Predicting Bio-indicators of Aquatic Ecosystems Using the Support Vector Machine Model in the Taizi River, China
Abstract
:1. Introduction
2. Materials and Methods
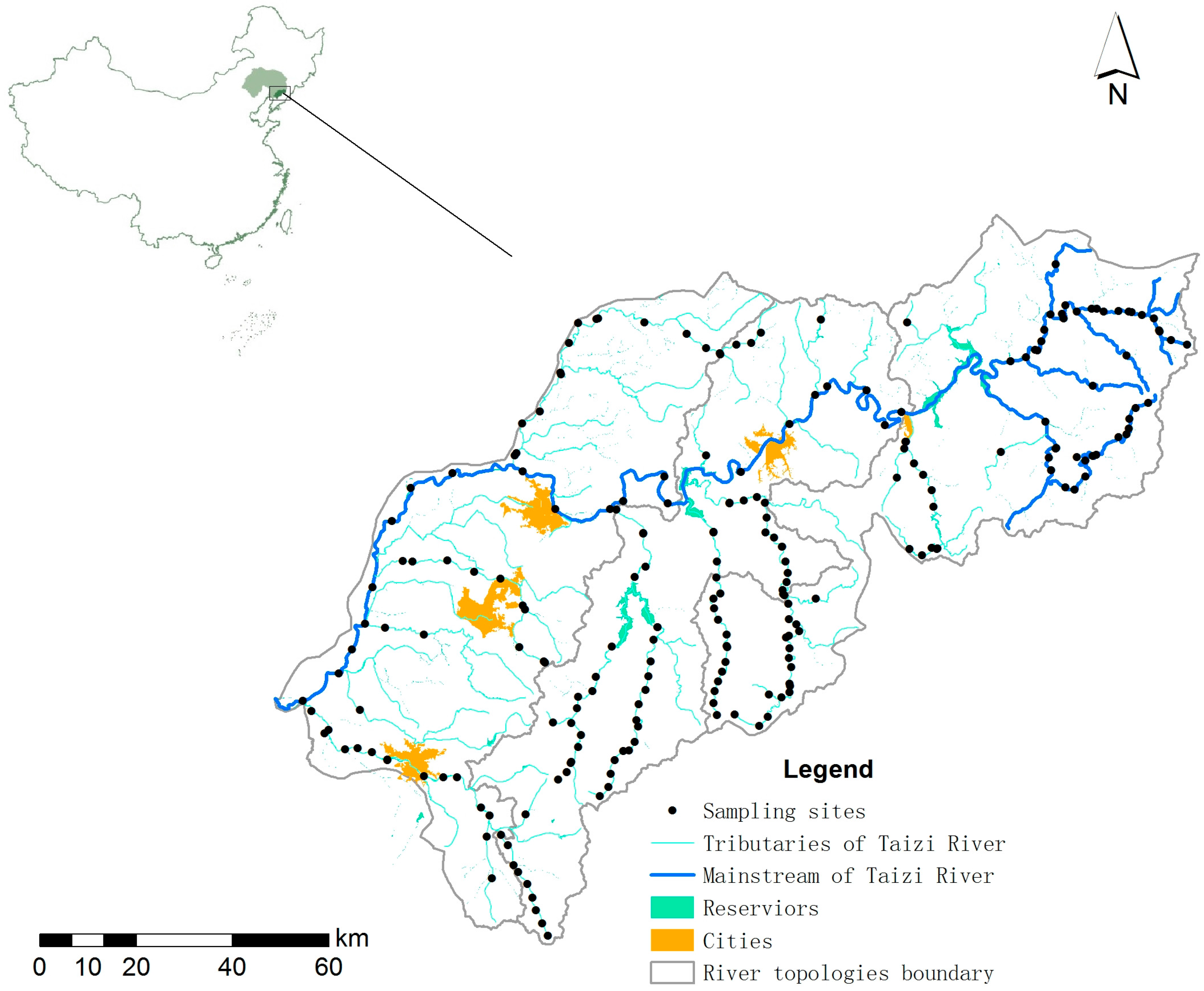
2.1. Study Area
2.2. The Available Dataset
2.3. Theoretical Background of Applied Models
2.4. Performance
2.5. Sensitivity Analysis
3. Results
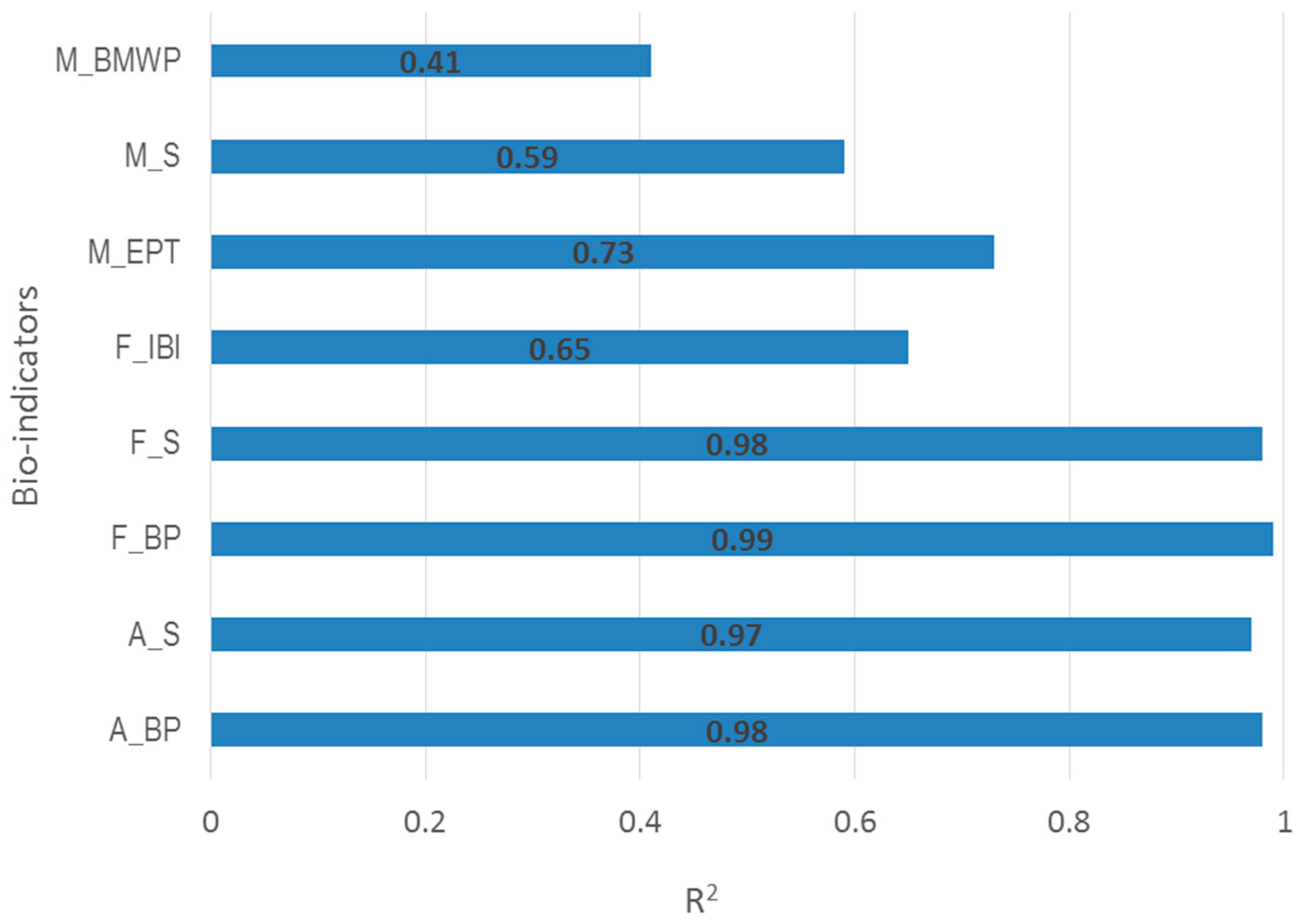
3.1. Determination of Optimal Model
3.2. Sensitivity Analysis
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Postel, S.; Carpenter, S. Freshwater ecosystem services. In Nature’s Services: Societal Dependence on Natural Ecosystems; Daily, G.C., Ed.; Island Press: Washington, DC, USA, 1997. [Google Scholar]
- Writing, C.; Walter, T.; Mooney, H.; Cropper, A. Millennium Ecosystem Assessment Synthesis Report; Island Press: Washington, DC, USA, 2005. [Google Scholar]
- Giorgio, A.; Bonis, S.D.; Guida, M. Macroinvertebrate and diatom communities as indicators for the biological assessment of river Picentino (Campania, Italy). Ecol. Indic. 2016, 64, 85–91. [Google Scholar] [CrossRef]
- Stevenson, R.J.; Pan, T.D. Assessing environmental conditions in rivers and streams with diatoms. In The Diatoms: Applications for the Environmental and Earth Sciences; Stoermer, E.F., Smol, J.P., Eds.; Cambridge University Press: Cambridge, UK, 2004. [Google Scholar]
- Arthington, A.H.; Bunn, S.E.; Poff, L.R.; Naiman, R.J. The challenge of providing environmental flow rules to sustain river ecosystems. Ecol. Appl. 2006, 16, 1311–1318. [Google Scholar] [CrossRef]
- Álvarez-Cabria, M.; Barquín, J. Macroinvertebrate community dynamics in a temperate European Atlantic river. Do they conform to general ecological theory? Hydrobiologia 2011, 658, 277–291. [Google Scholar] [CrossRef]
- Qu, X.D.; Wu, N.C.; Tang, T. Effects of heavy metals on benthic macroinvertebrate communities in high mountain streams. Int. J. Lim. 2010, 46, 291–302. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, F.; Meng, W.; Wang, X.Q. Water quality assessment and source identification of Daliao river basin using multivariate statistical methods. Environ. Monit. Assess. 2009, 152, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Bu, H.M.; Zhang, Y.; Meng, W. Classification of rivers based on water quality assessment using factor analysis in Taizi River basin, northeast China. Environ. Earth. Sci. 2013, 69, 909–919. [Google Scholar] [CrossRef]
- MEP (Ministry of Environmental Protection of the People’s Republic of China). National 12th Fiver-Year Environment Protection Plan 2012. Available online: http://zfs.mep.gov.cn/fg/gwyw/201112/t20111221_221570.htm (accessed on 24 May 2017). (In Chinese)
- Lee, J.H.W.; Huang, Y.; Dickman, M.; Jayawardena, A.W. Neural network modeling of coastal algal blooms. Ecol. Model. 2003, 159, 179–201. [Google Scholar] [CrossRef]
- Park, Y.; Cho, K.H.; Park, J.; Cha, S.M.; Kim, J.H. Development of early-warning protocol for predicting chlorophyll-a concentration using machine learning models in freshwater and estuarine reservoirs, Korea. Sci. Total Environ. 2015, 502, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Lee, J.H.W. Continuous monitoring of short term dissolved oxygen and algal dynamics. Water Res. 1995, 29, 2789–2796. [Google Scholar] [CrossRef]
- Yabunaka, K.; Hosomi, M.; Murakami, A. Novel application of a back-propagation artificial neural network model formulated to predict algal bloom. Water. Sci. Technol. 1997, 36, 89–97. [Google Scholar] [CrossRef]
- Liu, S.Y.; Tai, H.J.; Ding, Q.S.; Li, D.L.; Xu, L.Q.; Wei, Y.G. A hybrid approach of support vector regression with genetic algorithm optimization for aquaculture water quality prediction. Math. Comput. Model. 2013, 3–4, 458–465. [Google Scholar] [CrossRef]
- Singh, K.P.; Basant, N.; Gupta, S. Support vector machines in water quality management. Anal. Chim. Acta 2011, 703, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Granata, F.; Papirio, S.; Giovanni, E.; Gargano, R.; Marinis, G.D. Machine Learning Algorithms for the Forecasting of Wastewater Quality Indicators. Water 2017, 9, 105. [Google Scholar] [CrossRef]
- Granata, F.; Gargano, R.; Marinis, G.D. Support Vector Regression for Rainfall-Runoff Modeling in Urban Drainage: A Comparison with the EPA’s Storm Water Management Model. Water 2016, 8, 69. [Google Scholar] [CrossRef]
- Hoang, T.H.; Lock, K.; Mouton, A.; Goethals, P.L.M. Application of classification trees and support vector machines to model the presence of macroinvertebrates in rivers in Vietnam. Ecol. Inf. 2010, 5, 140–146. [Google Scholar] [CrossRef]
- Michaela, B.; Han, D. Identification of support vector machines for runoff modelling. J. Hydroinform. 2004, 6, 265–280. [Google Scholar]
- Leigh, C.; Qu, X.; Zhang, Y.; Kong, W.J.; Meng, W.; Hanington, P.; Speed, R.; Gippel, C.; Bond, N.; et al. Assessment of River Health in the Liao River Basin (Taizi. Subcatchment.); International Water Centre: Brisbane, Australia, 2012. [Google Scholar]
- CRAES (Chinese Research Academy of Environmental Sciences). Taizi. Basin Background Report; Report to ACEDP Project; International Water Centre: Brisbane, Australia, 2010. [Google Scholar]
- Fan, J.; Semenzin, E.; Meng, W.; Giubilato, E.; Zhang, Y.; Critto, A.; Zabeo, A.; Zhou, Y.; Ding, S.; Wan, J.; et al. Ecological status classification of the Taizi River Basin, China: A comparison of integrated risk assessment approaches. Environ. Sci. Pollut. Res. 2015, 22, 14738–14754. [Google Scholar] [CrossRef] [PubMed]
- Moyle, P.B.; Cech, J.J. Fishes: An Introduction to Ichthyology, 2nd ed.; Prentice Hall: Englewood Cliffs, NJ, USA, 1988. [Google Scholar]
- Flores, M.J.L.; Zafaralla, M.T. Macroinvertebrate Composition, Diversityand Richness in Relation to the Water Quality Status of Mananga River, Cebu, Philippines. Philipp. Sci. Lett. 2012, 5, 103–113. [Google Scholar]
- Armitage, P.D.; Moss, D.; Wright, J.F.; Furse, M.T. The performance of a new biological water quality score system based on macroinvertebrates over a wide range of unpolluted running-water sites. Water Res. 1983, 17, 333–347. [Google Scholar] [CrossRef]
- Mandaville, S.M. Benthic Macroinvertebrates in Freshwaters-Taxa Tolerance Values, Metrics, and Protocols; Soil & Water Conservation Society of Metro Halifax (Project H-1): New York, NY, USA, 2002. [Google Scholar]
- Cortes, C.; Vapnik, V. Support-vector networks. Mach. Learn. 1995, 20, 273–297. [Google Scholar] [CrossRef]
- Vapnik, V.N. Statistical Learning Theory; Wiley: New York, NY, USA, 1998. [Google Scholar]
- Varley, A.; Tyler, A.; Smith, L.; Dale, P.; Davies, M. Remediating radium contaminated legacy sites: Advances made through machine learning in routine monitoring of “hot” particles. Sci. Total Environ. 2015, 521, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xu, Z.; Lu, W.; Zhang, X.Y. Determination of the spread parameter in the Gaussian kernel for classification and regression. Neurocomputing 2003, 55, 643–663. [Google Scholar] [CrossRef]
- Cherkassky, V.; Ma, Y. Practical selection of SVM parameters and noise estimation for SVM regression. Neural Netw. 2004, 17, 113–126. [Google Scholar] [CrossRef]
- Verrelst, J.; Muñoz, J.; Alonso, L.; Delegido, J.; Rivera, J.P.; Camps-Valls, G.; Moreno, J. Machine learning regression algorithms for biophysical parameter retrieval: opportunities for sentinel-2 and -3. Remote Sens. Environ. 2012, 118, 127–139. [Google Scholar] [CrossRef]
- Bäck, T. Evolutionary Algorithms in Theory and Practice: Evolution Strategies, Evolutionary Programming, Genetic Algorithms; Oxford University Press: Oxford, UK, 1996. [Google Scholar]
- Fischer, P. An experimental test of metabolic and behavioural responses of benthic fish species to different types of substrate. Can. J. Fish. Aquat. Sci. 2000, 57, 2336–2344. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, Y.; Ding, S.; Zhao, R.; Meng, W. Response of fish communities to environmental changes in an agriculturally dominated watershed (Liao River Basin) in northeastern China. Ecol. Eng. 2015, 76, 130–141. [Google Scholar] [CrossRef]
- Silva, F.L.; Moreira, D.C.; Ruiz, S.S.; Bochini, G.L. Diversity and abundance of aquatic macroinvertebrates in a lotic environment in Midwestern São Paulo State, Brazil. Ambient. AguaInterdiscip. J. Appl. Sci. 2009, 4, 37–44. [Google Scholar] [CrossRef]
- Marshall, S.; Elliott, M. Environmental influences on the fish assemblage of the Humber estuary, UK. Estuar. Coast. Shelf Sci. 1998, 46, 175–184. [Google Scholar] [CrossRef]
- Poxton, M.G.; Allouse, S.B. Water quality criteria for marine fisheries. Aguacult. Eng. 1982, 1, 153–191. [Google Scholar] [CrossRef]
- Li, Y.L.; Li, Y.F.; Xu, Z.X. Effect of Environmental Factors on Fish Community Structure in the Huntai river Basin at Multiple Scales. Environ. Sci. 2014, 35, 3504–3512. (In Chinese) [Google Scholar]
| (a) | |||
|---|---|---|---|
| Community Structure of Freshwater Biology | |||
| Biological Communities | Indicators of Community Structure | Indication | |
| Fish | Species richness (F_S) | These indicators are related to physical, chemical, biological and zoogeographic factors and long-term pressures [21,24] | |
| Index of biotic integrity (F_IBI) | |||
| Berger–Parker index (F_BP) | |||
| Macroinvertebrate | Families richness (M_S) | A measure of diversity of macroinvertebrate families, which reflects the general deterioration of water quality [25] | |
| Biological monitoring working party score (M_BMWP) | A procedure based on macroinvertebrate communities assessment for measuring water quality related to organic pollution [26] | ||
| Ephemeroptera, Plecopter,a and Trichopterafamily richness (M_EPT) | Displaying the taxa richness within the insect groups, which are considered to be sensitive to pollution [27] | ||
| Algae | Species richness (A_S) | Both reflecting the water quality deterioration related to eutrophication and organic pollution [21] | |
| Berger–Parker index (A_BP) | |||
| (b) | |||
| Environmental indicators | Impact typologies | ||
| Physico-Chemistry | |||
| Electricity conductivity (EC) | Salinization | ||
| Dissolvedoxygen (DO) | Organic pollution | ||
| Biological oxygen demand in fivedays (BOD5) | Organic pollution | ||
| Chemical oxygen demand (COD) | Organic pollution | ||
| Ammonia nitrogen (NH3-N) | Eutrophication | ||
| Total phosphorus (TP) | Eutrophication | ||
| Hydromorphology | |||
| Water quantity (WQ) | Alteration of hydrological regime | ||
| Channel change (CC) | Alteration of river continuity | ||
| Morphology diversity (MD) | Morphological alteration | ||
| Regression Model | C | ε | σ |
|---|---|---|---|
| A_BP | 12.54 | 0.1 | 49.87 |
| A_S | 41.19 | 0.01 | 99.88 |
| F_BP | 10.53 | 0.001 | 13.62 |
| F_IBI | 1.51 | 0.1 | 0.25 |
| F_S | 87.72 | 0.13 | 11.52 |
| M_BMWP | 3.714 | 0.33 | 0.08 |
| M_EPT | 0.64 | 0.22 | 2.27 |
| M_S | 0.48 | 0.24 | 0.44 |
| Variables | EC | DO | BOD5 | COD | NH3-N | TP | TN | WQ | CC | MD |
|---|---|---|---|---|---|---|---|---|---|---|
| A_BP | 0.98 | 0.96 | 0.96 | 0.97 | 0.97 | 0.94 | 0.98 | 0.97 | 0.95 | 0.98 |
| A_S | 0.96 | 0.92 | 0.95 | 0.96 | 0.95 | 0.93 | 0.93 | 0.95 | 0.91 | 0.95 |
| F_BP | 0.97 | 0.94 | 0.93 | 0.98 | 0.97 | 0.95 | 0.94 | 0.98 | 0.97 | 0.98 |
| F_IBI | 0.65 | 0.64 | 0.63 | 0.65 | 0.64 | 0.63 | 0.63 | 0.64 | 0.62 | 0.64 |
| F_S | 0.96 | 0.94 | 0.93 | 0.96 | 0.97 | 0.98 | 0.96 | 0.98 | 0.97 | 0.98 |
| M_BMWP | 0.40 | 0.39 | 0.35 | 0.36 | 0.41 | 0.38 | 0.39 | 0.9 | 0.40 | 0.40 |
| M_EPT | 0.69 | 0.67 | 0.66 | 0.66 | 0.71 | 0.67 | 0.69 | 0.72 | 0.65 | 0.71 |
| M_S | 0.57 | 0.55 | 0.58 | 0.58 | 0.57 | 0.54 | 0.56 | 0.57 | 0.57 | 0.58 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, J.; Wu, J.; Kong, W.; Zhang, Y.; Li, M.; Zhang, Y.; Meng, W.; Zhang, A.M. Predicting Bio-indicators of Aquatic Ecosystems Using the Support Vector Machine Model in the Taizi River, China. Sustainability 2017, 9, 892. https://doi.org/10.3390/su9060892
Fan J, Wu J, Kong W, Zhang Y, Li M, Zhang Y, Meng W, Zhang AM. Predicting Bio-indicators of Aquatic Ecosystems Using the Support Vector Machine Model in the Taizi River, China. Sustainability. 2017; 9(6):892. https://doi.org/10.3390/su9060892
Chicago/Turabian StyleFan, Juntao, Jin Wu, Weijing Kong, Yizhang Zhang, Mengdi Li, Yuan Zhang, Wei Meng, and And Mengheng Zhang. 2017. "Predicting Bio-indicators of Aquatic Ecosystems Using the Support Vector Machine Model in the Taizi River, China" Sustainability 9, no. 6: 892. https://doi.org/10.3390/su9060892





