Techno-Economic Forecasts of Lithium Nitrates for Thermal Storage Systems
Abstract
:1. Introduction
2. Thermal Energy Storage Systems and Materials
2.1. Classifications of TES Systems
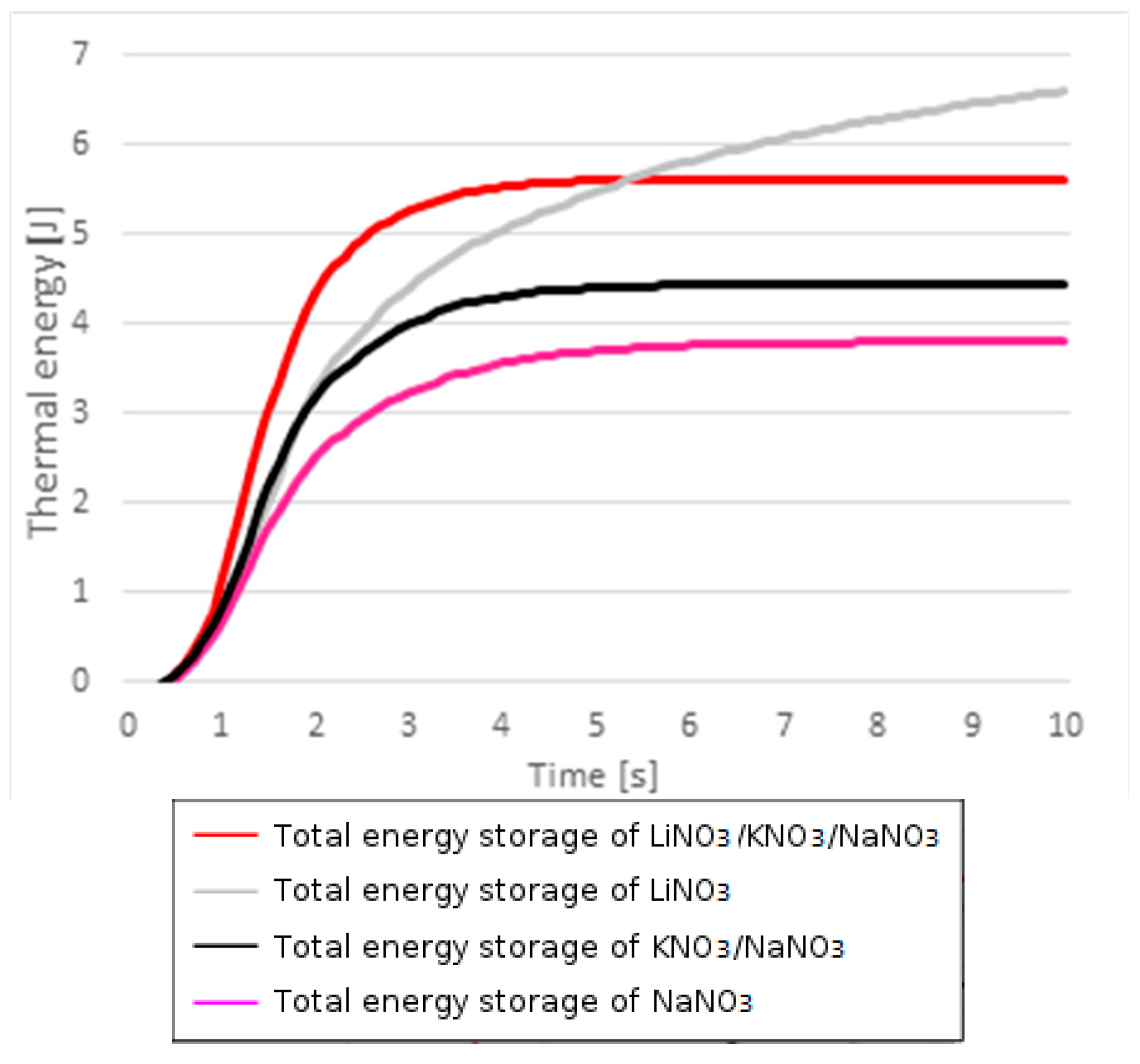
2.2. Improving TES: The Case for Lithium-Based Nitrates
3. Economic Evaluation of the Use of Lithium Nitrates
3.1. Description of Selected Plants
3.2. Levelized Cost of Energy (LCOE)
- It was assumed that the original TES system of the plants is able to work with the lithium based mixtures and that it can be stored in similar tank technology.
- The required amount of lithium based mixtures will be able to store the same quantity of thermal energy than solar salts. Then, for each new LCOE the tons and volume of lithium based mixture that were necessary to replace solar salt were estimated. These quantities were calculated using the thermal properties of each mixture mentioned in Table 1 and the following equation:where Q is heat, m is the mass of the selected heat storage medium and Cp is the specific heat of the material. Ti and Tf are the initial and final temperature of the heat storage medium respectively.
- The maximum stored sensible heat of solar salt of each CSP plant, Q in Equation (2), was obtained taking into account the corresponding working temperature range.
- The tons of lithium based mixtures, m in Equation (2), required to store the same quantity of thermal heat (Q) than with solar salt were calculated. The working temperature range was modified depending on the nitrate salt, considering that lower melting points will allow a lower limit.
- The costs of tanks to store the required lithium based mixtures calculated in step 2 were estimated. The costs were obtained by reducing the original cost of the tank in the same proportion as the required volume of storage material was reduced in comparison with solar salt. It was assumed that the lithium based nitrates might be kept in the same type of tanks used for solar salts.
- The I0 of each plant was replaced by the sum of the original total investment of the plant without the cost of the tanks and salts, and the new cost of tanks and salts.
- In each of the lithium based nitrates scenarios an increase of 2% in generated electricity was assumed as an effect of lowering the energy use of heat trace due to lower melting point as estimated in [44].
- In every scenario an analysis period of 30 years and a discount rate of 10% and 7% were considered.
3.3. Cost Comparison: Solar Salt vs. Lithium Mixture
4. The Impact of Uncertainty and Future Market Prospects
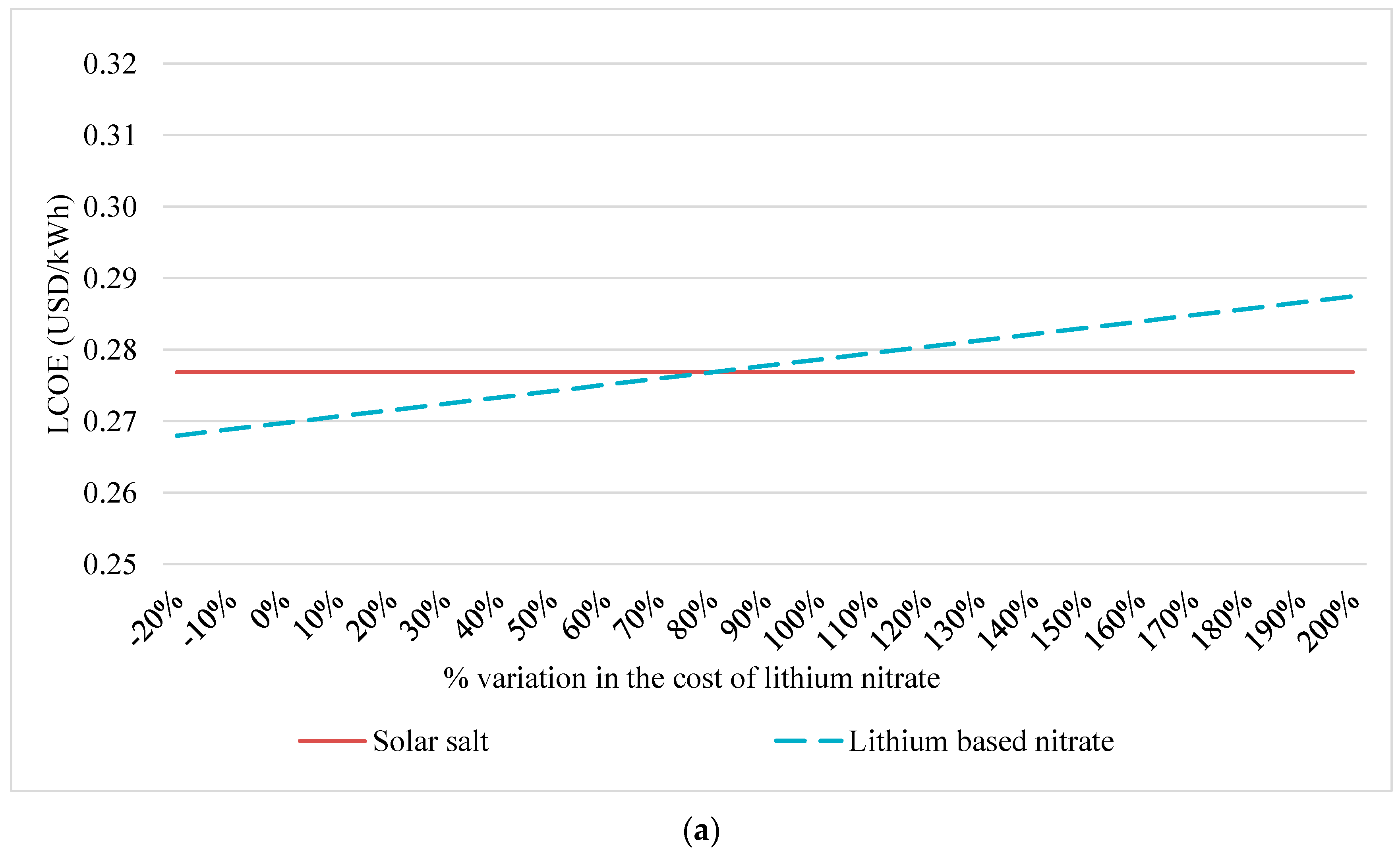
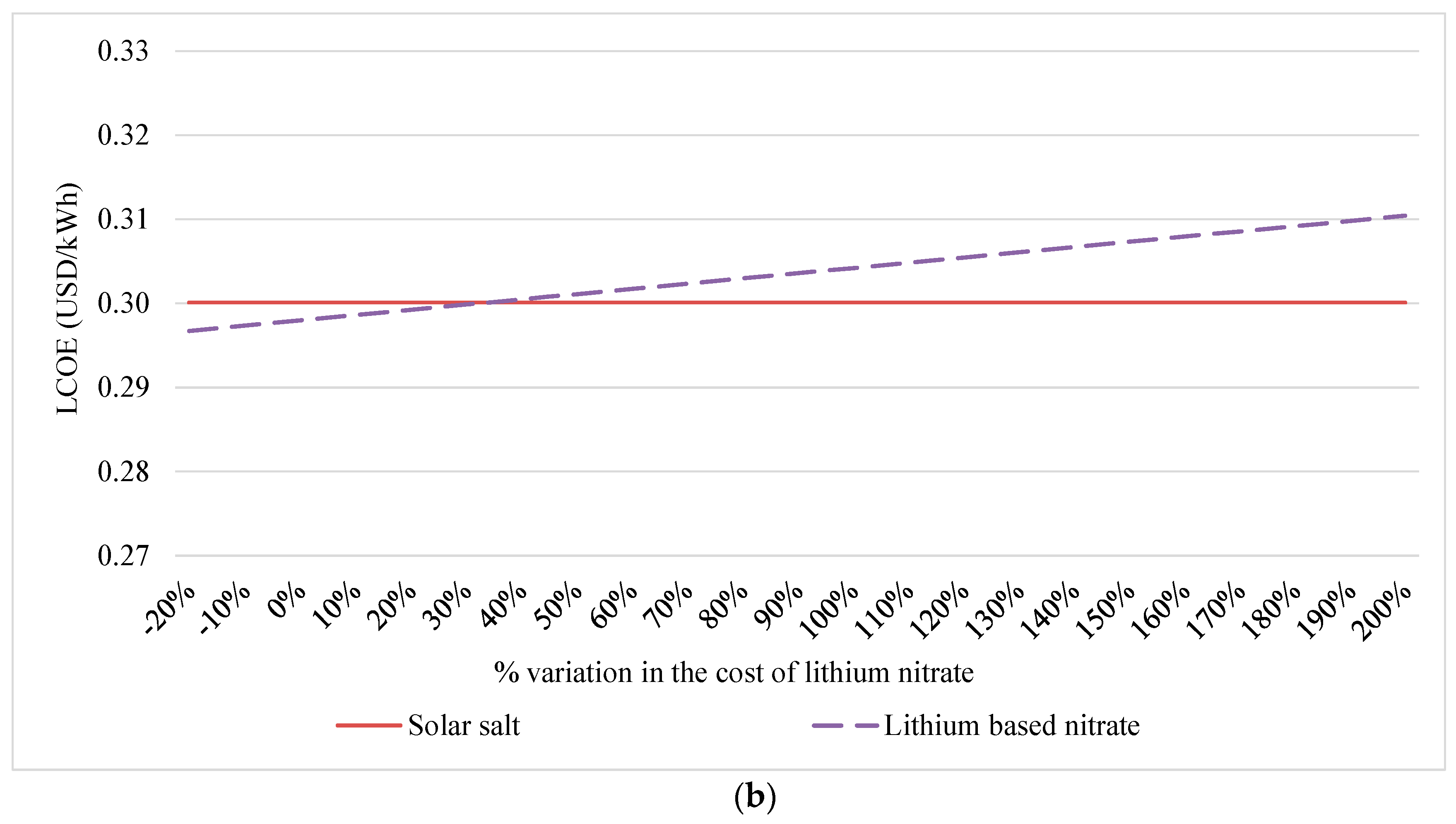
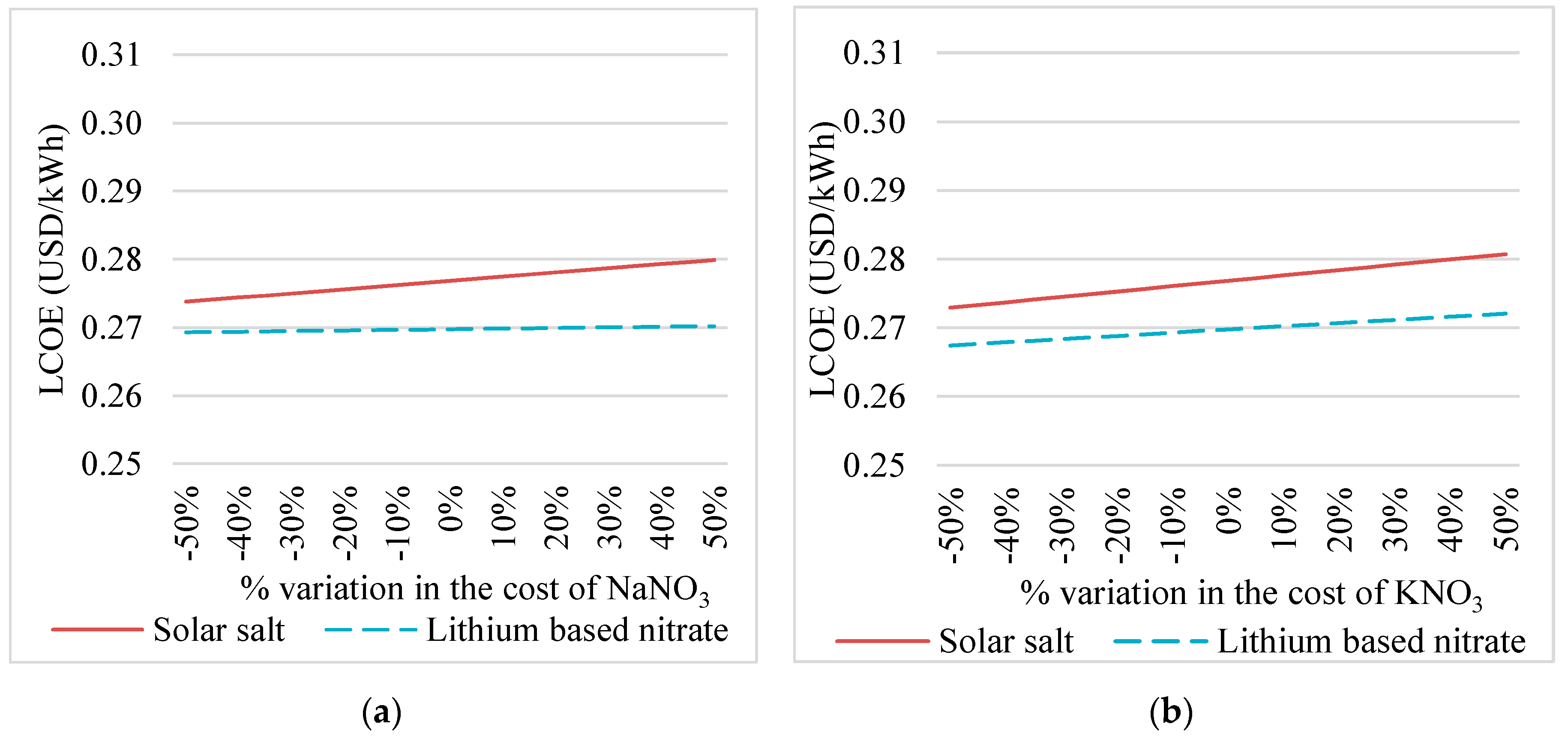
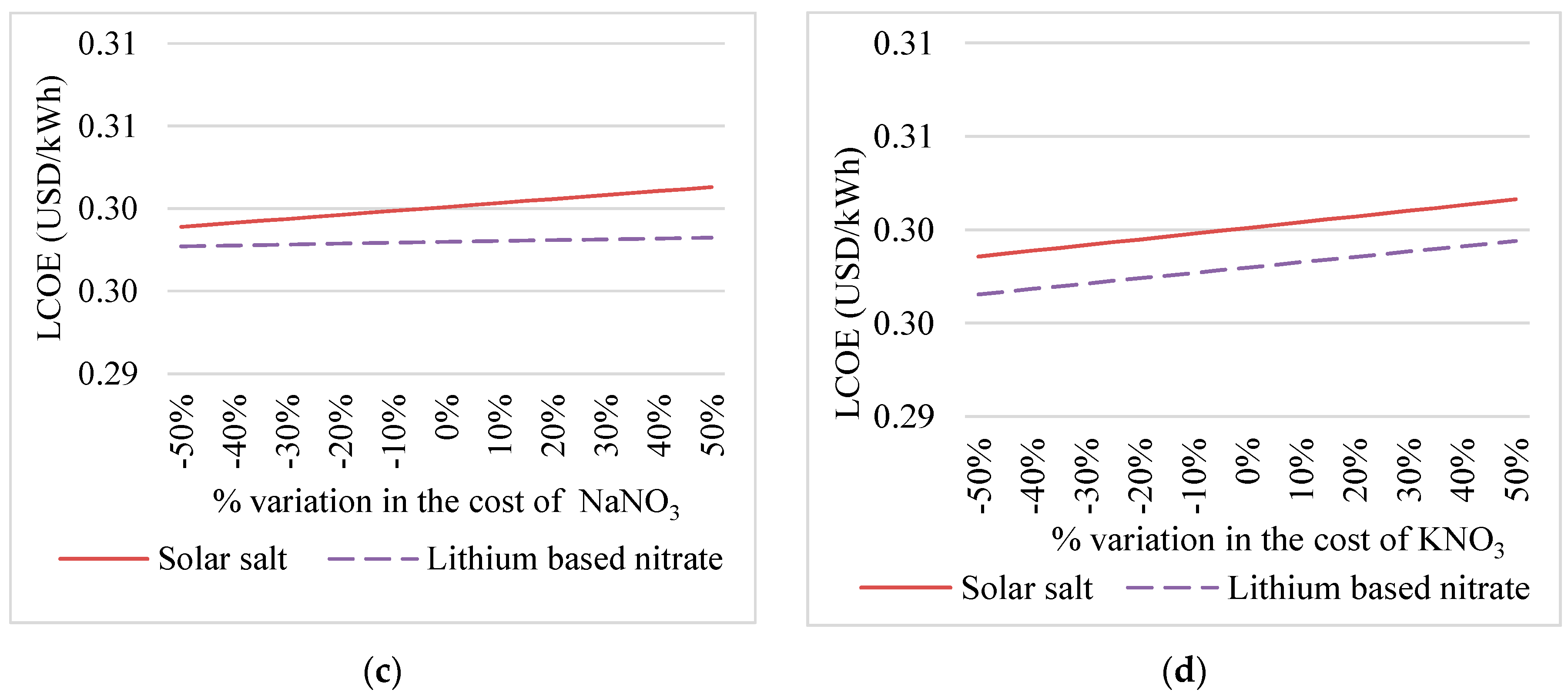
4.1. Sensitivity Analysis
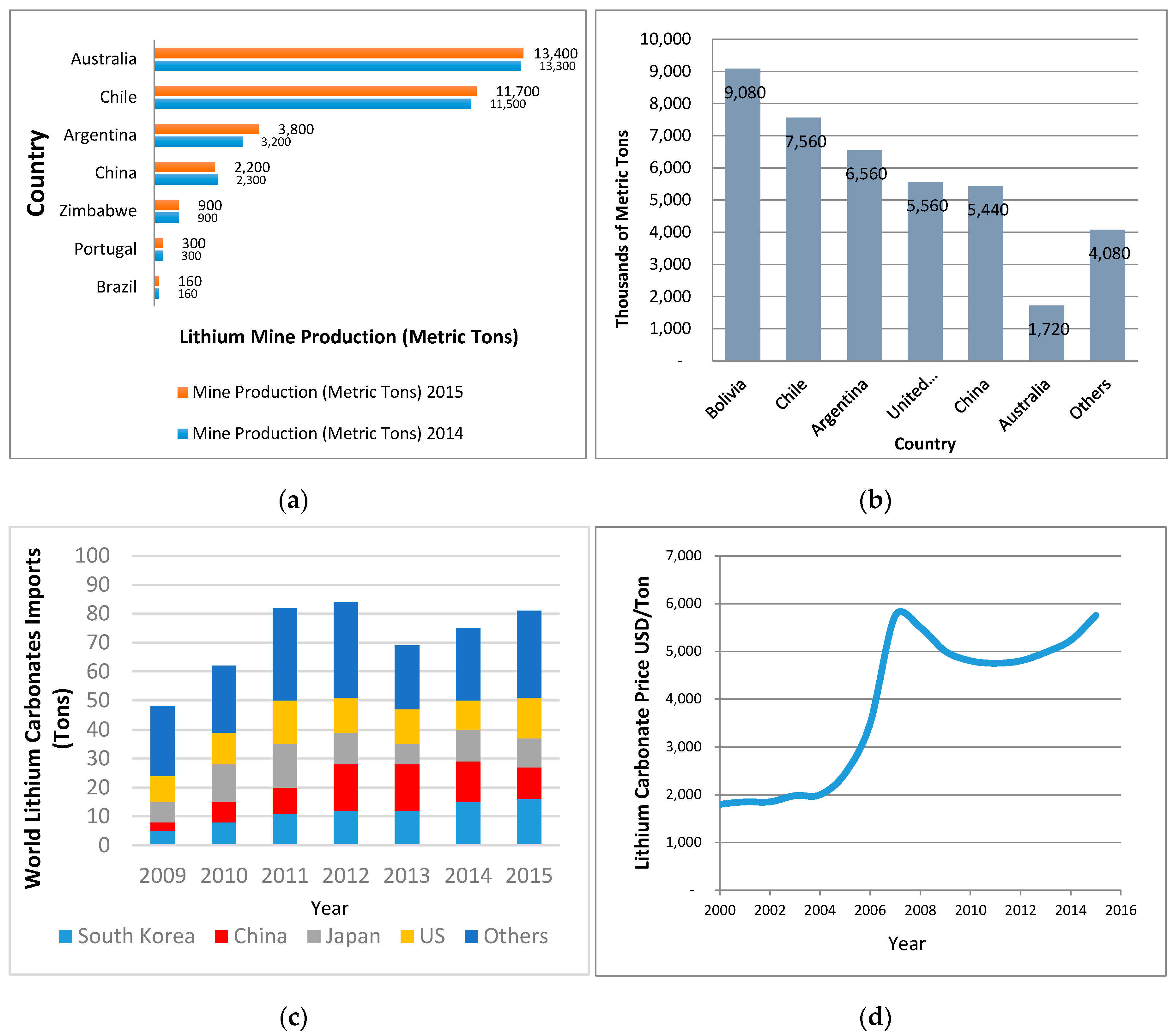
4.2. Lithium Market and Projections
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- U.S. Department of Energy. 2010 Solar Technologies Market Report. Available online: http://www.nrel.gov/docs/fy12osti/51847.pdf (accessed on 19 August 2016).
- Electricidad la Revista Energética de Chile. Proyecto Solar Cerro Dominador Presenta Avance de Construcción del 9%. Available online: http://www.revistaei.cl/2014/08/14/proyecto-solar-cerro-dominador-presenta-avance-de-construccion-del-9/# (accessed on 21 January 2016). (In Spanish).
- Gil, A; Medrano, M.; Martorell, I.; Lazaro, A.; Dolado, P.; Zalba, B.; Cabeza, L.F. State of the art on high temperature thermal energy storage for power generation. Part 1—Concepts, materials and modellization. Renew. Sustain. Energy Rev. 2010, 14, 31–55. [Google Scholar] [CrossRef]
- Fernandes, D.; Pitié, F.; Cáceres, G.; Baeyens, J. Thermal energy storage: “How previous findings determine current research priorities”. Energy 2012, 39, 246–257. [Google Scholar] [CrossRef]
- Zhang, H.L.; Baeyens, J.; Degrève, J.; Cacères, G. Concentrated solar power plants: Review and design methodology. Renew. Sustain. Energy Rev. 2013, 22, 466–481. [Google Scholar] [CrossRef]
- Tamme, R. Optimized Industrial Process Heat and Power Generation with Thermal Energy Storage. Technical Report. Available online: http://www.iea-eces.org/files/annex_19_finalreport-07-2010.pdf (accessed on 19 August 2016).
- Tian, Y.; Zhao, C.Y. A Review of Solar Collectors and Thermal Energy Storage in Solar Thermal Applications. Appl. Energy 2013, 104, 538–553. [Google Scholar] [CrossRef]
- Liu, M.; Saman, W.; Bruno, F. Review on Storage Materials and Thermal Performance Enhancement Techniques for High Temperature Phase Change Thermal Storage Systems. Renew. Sustain. Energy Rev. 2012, 16, 2118–2132. [Google Scholar] [CrossRef]
- Zalba, B.; Marin, J.M.; Cabeza, L.F.; Mehling, H. Review on Thermal Energy Storage with Phase Change: Materials, Heat Transfer Analysis and Applications. Appl. Therm. Eng. 2003, 23, 251–283. [Google Scholar] [CrossRef]
- Zhang, H.L.; Baeyens, J.; Cáceres, G.; Degrève, J.; Lv, Y.Q. Thermal Energy Storage: Recent Developments and Practical Aspects. Prog. Energy Combust. Sci. 2016, 53, 1–40. [Google Scholar] [CrossRef]
- Abedin, A.H.; Rosen, M.A. A Critical Review of Thermochemical Energy Storage Systems. Open Renew. Energy J. 2011, 4, 42–46. [Google Scholar] [CrossRef]
- Vignarooban, K.; Xu, X.; Arvay, A.; Hsua, K.; Kannan, A.M. Heat Transfer Fluids for Concentrating Solar Power Systems—A review. Appl. Energy 2015, 146, 383–396. [Google Scholar] [CrossRef]
- Kearney, D.; Herrmann, U.; Nava, P.; Kelly, B.; Mahoney, R.; Pacheco, J.; Cable, R.; Potrovitza, N.; Blake, D.; Price, H. Assessment of a molten salt heat transfer fluid in a parabolic trough solar field. J. Sol. Energy Eng. 2003, 125, 170–176. [Google Scholar] [CrossRef]
- Brems, A.; Cáceres, G.; Dewil, R.; Baeyens, J.; Pitié, F. Heat transfer to the riser-wall of a circulating fluidised bed (CFB). Energy 2013, 50, 493–500. [Google Scholar] [CrossRef]
- Zhang, H.L.; Baeyens, J.; Degrève, J.; Cáceres, G.; Segal, R.; Pitié, F. Latent heat storage with tubular-encapsulated phase change materials (PCMs). Energy 2014, 76, 66–72. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Lu, W.; Tian, Y. Heat transfer enhancement for thermal energy storage using metal foams embedded within phase change materials (PCMs). Sol. Energy 2010, 84, 1402–1412. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Gutierrez, A.; Barreneche, C.; Ushak, S.; Fernández, A.G.; Fernández, A.I.; Grágeda, M. Lithium in termal energy storage: A state-of-the-art review. Renew. Sustain. Energy Rev. 2015, 42, 1106–1112. [Google Scholar] [CrossRef]
- Reddy, R.G. Novel Molten Salts Thermal Energy Storage for Concentrating Solar Power Generation. Solar Energy Technologies Program Peer Review. Available online: http://www1.eere.energy.gov/solar/review_meeting/pdfs/prm2010_ualabama.pdf (accessed on 29 August 2016).
- Bradshaw, R.W.; Siegel, N.P. Molten nitrate salt development for thermal energy storage in parabolic trough solar power systems. In Proceedings of the ASME 2008 2nd International Conference on Energy Sustainability, Jacksonville, FL, USA, 10–14 August 2008; pp. 631–637. [Google Scholar]
- Bauer, T.; Pfleger, N.; Laing, D.; Steinmann, W.; Eck, M.; Kaesche, S. High-Temperature Molten Salts for Solar Power Application. In Molten Salts Chemistry; Lantelme, H., Groult, F., Eds.; Elsevier Inc.: Oxford, UK, 2013; pp. 415–438. [Google Scholar]
- Ulloa, R. Technical Viability of Phase Change Materials with Metal Foams to Be Used as Thermal Energy Storage in Concentrated Solar Power Plants. Dissertation for the Title of Civil Industrial Engineering, Adolfo Ibáñez University, Santiago, Chile, June 2015. [Google Scholar]
- Lopez, J.; Caceres, G.; Palomo Del Barrio, E.; Jomaa, W. Confined melting in deformable porous media: A first attempt to explain the graphite/salt composites behaviour. Int. J. Heat Mass Tran. 2010, 53, 1195–1207. [Google Scholar] [CrossRef]
- Pit1ie, F.; Zhao, C.Y.; Caceres, G. Thermo-mechanical analysis of ceramic encapsulated phase-change-material (PCM) particles. Energy Environ. Sci. 2011, 4, 2117–2124. [Google Scholar] [CrossRef]
- Parrado, C.; Cáceres, G.; Bize, F.; Bubnovich, V.; Baeyens, J.; Degreve, J.; Zhang, H.L. Thermo-mechanical analysis of copper-encapsulated NaNO3-KNO3. Chem. Eng. Res. Des. 2015, 93, 224–231. [Google Scholar] [CrossRef]
- Fernández, A.G.; Ushak, S.; Galleguillos, H.; Pérez, F.J. Development of new molten salts with LiNO3 and Ca(NO3)2 for energy storage in CSP plants. Appl. Energ 2014, 119, 131–140. [Google Scholar] [CrossRef]
- Fernández, A.G.; Pérez, F.J. Improvement of the corrosion properties in ternary molten nitrate salts for direct energy storage in CSP plants. Sol. Energy 2016, 134, 468–478. [Google Scholar] [CrossRef]
- Fernández, A.G.; Gomez, J.; Galleguillos, H.; Fuentealba, E. Thermophysical Properties and Corrosion Characterization of Low Cost Lithium Containing Nitrate Salts Produced in Northern Chile for Thermal Energy Storage. Available online: http://aip.scitation.org/doi/pdf/10.1063/1.4949112 (accessed on 29 August 2016).
- Cáceres, G.; Montané, M.; Nasirov, S.; O’Ryan, R. Review of Thermal Materials for CSP Plants and LCOE Evaluation for Performance Improvement using Chilean Strategic Minerals: Lithium Salts and Copper Foams. Sustainability 2016, 8, 106. [Google Scholar] [CrossRef]
- Reddy, R. Novel Molten Salts Thermal Energy Storage for Concentrating Solar Power Generation. Technical Report. Available online: https://www.osti.gov/scitech/biblio/1111584/ (accessed on 27 December 2016).
- Jäger-Waldau, A.; Monforti-Ferrario, F.; Banja, M.; Lacal, R. Renewable Energy Snapshots 2013. JRC Scientific and Policy Reports. Available online: http://publications.jrc.ec.europa.eu/repository/bitstream/JRC84762/jrc%20res%20final%20snapshots%202013%20.pdf (accessed on 30 August 2016).
- Energy News. Extresol 3 Rendimiento Controlado. Available online: http://www.energynews.es/articulostecnicos/Articuloextresol.pdf (accessed on 29 August 2016).
- National Renewable Energy Laboratory. Concentrating Solar Power Projects. Available online: http://www.nrel.gov/csp/solarpaces/ (accessed on 29 August 2016).
- Torresol Energy. Available online: http://www.torresolenergy.com/TORRESOL/gemasolar-plant/en (accessed on 29 August 2016).
- Azcárraga, G. Evaluating the Effectiveness of Molten Salt Storage with Solar Plants. Available online: https://www.ises.org/fileadmin/user_upload/PDF/Molten_salt_tower_plant_GA_Azcarraga.pdf (accessed on 29 August 2016).
- Cáceres, G.; Anrique, N.; Girard, A.; Degreve, J.; Baeyens, J.; Zhang, H.L. Performance of molten salt solar power towers in Chile. J. Renew. Sustain. Energ 2013, 5. [Google Scholar] [CrossRef]
- International Renewable Energy Agency. Renewable Power Generation Costs in 2014. Available online: http://www.irena.org/documentdownloads/publications/irena_re_power_costs_2014_report.pdf (accessed on 29 August 2016).
- International Renewable Energy Agency. Renewable Energy Technologies: Cost Analysis Series, Concentrating Solar Power. Available online: http://www.irena.org/documentdownloads/publications/re_technologies_cost_analysis-csp.pdf (accessed on 19 August 2016).
- Turchi, C.; Mehos, M.; Ho, C.; Kolb, G. Current and Future Costs for Parabolic Trough and Power Tower Systems in the US Market. Available online: http://www.nrel.gov/docs/fy11osti/49303.pdf (accessed on 19 August 2016).
- International Energy Agency. Technology Roadmap, Solar Thermal Electricity. Available online: https://www.iea.org/publications/freepublications/publication/technologyroadmapsolarthermalelectricity_2014edition.pdf (accessed on 29 August 2016).
- Kost, C.; Mayer, J.; Thomsen, J.; Hartmann, N.; Senkpiel, C.; Philipps, S.; Nold, S.; Lude, S.; Saad, N.; Schlegl, T. Levelized Cost of Electricity Renewable Energy Technologies. Study. Available online: https://www.ise.fraunhofer.de/en/publications/veroeffentlichungen-pdf-dateien-en/studien-und-konzeptpapiere/study-levelized-cost-of-electricity-renewable-energies.pdf (accessed on 29 August 2016).
- Short, W.; Packey, D.J.; Holt, T. A manual for the Economic Evaluation of Energy Efficiency and Renewable Energy Technologies. Technical Report NREL/TP-462-5173. Available online: http://www.nrel.gov/docs/legosti/old/5173.pdf (accessed on 29 August 2016).
- Darling, S.B.; You, F.; Veselka, T.; Velosa, A. Assumptions and the levelized cost of energy for photovoltaics. Energy Environ. Sci. 2011, 4, 3133–3139. [Google Scholar] [CrossRef]
- Branker, K.; Pathak, M.J.M.; Pearce, J.M. A review of solar photovoltaic levelized cost of electricity. Renew. Sustain. Energ Rev. 2011, 15, 4470–4482. [Google Scholar] [CrossRef]
- Centro Nacional para la Innovación y Fomento de las Energías Sustentables. Estudio de Prefactibilidad para el Desarrollo del Litio como Elemento de Transporte y Almacenamiento Térmico de la Energía Solar. Available online: http://cifes.gob.cl/wp-content/uploads/2014/08/Informe-Prefactibilidad_Solar_Litio.pdf (accessed on 29 August 2016).
- Corral, N.; Anrique, N.; Fernandes, D.; Parrado, C.; Caceres, G. Power, placement and LEC evaluation to install CSP plants in northern Chile. Renew. Sustain. Energ Rev. 2012, 16, 6678–6685. [Google Scholar] [CrossRef]
- Fox-Davies Resources Specialist. The Lithium Market. Available online: http://doc.xueqiu.com/1497add8471193fc2e583642.pdf (accessed on 29 August 2016).
- U.S. Geological Survey. Mineral Commodity Summaries. Available online: http://minerals.usgs.gov/minerals/pubs/mcs/2016/mcs2016.pdf (accessed on 29 August 2016).
- Maxwell, P. Transparent and opaque pricing: The interesting case of lithium. Resour. Pol. 2015, 45, 92–97. [Google Scholar] [CrossRef]
- Romei, V. Lithium Price on the Rise, FT Data. Available online: http://blogs.ft.com/ftdata/2016/04/14/lithium-price-on-the-rise/ (accessed on 29 August 2016).
- Citi GPS. Lithium: The Future Is Electric. Investment Themes in 2016: New normal or No Normal. Available online: https://www.citi.com/commercialbank/insights/assets/docs/investment-themes-in-2016.pdf (accessed on 11 May 2017).
- Miedema, J.H.; Moll, H.C. Lithium Availability in the EU27 for Battery-Driven Vehicles: The Impact of Recycling and Substitution on the Confrontation between Supply and Demand until2050. Resour. Pol. 2013, 38, 204–211. [Google Scholar] [CrossRef]
- Ker, P. Lithium Prices Tipped to Rise 20 Per Cent by 2017 on Demand for Electric Cars. Available online: http://www.smh.com.au/business/mining-and-resources/lithium-prices-tipped-to-rise-by-20-per-cent-by-2017-on-demand-for-electric-cars-20151026-gkid4z.html (accessed on 29 August 2016).
- Statista. The Statistical Portal. Forecast of Annual Price Averages for Lithium Chemicals Worldwide from 2015 to 2025 (in U.S. Dollars per Kilogram). Available online: https://www.statista.com/statistics/452028/average-annual-price-projection-for-lithium-chemicals-globally/ (accessed on 29 August 2016).
- Hocking, M.; Kan, J.; Young, P.; Terry, C.; Begleiter, D. Industry Lithium 101; Deutsche Bank Markets Research; Deutsche Bank AG/Sydney: Sydney, Australia, 2016. [Google Scholar]
| Mixture | Melting Point | Thermal Stability | Heat Capacity | Density | Price |
|---|---|---|---|---|---|
| (°C) | (°C) | (kJ/kg K) | (kg/m3) | (USD/kg) | |
| 40% KNO3 + 60% NaNO3 | 222 | 588.5 | 1.50 | 1899 | 0.72 |
| 25.92% LiNO3 + 20.01% NaNO3 + 54.07% KNO3 | 117 | 435 | 1.63 | 1720 | 1.68 |
| KNO3 + NaNO2 + LiNO2 + NaNO3 | 79 | N/A | 1.50 | 1780 | 1.93 |
| KNO3 + LiNO3 + NaNO3 + MgK | 101 | 385 | 1.58 | 1710 | 1.54 |
| LiNO3 + NaNO2 + NaNO3 + KNO3 | 99 | 425 | 1.56 | 1780 | 1.81 |
| LiNO3 + NaNO2 + NaNO3 + KNO2 + KNO3 | 95.7 | 435 | 1.55 | 1780 | 1.80 |
| 30% LiNO3 + 60% KNO3 + 10% Ca(NO3)2 | 132 | 567.2 | 1.4 | 1773 | 1.83 |
| 53% KNO3 + 29% LiNO3 + 18% NaNO3 | 120 | 540 | 1.64 | 1780 | 1.79 |
| 20% LiNO3 + 52% KNO3 + 28% NaNO3 | 130 | 600.5 | 1.09 | N/A | 1.47 |
| Parameter | Extresol 3 | Gemasolar |
|---|---|---|
| Capacity (MWe) | 50 [30,32] | 19.9 [32,33,35] |
| TES concept | Active indirect [32] | Active direct [34,35] |
| TES capacity (Hours) | 7.5 [30,31] | 15 [32,33,35] |
| Type of HSM | Solar salt [31] | Solar salt [30,35] |
| Amount of HSM (tons) | 28,500 [31] | 7900 [34] |
| Diameter of TES tanks (m) | 38 [31] | 23 [34] |
| Height of TES tanks (m) | 14 [31] | 10.5 [34] |
| Annual Generation (MWhe) | 158,000 [32] | 110,000 [32,33,34,35] |
| Type of HTF | Diphenyl/Biphenyl oxide [32] | Solar salt [32,34] |
| Min. working temperature (°C) | 293 [32] | 290 [32] |
| Max. working temperature (°C) | 393 [32] | 565 [32,35] |
| Parameter | Extresol 3 | Gemasolar |
|---|---|---|
| Total investment (MM USD) | 390 [30,32] | 299 [30,32] |
| Solar salts cost (MM USD) 1 | 20.8 | 5.7 |
| Tanks cost (MM USD) | 7.1 | 1.8 |
| Annual O&M (MM USD) | 2.4 [36,37] | 1.3 [36,38] |
| 40% KNO3 + 60% NaNO3 (Solar Salt) | 25.92% LiNO3 + 20.01% NaNO3 + 54.07% KNO3 | KNO3 + LiNO3 + NaNO3 + MgK | LiNO3 + NaNO2 + NaNO3 + KNO2 + KNO3 | 53% KNO3 + 29% LiNO3 + 18% NaNO3 | ||
|---|---|---|---|---|---|---|
| Salts volume | m3 | 15,271 | 7543 | 7266 | 6964 | 7352 |
| Total TES Module cost | MM USD | 27.86 | 25.35 | 22.46 | 25.50 | 26.78 |
| LCOE (d = 10%) | USD/kWh | 0.2768 | 0.2698 | 0.2679 | 0.2699 | 0.2707 |
| LCOE variation (d = 10%) 1 | % | - | ↓ 2.6% | ↓ 3.2% | ↓ 2.5% | ↓ 2.2% |
| LCOE (d = 7%) | USD/kWh | 0.2139 | 0.2085 | 0.2070 | 0.2085 | 0.2092 |
| LCOE variation (d = 7%) 1 | % | - | ↓ 2.5% | ↓ 3.2% | ↓ 2.5% | ↓ 2.2% |
| 40% KNO3 + 60% NaNO3 (Solar Salt) | 30% LiNO3 + 60% KNO3 + 10% Ca(NO3)2 | 53% KNO3 + 29% LiNO3 + 18% NaNO3 | ||
|---|---|---|---|---|
| Salts volume | m3 | 4160 | 3593 | 3188 |
| Total TES Module cost | MM USD | 7.77 | 13.47 | 11.75 |
| LCOE (d = 10%) | USD/kWh | 0.3001 | 0.2996 | 0.2980 |
| LCOE variation (d = 10%) 1 | % | - | ↓ 0.16% | ↓ 0.71 % |
| LCOE (d = 7%) | USD/kWh | 0.2308 | 0.2304 | 0.2291 |
| LCOE variation (d = 7%)1 | % | - | ↓ 0.19% | ↓ 0.72% |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montané, M.; Cáceres, G.; Villena, M.; O’Ryan, R. Techno-Economic Forecasts of Lithium Nitrates for Thermal Storage Systems. Sustainability 2017, 9, 810. https://doi.org/10.3390/su9050810
Montané M, Cáceres G, Villena M, O’Ryan R. Techno-Economic Forecasts of Lithium Nitrates for Thermal Storage Systems. Sustainability. 2017; 9(5):810. https://doi.org/10.3390/su9050810
Chicago/Turabian StyleMontané, Macarena, Gustavo Cáceres, Mauricio Villena, and Raúl O’Ryan. 2017. "Techno-Economic Forecasts of Lithium Nitrates for Thermal Storage Systems" Sustainability 9, no. 5: 810. https://doi.org/10.3390/su9050810





