteria. PLoS ONE 2011, 6, e18127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stavri, M.; Piddock, L.J.V.; Gibbons, S. Bacterial efflux pump inhibitors from natural sources. J. Antimicrob. Chemother. 2007, 59, 1247–1260. [Google Scholar] [CrossRef] [PubMed]
- Piddock, L.J.V. Clinically relevant chromosomally encoded multidrug resistance efflux pumps in bacteria. Clin. Microbiol. Rev. 2006, 19, 382–402. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.C.J.; Williamson, E.M.; Wareham, N.; Kaatz, G.W.; Gibbons, S. Antibacterials and modulators of bacterial resistance from the immature cones of Chamaecyparis lawsoniana. Phytochemistry 2007, 68, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Akinpelu, D.A.; Aiyegoro, O.A.; Okoh, A.I. In vitro antimicrobial and phytochemical properties of extract of stem bark of Afzelia africana (Smith). Afr. J. Biotechnol. 2008, 7, 3665–3670. [Google Scholar]
- Oloke, J.K. The Antibacterial and Antifungal Effect of the Volatile Oil and Partially Purified Extracts of Aframomum melegueta K. Schum. Ph.D. Thesis, Department of Microbiology, University of Ife, Ile-Ife, Nigeria, 1989. [Google Scholar]
- Akinpelu, D.A.; Alayande, K.A.; Aiyegoro, O.A.; Akinpelu, O.F.; Okoh, A.I. Probable mechanisms of biocidal action of Cocos nucifera Husk extract and fractions on bacteria isolates. BMC Complement. Altern. Med. 2015, 15, 116. [Google Scholar] [CrossRef] [PubMed]
- Aiyegoro, O.A.; Akinpelu, D.A.; Okoh, A.I. In vitro potentials of the stem bark of red water tree (Erythrophyleum suaveolens). J. Biol. Sci. 2007, 7, 1233–1238. [Google Scholar]
- Davis, B.D.; Chen, L.; Tai, P.C. Misread protein creates memnbrane channels: An essential steps in the bacterial action of aminoglycosides. Proc. Natl. Acad. Sci. USA 1986, 83, 6164–6168. [Google Scholar] [CrossRef] [PubMed]
- Druy, M.A. Applications for mid-IR Spectroscopy in pharmaceutical process environment. Spectroscopy 2004, 19, 60–63. [Google Scholar]
- Nakanishi, K.; Solomon, P.H. Infrared Absorption Spectroscopy, 2nd ed.; Holden-Day, Inc.: San Francisco, CA, USA, 1977. [Google Scholar]
- Koeppen, B.H.; Basson, D.S. The anthocyanin pigments of barlinka grapes. Phytochemistry 1966, 5, 183–187. [Google Scholar] [CrossRef]
- Jose, C.I.; Phadke, P.S.; Rama Rao, A.V. Infrared spectra of flavones and isoflavones: Effect of iodine and boron trifluoride on carbonyl frequencies. Spectrochim. Acta A Mol. Spectrosc. 1974, 30, 1199–1206. [Google Scholar] [CrossRef]
- Ficarra, R.; Tommasini, S.; Raneri, D.; Calabro, M.L.; Dibella, M.R.; Rusticherri, C.; Gamberini, M.C. Study of flavonoids/β-cyclodextrins inclusion complexes by NMR, FT-IR, DSC, X-ray investigation. J. Pharm. Biomed. Anal. 2002, 29, 1005–1014. [Google Scholar] [CrossRef]
- Udobi, C.E.; Onaolapo, J.A. Bioactive compounds of the bark of P. biglobosa. J. Appl. Pharm. Sci. 2012, 2, 133–137. [Google Scholar]
- Millogo-Kone, H.; Guissou, I.P.; Nacoulma, O.; Traore, A.S. Antimicrobial effects of the stem bark extracts of Parkia biglobosa (Jacq.) Benth. on shigellae. Afr. J. Tradit. Complement. Altern. Med. 2007, 4, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Corrado, T.; Carmela, S.; Onofrio, D.L. Bioactive constituents of the bark of Parkia biglobosa. Fitoterapia 2000, 71, 118–125. [Google Scholar]
- Gernmah, D.I.; Atolagbe, M.O.; Echegwo, C.C. Nutritional composition of the African locust bean (Parkia biglobosa) fruit pulp. Niger. Food J. 2007, 25, 190–196. [Google Scholar] [CrossRef]
- Tijani, A.Y.; Okhale, S.E.; Salawu, T.A.; Onigbanjo, H.O.; Obianodo, L.A.; Akingbasote, J.A.; Salawu, O.A.; Okogun, J.I.; Kunle, F.O.; Emeje, M. Antidiarrheal and antibacterial properties of crude aqueous stem bark extract and fractions of P. biglobosa (Jacq.) R.Br Ex G. Don. Afr. J. Pharm. Pharmacol. 2009, 7, 347–353. [Google Scholar]
- Builders, M.I.; Isichie, C.O.; Aguiyi, J.C. Toxicity studies of the extracts of Parkia biglobosa stem bark in rats. Br. J. Pharm. Res. 2012, 2, 1–16. [Google Scholar] [CrossRef]
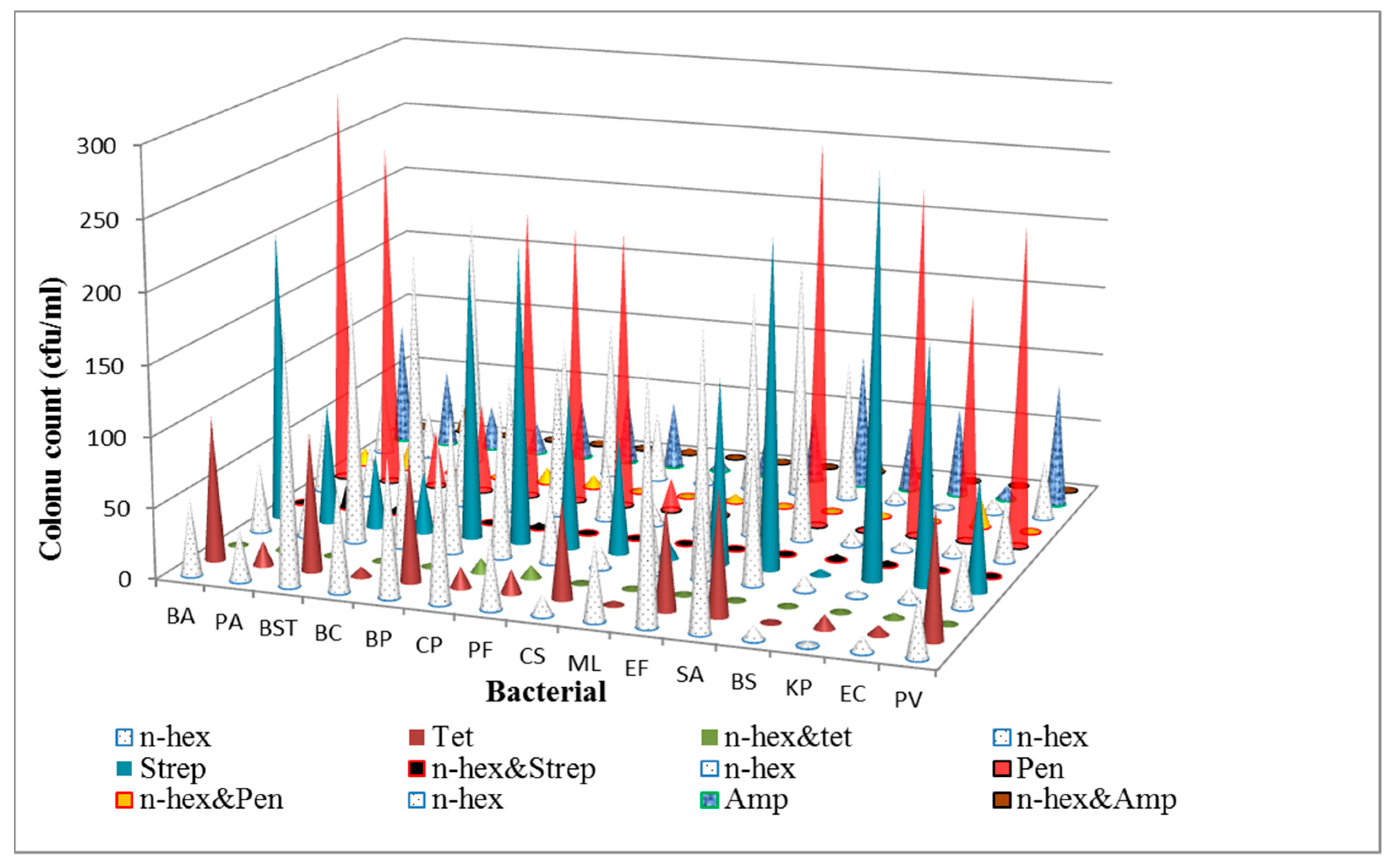
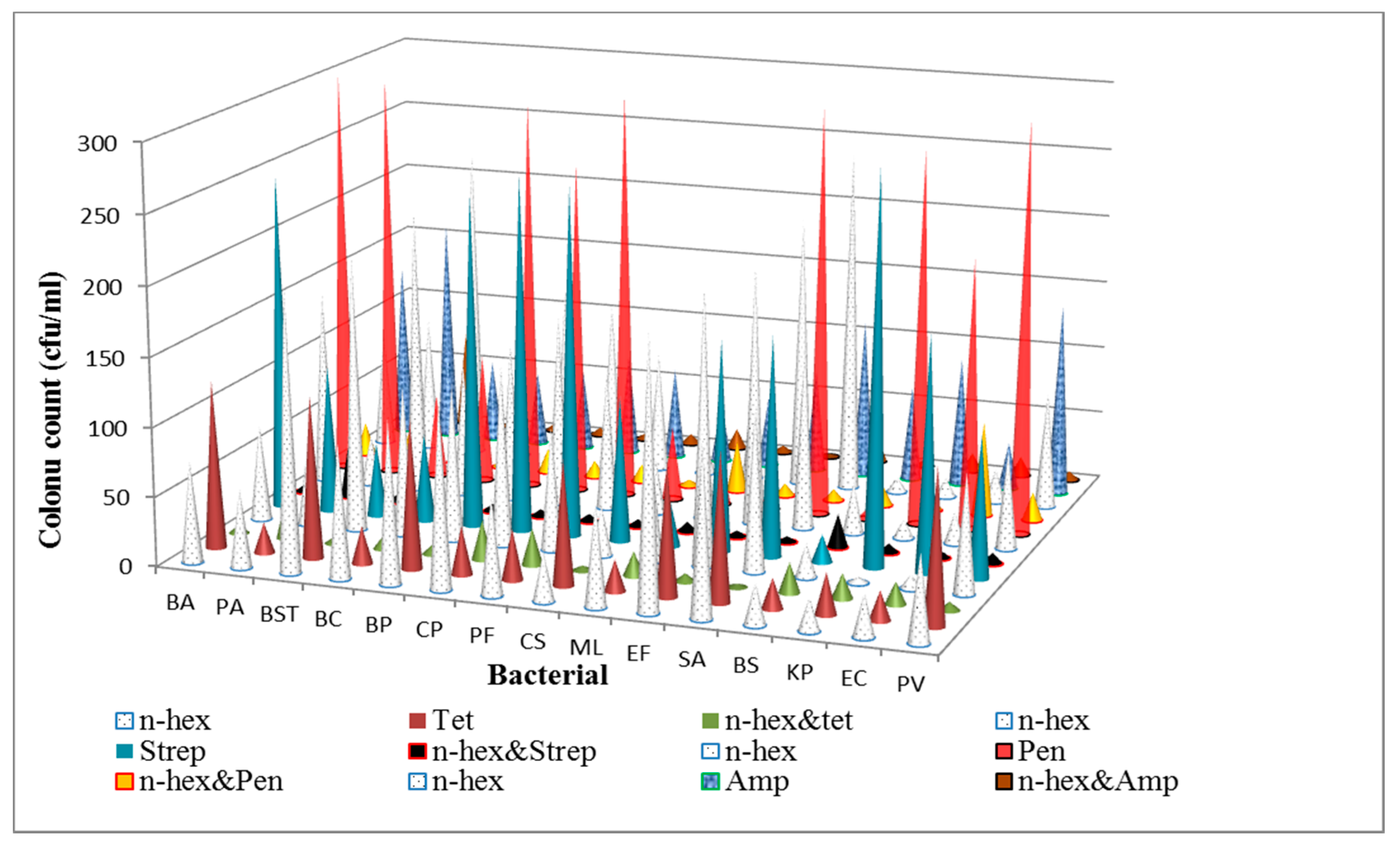



| Bacterial Isolates | TET + N-HEX | STREP + N-HEX | PEN + N-HEX | AMP + N-HEX |
|---|---|---|---|---|
| B. anthracis (LIO) | −2.86 ± 0.01(S) | −2.81 ± 0.02(S) | −1.34 ± 0.10(I) | −2.80 ± 0.17(S) |
| Ps. aureginosa (NCIB 950) | −0.70 ± 0.05(I) | −0.32 ± 0.05(I) | −0.49 ± 0.05(I) | −0.52 ± 0.05(I) |
| B. stearothermophillus (NCIB 8222) | −3.98 ± 0.00(S) | −2.07 ± 0.10(S) | −2.91 ± 0.10(S) | −3.01 ± 0.13(S) |
| B. cereus (NCIB 6349) | −0.93 ± 0.17(I) | −2.73 ± 0.09(S) | −2.27 ± 0.16(S) | −2.17 ± 0.11(S) |
| B. polymyxa (LIO) | −2.96 ± 0.22(S) | −3.57 ± 0.14(S) | −1.90 ± 0.01(I) | −2.27 ± 0.13(S) |
| C. pyogenes (LIO) | −0.35 ± 0.04(I) | −2.87 ± 0.10(S) | −2.22 ± 0.06(S) | −2.78 ± 0.17(S) |
| Ps. fluorescence (NCIB 3756) | −0.56 ± 0.14(I) | −3.34 ± 0.10(S) | −2.90 ± 0.04(S) | −2.13 ± 0.10(S) |
| C. sporogenes (NCIB 532) | −2.11 ± 0.14(S) | −2.29 ± 0.10(S) | −2.15 ± 0.10(S) | −1.45 ± 0.08(I) |
| M. luteus (NCIB 196) | −0.45 ± 0.14(I) | −1.68 ± 0.10(I) | −0.28 ± 0.06(I) | −2.29 ± 0.27(S) |
| E. faecalis (NCIB 775) | −3.15 ± 0.01(S) | −3.88 ± 0.11(S) | −1.46 ± 0.14(I) | −3.46 ± 0.17(S) |
| Staph. aureus (NCIB 8588) | −3.65 ± 0.19(S) | −3.55 ± 0.11(S) | −3.83 ± 0.17(S) | −1.82 ± 0.04(I) |
| B. subtilis (NCIB 3610) | −0.05 ± 0.00(I) | 0.01 ± 0.04(I) | 0.26 ± 0.01(I) | −0.81 ± 0.05(I) |
| K. pneumoniae (NCIB 418) | −0.38 ± 0.38(I) | −0.17 ± 0.17(I) | −0.97 ± 0.97(I) | −0.33 ± 0.33(I) |
| E. coli (NCIB 86) | −0.53 ± 0.06(I) | −1.08 ± 0.12(I) | 0.63 ± 0.04(I) | −1.28 ± 0.06(I) |
| P. vulgaris (LIO) | −2.76 ± 0.17(S) | −2.87 ± 0.13(S) | −2.61 ± 0.10(S) | −2.46 ± 0.08(S) |
| Bacterial Isolates | TET + N-HEX | STREP + N-HEX | PEN + N-HEX | AMP + N-HEX |
|---|---|---|---|---|
| B. anthracis (LIO) | −2.18 ± 0.01(S) | −2.21 ± 0.14(S) | −1.55 ± 0.03(I) | −2.08 ± 0.10(S) |
| Ps. aureginosa (NCIB 950) | 0.31 ± 0.05(I) | −0.10 ± 0.03(I) | −0.35 ± 0.04(I) | −0.29 ± 0.02(I) |
| B. stearothermophillus (NCIB 8222) | −2.75 ± 0.00(S) | −2.00 ± 0.10(S) | −2.15 ± 0.10(S) | −2.28 ± 0.13(S) |
| B. cereus (NCIB 6349) | −0.86 ± 0.04(I) | −2.01 ± 0.09(S) | −2.25 ± 0.11(S) | −1.93 ± 0.11(I) |
| B. polymyxa (LIO) | −2.38 ± 0.10(S) | −2.63 ± 0.14(S) | −1.64 ± 0.03(I) | −2.00 ± 0.65(S) |
| C. pyogenes (LIO) | −0.18 ± 0.03(I) | −2.78 ± 0.09(S) | −2.14 ± 0.08(S) | −2.47 ± 0.11(S) |
| Ps. fluorescence (NCIB 3756) | −0.29 ± 0.02(I) | −2.41 ± 0.10(S) | −1.80 ± 0.04(I) | −1.95 ± 0.12(I) |
| C. sporogenes (NCIB 532) | −1.98 ± 0.14(I) | −1.67 ± 0.10(I) | −1.86 ± 0.10(I) | −0.48 ± 0.08(I) |
| M. luteus (NCIB 196) | −0.22 ± 0.02(I) | −1.34 ± 0.08(I) | 0.12 ± 0.05(I) | −2.01 ± 0.09(S) |
| E. faecalis (NCIB 775) | −2.40 ± 0.01(S) | −3.06 ± 0.11(S) | −0.53 ± 0.14(I) | −3.10 ± 0.17(S) |
| Staph. aureus (NCIB 8588) | −3.32 ± 0.19(S) | −2.87 ± 0.11(S) | −2.44 ± 0.17(S) | −1.45 ± 0.04(I) |
| B. subtilis (NCIB 3610) | 0.00 ± 0.00(I) | 0.16 ± 0.04(I) | 0.41 ± 0.01(I) | −0.77 ± 0.05(I) |
| K. pneumoniae (NCIB 418) | −0.20 ± 0.05(I) | 0.42 ± 0.19(I) | 0.03 ± 0.15(I) | −0.04 ± 0.11(I) |
| E. coli (NCIB 86) | −0.24 ± 0.06(I) | −0.52. ± 0.12(I) | 0.94 ± 0.04(I) | −0.45 ± 0.06(I) |
| P. vulgaris (LIO) | −2.05 ± 0.17(S) | −2.10 ± 0.13(S) | −1.07 ± 0.10(I) | −2.23 ± 0.08(S) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abioye, O.E.; Akinpelu, D.A.; Okoh, A.I. Synergistic Effects of n-Hexane Fraction of Parkia biglobosa (Jacq.) Bark Extract and Selected Antibiotics on Bacterial Isolates. Sustainability 2017, 9, 228. https://doi.org/10.3390/su9020228
Abioye OE, Akinpelu DA, Okoh AI. Synergistic Effects of n-Hexane Fraction of Parkia biglobosa (Jacq.) Bark Extract and Selected Antibiotics on Bacterial Isolates. Sustainability. 2017; 9(2):228. https://doi.org/10.3390/su9020228
Chicago/Turabian StyleAbioye, Oluwatayo E., David A. Akinpelu, and Anthony I. Okoh. 2017. "Synergistic Effects of n-Hexane Fraction of Parkia biglobosa (Jacq.) Bark Extract and Selected Antibiotics on Bacterial Isolates" Sustainability 9, no. 2: 228. https://doi.org/10.3390/su9020228






