Impact of Biochar Formulation on the Release of Particulate Matter and on Short-Term Agronomic Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Particle Matter Experiment
2.2. Pot Experiment
3. Results
3.1. Particle Matter Experiment
3.2. Pot Experiment
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lehmann, J. Bio-energy in the black. Front. Ecol. Environ. 2007, 5, 381–387. [Google Scholar] [CrossRef]
- Smith, P. Soil carbon sequestration and biochar as negative emission technologies. Glob. Chang. Biol. 2016, 22, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Woolf, D.; Amonette, J.E.; Street-Perrott, F.A.; Lehmann, J.; Joseph, S. Sustainable biochar to mitigate global climate change. Nature 2010, 1, 56. [Google Scholar] [CrossRef]
- Baronti, S.; Vaccari, F.P.; Miglietta, F.; Calzolari, C.; Lugato, E.; Orlandini, S.; Pini, R.; Zulian, C.; Genesio, L. Impact of biochar application on plant water relations in Vitis vinifera (L.). Eur. J. Agron. 2014, 53, 38–44. [Google Scholar] [CrossRef]
- Vaccari, F.P.; Maienza, A.; Miglietta, F.; Baronti, S.; di Lonardo, S.; Giagnoni, L.; Lagomarsino, A.; Pozzi, A.; Pusceddu, E.; Ranieri, R.; et al. Biochar stimulates plant growth but not fruit yield of processing tomato in a fertile soil. Agric. Ecosyst. Environ. 2015, 207, 163–170. [Google Scholar] [CrossRef]
- Laird, D.; Fleming, P.; Wang, B.; Horton, R.; Karlen, D. Biochar impact on nutrient leaching from a Midwestern agricultural soil. Geoderma 2010, 158, 3–4. [Google Scholar] [CrossRef]
- Ventura, M.; Sorrenti, G.; Panzacchi, P.; George, E.; Tonon, G. Biochar reduces short-term nitrate leaching from a horizon in an apple orchard. J. Environ. Qual. 2013, 42, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Vaccari, F.P.; Baronti, S.; Lugato, E.; Genesio, L.; Castaldi, S.; Fornasier, F.; Miglietta, F. Biochar as a strategy to sequester carbon and increase yield in durum wheat. Eur. J. Agron. 2011, 34, 231–238. [Google Scholar] [CrossRef]
- Jeffery, S.; Verheijen, F.G.; van der Velde, M.; Bastos, A.C. A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric. Ecosyst. Environ. 2011, 144, 175–187. [Google Scholar] [CrossRef]
- Genesio, L.; Miglietta, F.; Baronti, S.; Vaccari, F.P. Biochar increases vineyard productivity without affecting grape quality: Results from a four years field experiment in Tuscany. Agric. Ecosyst. Environ. 2015, 201, 20–25. [Google Scholar] [CrossRef]
- Roberts, K.G.; Gloy, B.A.; Joseph, S.; Scott, N.R.; Lehmann, J. Life cycle assessment of biochar systems: Estimating the energetic, economic, and climate change potential. Environ. Sci. Technol. 2009, 44, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Genesio, L.; Vaccari, F.P.; Miglietta, F. Black Carbon aerosol from biochar threats its negative emission potential. Glob. Chang. Biol. 2016, 22, 2313–2314. [Google Scholar] [CrossRef] [PubMed]
- Spokas, K.A.; Novak, J.M.; Masiello, C.A.; Johnson, M.G.; Colosky, E.C.; Ippolito, J.A.; Trigo, C. Physical disintegration of biochar: An overlooked process. Environ. Sci. Technol. Lett. 2014, 1, 326–332. [Google Scholar] [CrossRef]
- Olchevski, S.; Ravi, S.; Li, J.; Sharratt, B. Particulate emission from biochar amended soils: A potential health hazard? In Proceedings of the 2015 GSA Annual Meeting, Baltimore, MD, USA, 1–4 November 2015. [Google Scholar]
- Ravi, S.; Sharratt, B.S.; Li, J.; Olshevski, S.; Meng, Z.; Zhang, J. Particulate matter emissions from biochar-amended soils as a potential tradeoff to the negative emission potential. Sci. Rep. 2016. [Google Scholar] [CrossRef] [PubMed]
- Bond, T.C.; Doherty, S.J.; Fahey, D.W.; Forster, P.M.; Berntsen, T.; DeAngelo, B.J.; Flanner, M.G.; Ghan, S.; Kärcher, B.; Koch, D.; et al. Bounding the role of black carbon in the climate system: A scientific assessment. J. Geophys. Res. Atmos. 2013, 118, 5380–5552. [Google Scholar] [CrossRef]
- Myhre, G.; Shindell, D.; Bréon, F.-M.; Collins, W.; Fuglestvedt, J.; Huang, D.; Koch, J.-F.; Lamarque, D.; Lee, B.; Mendoza, T.; et al. Anthropogenic and Natural Radiative Forcing. In Climate Change 2013: The Physical Science Basis; Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Novak, J.M.; Busscher, W.J.; Laird, D.L.; Ahmedna, M.; Watts, D.W.; Niandou, M.A. Impact of biochar amendment on fertility of a southeastern Coastal Plain soil. Soil Sci. 2009, 174, 105–112. [Google Scholar] [CrossRef]
- Spokas, K.A.; Cantrell, K.B.; Novak, J.M.; Archer, D.W.; Ippolito, J.A.; Collins, H.P.; Boateng, A.A.; Lima, I.M.; Lamb, M.C.; McAloon, A.J.; et al. Biochar: A synthesis of its agronomic impact beyond carbon sequestration. J. Environ. Qual. 2012, 41, 973–989. [Google Scholar] [CrossRef] [PubMed]
- Andrenelli, M.C.; Maienza, A.; Genesio, L.; Miglietta, F.; Pellegrini, S.; Vaccari, F.P.; Vignozzi, N. Field application of pelletized biochar: Short term effect on the hydrological properties of a silty clay loam soil. Agric. Water Manag. 2016, 163, 190–196. [Google Scholar] [CrossRef]
- Silva, F.C.; Borrego, C.; Keizer, J.J.; Amorim, J.H.; Verheijen, F.G.A. Effects of moisture content on wind erosion thresholds of biochar. Atmos. Environ. 2015, 123, 121–128. [Google Scholar] [CrossRef]
- Singh, B.P.; Fang, Y.Y.; Boersma, M.; Collins, D.; van Zwieten, L.; Macdonald, L.M. In Situ persistence and migration of biochar carbon and its impact on native carbon emission in contrasting soils under managed temperate pastures. PLoS ONE 2015, 10, e0141560. [Google Scholar] [CrossRef] [PubMed]
- Prendergast-Miller, M.T.; Duvall, M.; Sohi, S.P. Biochar-root interaction are mediated by biochar nutrient content and impacts on soil nutrient availability. Eur. J. Soil Sci. 2014, 65, 173–185. [Google Scholar] [CrossRef]
- Sohi, S.P.; Krull, E.; Lopez-Capel, E.; Bol, R. A review of biochar and its use and function in soil. Adv. Agron. 2010, 105, 47–48. [Google Scholar] [CrossRef]
- Sigua, G.C.; Novak, J.M.; Watts, D.W.; Cantrell, K.B.; Shumaker, P.D.; Szögi, A.A.; Johnson, M.G. Carbon mineralization in two ultisols amended with different sources and particle sizes of pyrolyzed biochar. Chemosphere 2014, 103, 313–321. [Google Scholar] [CrossRef] [PubMed]
- International Biochar Initiative. Standardized Product Definition and Product Testing Guidelines for Biochar That Is Used in Soil IBI-STD-01; IBI: Kiel, Germany, 2012. [Google Scholar]
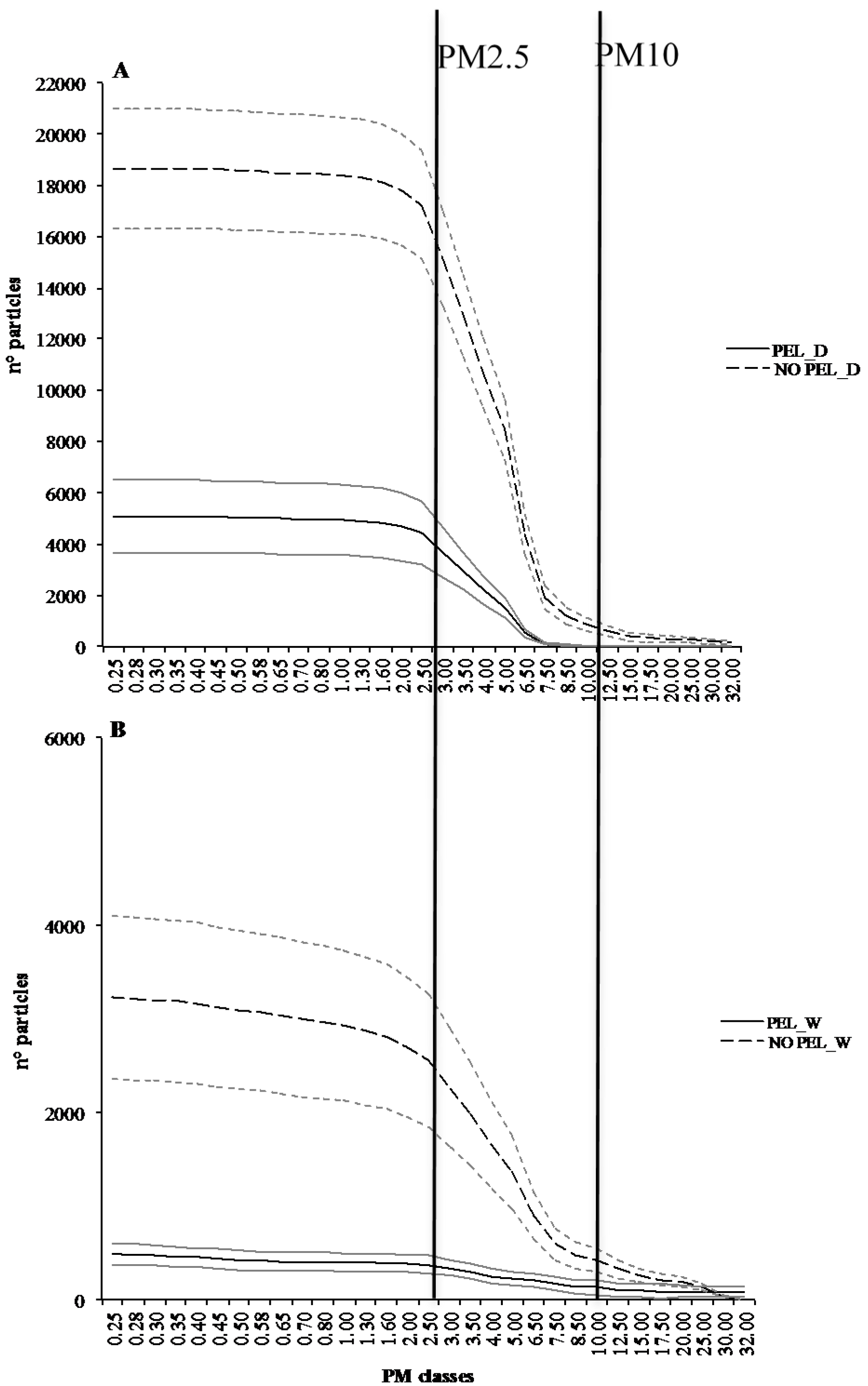
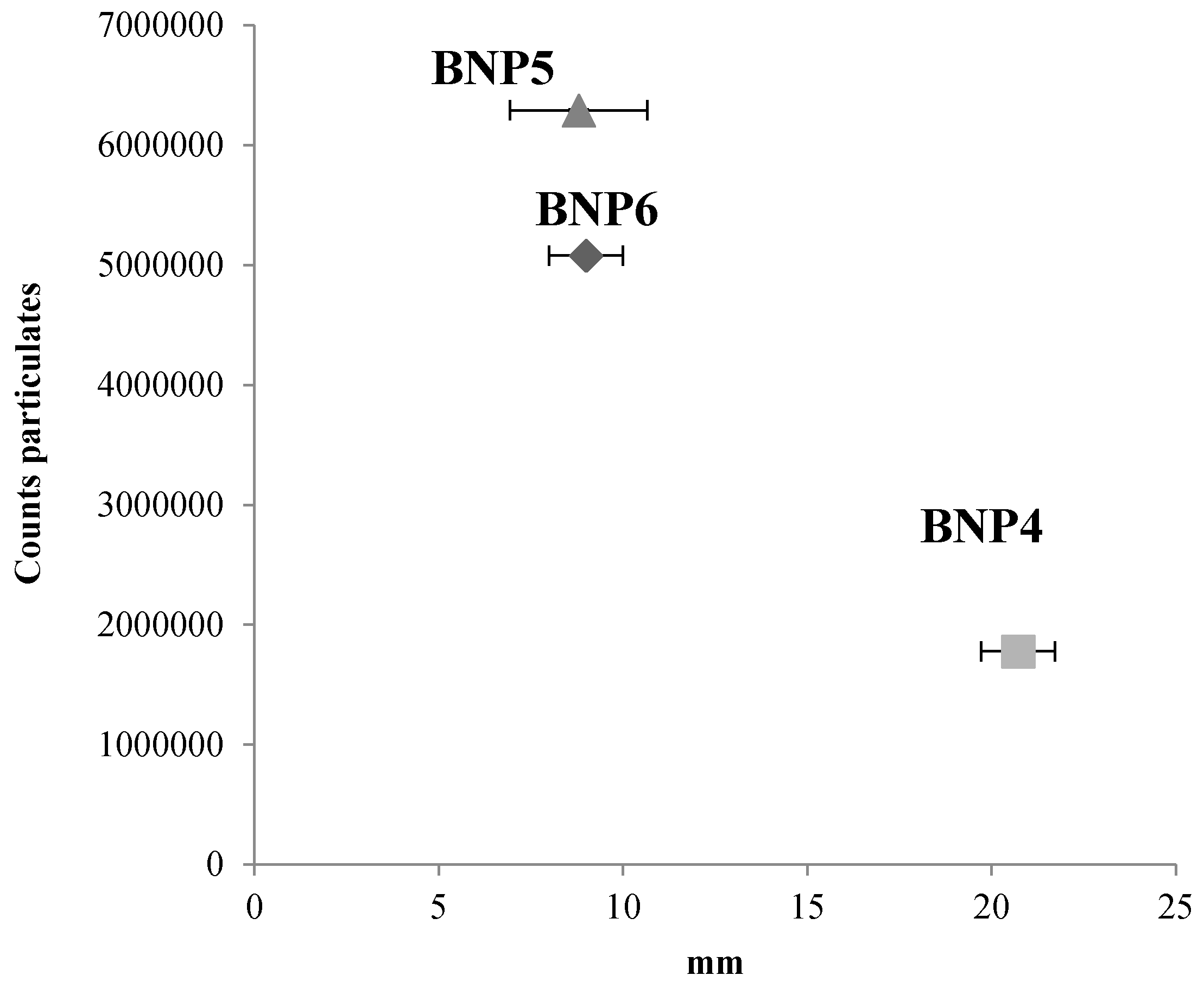
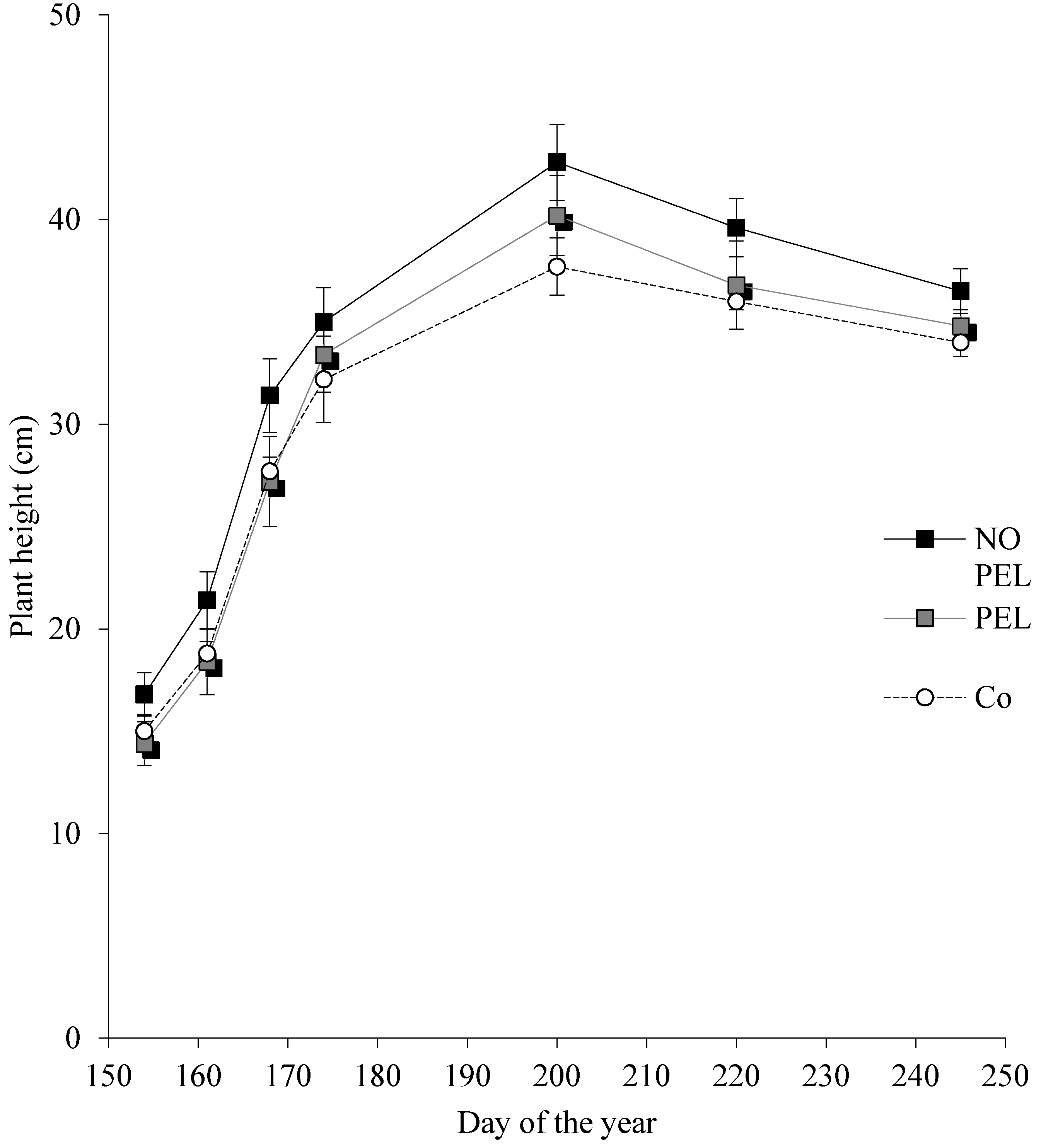
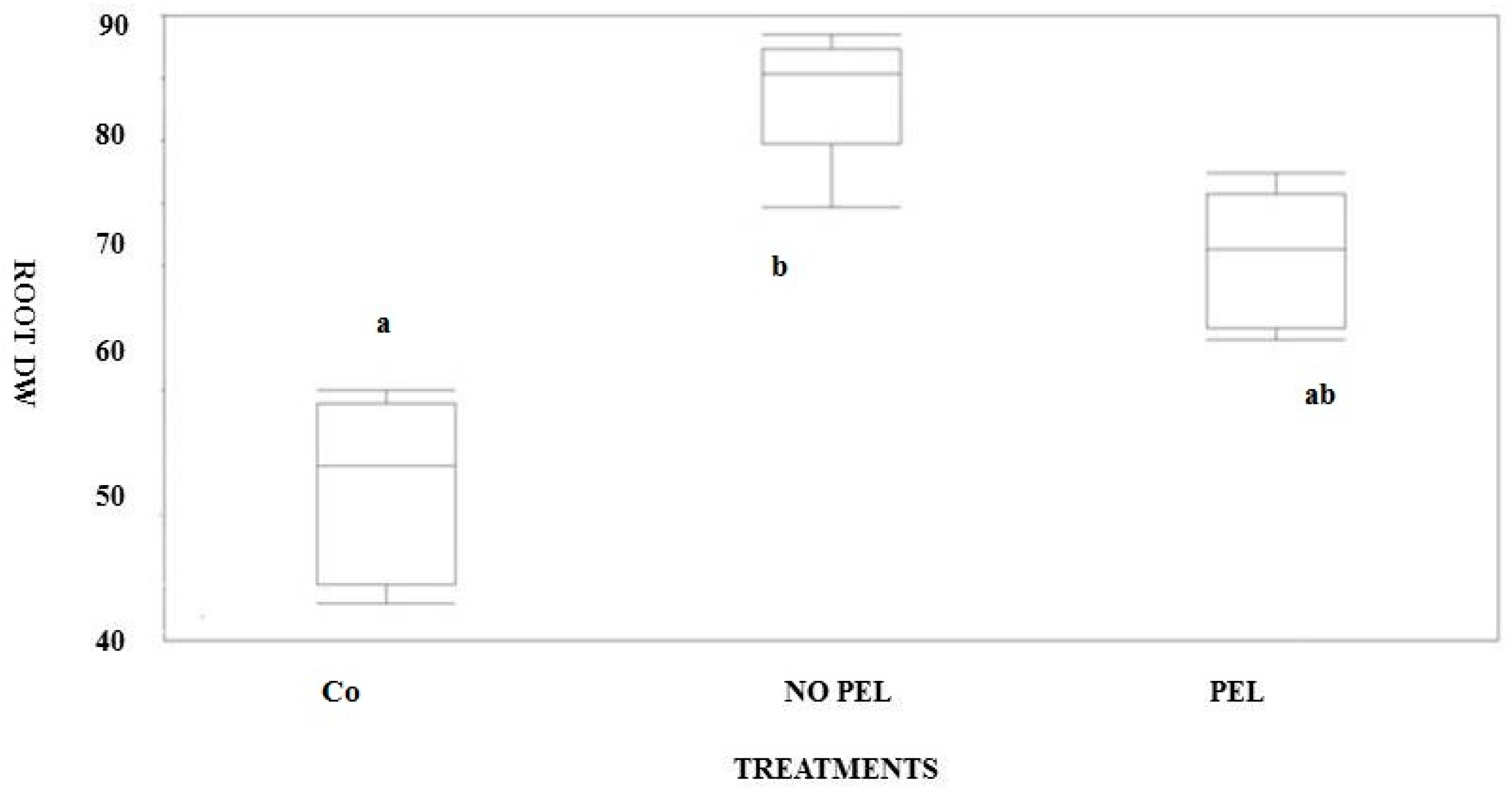
| Feedstock | Acr. | Process | Temp (°C) | Formulation | C (%) | N (%) | Bulk Dens. (kg cm−3) | pH |
|---|---|---|---|---|---|---|---|---|
| Kenaf | BP1 | pyrolysis | 550 | PEL | 86.7 | 1.9 | 0.8 | 9.8 |
| Poplar wood | BP2 | gasification | 1200 | PEL | 90.2 | 1.5 | 0.6 | 7.8 |
| Wheat bran | BP3 | gasification | 1200 | PEL * | 50.4 | 1.2 | 0.5 | 11.2 |
| Almond husk | BNP4 | pyrolysis | 500 | NO PEL | 60.3 | 5.3 | 0.4 | 10.1 |
| Maple tree | BNP5 | pyrolysis | 600 | NO PEL | 90.1 | 0.9 | 0.2 | 8.3 |
| Wheat straw | BNP6 | pyrolysis | 700 | NO PEL | 48.6 | 1.1 | 0.6 | 10.8 |
| Mesh Classes (mm) | BP1 (%) | BP2 (%) | BP3 (%) | BNP4 (%) | BNP5 (%) | BNP6 (%) |
|---|---|---|---|---|---|---|
| >2 | 99.3 | 98.7 | 74.3 | 17.1 | 82.4 | 64.2 |
| >1 | 0.4 | 0.6 | 10.0 | 16.0 | 2.9 | 14.2 |
| >0.5 | 0.3 | 0.4 | 7.0 | 18.9 | 6.2 | 8.9 |
| >0.25 | 0.0 | 0.3 | 5.3 | 26.5 | 3.3 | 6.8 |
| <0.25 | 0.0 | 0.0 | 3.4 | 21.5 | 5.2 | 5.9 |
| Parameter | Unit | Wheat Bran Feedstock | Wheat Bran Biochar |
|---|---|---|---|
| pH | - | - | 11.2 |
| C | % | 34.6 | 50.4 |
| N | % | 2.2 | 1.2 |
| Ca | g kg−1 | 1.0 | 3.6 |
| K | g kg−1 | 4.5 | 14.2 |
| Mg | g kg−1 | 3.9 | 4.8 |
| Na | g kg−1 | 0.1 | 0.9 |
| P | g kg−1 | 9.1 | 9.8 |
| Cu | mg kg−1 | <100 | <100 |
| Fe | mg kg−1 | <120 | <120 |
| Mesh Classes (mm) | PEL (%) | NO PEL (%) |
|---|---|---|
| >2 | 74.3 | 48.2 |
| >1 | 10.0 | 21.5 |
| >0.5 | 7.0 | 13.5 |
| >0.25 | 5.3 | 9.6 |
| <0.25 | 3.4 | 7.2 |
| Soil Characteristics | |
|---|---|
| Sand (g kg−1) 2 mm >> 0.05 mm | 501 |
| Silt (g kg−1) 0.055 >> 0.02 mm | 433 |
| Clay (g kg−1) < 0.02 | 67 |
| Bulk density (Mgm−3) | 1.2 |
| C% | 2 |
| N% | 1.5 |
| CEC (m equiv./100 g) | 19 |
| pH | 5.5 |
| Treatment | pH | CEC meq/100 g | CS (%) | NS (%) | CP (%) | NP (%) |
|---|---|---|---|---|---|---|
| CO | 5.5 a (0.1) | 19 a (0.5) | 2.1 a (0.2) | 1.0 ns (0.1) | 32.4 ns (0.5) | 2.2 a (0.5) |
| PEL | 6.4 b (0.5) | 21 ab (0.1) | 2.7 b (0.4) | 1.2 ns (0.1) | 33.6 ns (1.2) | 2.1 a (0.1) |
| NO PEL | 6.4 b (0.4) | 23 b (0.3) | 2.7 b (0.2) | 1.2 ns (0.1) | 36.3 ns (2.4) | 3.7 b (0.1) |
| Treat. | TPa (g plant−1) | MPb (g plant−1) | NMPc (g plant−1) | Fwd (g fruit−1) | Fde (cm fruit−1) | ORf (°Brix) | pHg |
|---|---|---|---|---|---|---|---|
| CO | 460.8 a (22.4) | 170.5 a (7.5) | 276.3 ns (23.2) | 20.3 ns (1.5) | 1.4 ns (0.1) | 4.2 ns (0.2) | 4.5 ns (0.3) |
| PEL | 500.3 a (18.9) | 185.5 ab (6.3) | 314.8 ns (25.8) | 23.2 ns (2.5) | 1.5 ns (0.1) | 4.2 ns (0.2) | 4.1 ns (0.1) |
| NO PEL | 800.5 b (19.3) | 470.1 c (8.3) | 330.4 ns (32.4) | 23.5 ns (1.9) | 1.4 ns (0.1) | 4.3 ns (0.2) | 4.1 ns (0.1) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maienza, A.; Genesio, L.; Acciai, M.; Miglietta, F.; Pusceddu, E.; Vaccari, F.P. Impact of Biochar Formulation on the Release of Particulate Matter and on Short-Term Agronomic Performance. Sustainability 2017, 9, 1131. https://doi.org/10.3390/su9071131
Maienza A, Genesio L, Acciai M, Miglietta F, Pusceddu E, Vaccari FP. Impact of Biochar Formulation on the Release of Particulate Matter and on Short-Term Agronomic Performance. Sustainability. 2017; 9(7):1131. https://doi.org/10.3390/su9071131
Chicago/Turabian StyleMaienza, Anita, Lorenzo Genesio, Marco Acciai, Franco Miglietta, Emanuela Pusceddu, and Francesco Primo Vaccari. 2017. "Impact of Biochar Formulation on the Release of Particulate Matter and on Short-Term Agronomic Performance" Sustainability 9, no. 7: 1131. https://doi.org/10.3390/su9071131





