Making Marine Noise Pollution Impacts Heard: The Case of Cetaceans in the North Sea within Life Cycle Impact Assessment
Abstract
:1. Introduction
2. Material and Methods
2.1. Choice of Impact Pathway and Affected Species
2.2. Constructing the Characterization Factor
2.2.1. Sound Propagation and Fate Factor
2.2.2. Affected Animals and Modelling of a Midpoint Characterization Factor
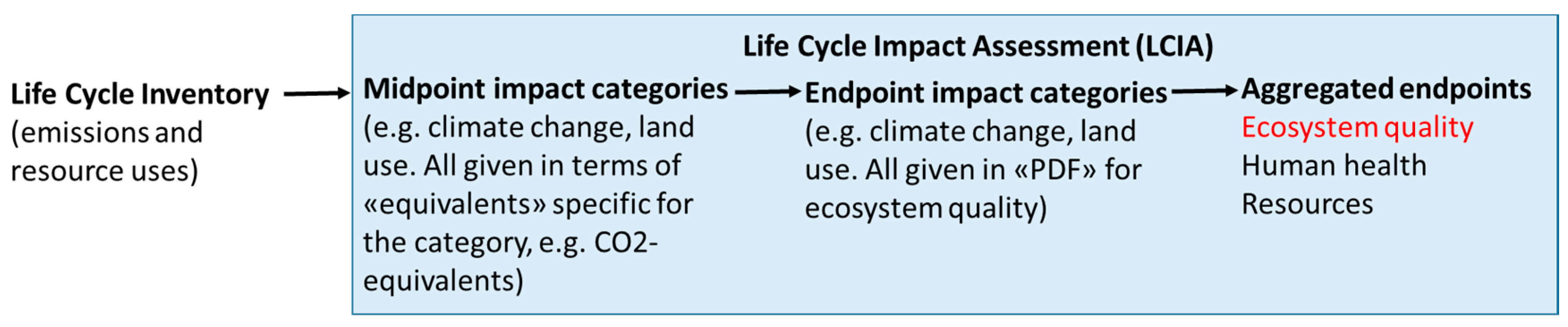
2.2.3. Endpoint Modelling
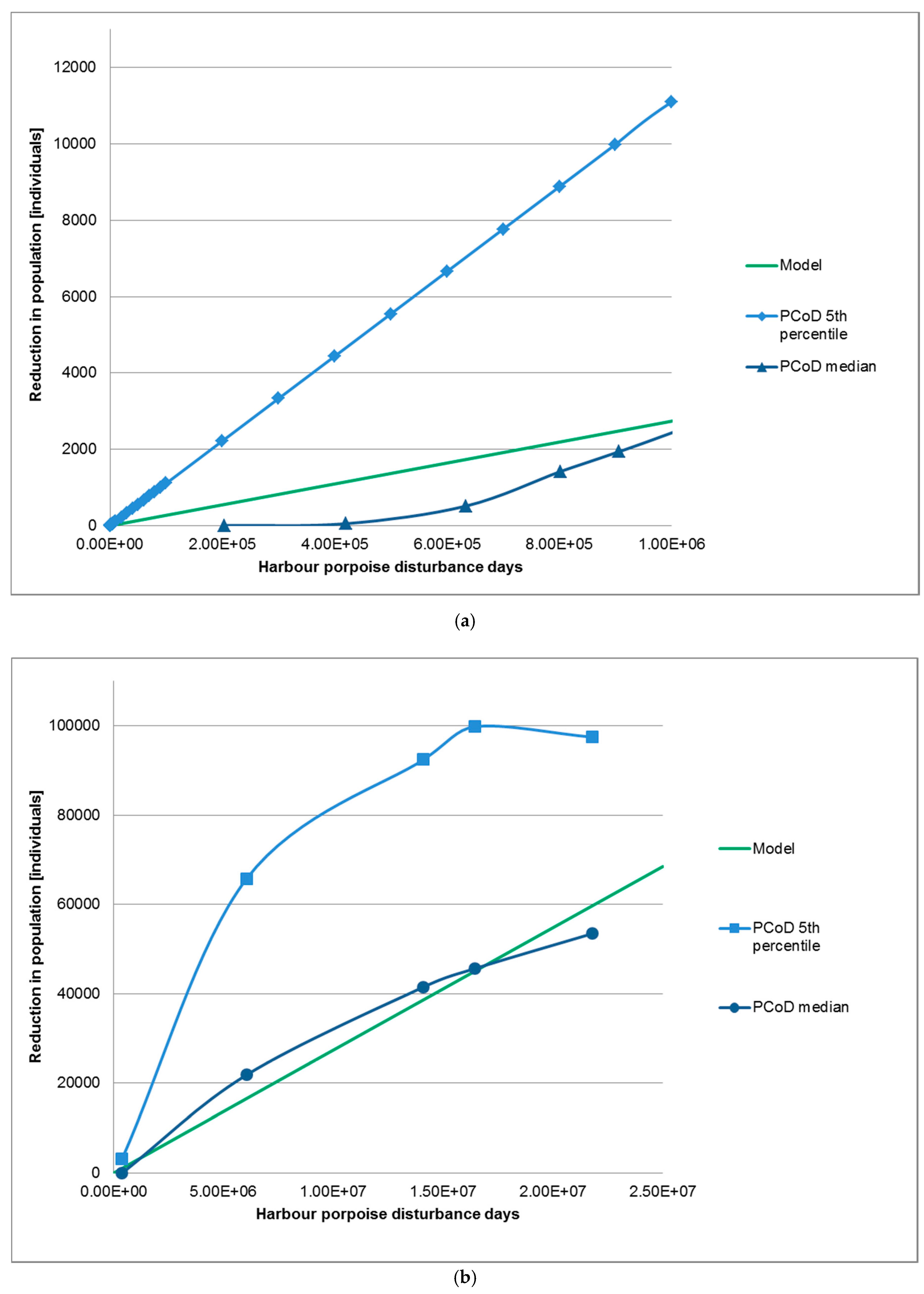
2.3. Verification of the Method
2.4. Expansion to other Cetacean Species
2.4.1. Threshold Values
2.4.2. Abundance and Population Density Data
2.5. Case-Study
3. Results
3.1. Sound Propagation
3.2. Verification of Approach
3.3. Characterization Factors
3.4. Comparison with other Impact Categories
4. Discussion
4.1. Choice of Impact Pathway
4.2. Characterization Factor Development
4.2.1. Sound Propagation Model
4.2.2. Disturbance Days
4.2.3. Endpoint Characterization Factor
4.3. Application to other Cetacean Species
4.4. Case-Study
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Costanza, R. The ecological, economic, and social importance of the oceans. Ecol. Econ. 1999, 31, 199–213. [Google Scholar] [CrossRef]
- The State of World Fisheries and Aquaculture. Fisheries and Aquaculture Department, Food and Agriculture Organization; FAO: Rome, Italy, 2014. [Google Scholar]
- HLPE. Sustainable Fisheries and Aquaculture for Food Security and Nutrition; High Level Panel of Experts of Food Security and Nutrition of the Committee on World Food Security: Rome, Italy, 2014; Available online: http://www.fao.org/3/a-i3844e.pdf (accessed on 27 June 2017).
- Tanzer, J.; Phua, C.; Jeffries, B.; Lawrence, A.; Gonzales, A.; Gamblin, P.; Roxburgh, T. Living Blue Planet Report Species, Habitats and Human Well-Being; WWF International: Gland, Switzerland, 2015. [Google Scholar]
- IPCC. Climate Change 2013: The Physical Science Basis; Contribution of Working Group I to the Fifth Assessment Report of the IPCC; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- BCG. BCG Economic Valuation: Methodology and Sources. Reviving the Ocean Economy: The Case for Action; Boston Consulting Group, Global Change Institute and WWF International: Gland, Switzerland, 2015. [Google Scholar]
- Wiber, M.G.; Young, S.; Wilson, L. Impact of Aquaculture on Commercial Fisheries: Fishermen’s Local Ecological Knowledge. Hum. Ecol. 2012, 40, 29–40. [Google Scholar] [CrossRef]
- Wysocki, L.E.; Davidson, J.W.; Smith, M.E.; Frankel, A.S.; Ellison, W.T.; Mazik, P.M.; Popper, A.N.; Bebak, J. Effects of aquaculture production noise on hearing, growth, and disease resistance of rainbow trout Oncorhynchus mykiss. Aquaculture 2007, 272, 687–697. [Google Scholar] [CrossRef]
- Tournadre, J. Anthropogenic pressure on the open ocean: The growth of ship traffic revealed by altimeter data analysis. Geophys. Res. Lett. 2014, 41, 7924–7932. [Google Scholar] [CrossRef]
- Maribus. World Ocean Review 3: Living with Oceans: Marine Resources—Opportunities and Risks; Maribus GmbH: Hamburg, Germany, 2014. [Google Scholar]
- McCauley, R.D.; Fewtrell, J.; Popper, A.N. High intensity anthropogenic sound damages fish ears. J. Acoust. Soc. Am. 2003, 113, 638–642. [Google Scholar] [CrossRef] [PubMed]
- Kunc, H.P.; McLaughlin, K.E.; Schmidt, R. Aquatic noise pollution: Implications for individuals, populations, and ecosystems. Proc. R. Soc. B 2016, 283. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, A.D.; Pembroke, A.E.; Popper, A.N. Information gaps in understanding the effects of noise on fishes and invertebrates. Rev. Fish Biol. Fish. 2015, 25, 39–64. [Google Scholar] [CrossRef]
- Slabbekoorn, H.; Bouton, N.; van Opzeeland, I.; Coers, A.; ten Cate, C.; Popper, A.N. A noisy spring: The impact of globally rising underwater sound levels on fish. Trends Ecol. Evol. 2010, 25, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Popper, A.N. Effects of Anthropogenic Sounds on Fishes. Fisheries 2003, 28, 24–31. [Google Scholar] [CrossRef]
- Southall, B.L.; Bowles, A.E.; Ellison, W.T.; Finneran, J.J.; Gentry, R.L.; Greene, C.R.; Kastak, D.; Ketten, D.R.; Miller, J.H.; Nachtigall, P.E.; et al. Marine Mammal Noise Exposure Criteria: Initial Scientific Recommendations. Aquat. Mamm. 2007, 33, 411–414. [Google Scholar] [CrossRef]
- Warner, R.M. Protecting the diversity of the depths: Environmental regulation of bioprospecting and marine scientific research beyond national jurisdiction. Ocean Yearb. 2008, 22, 411–443. [Google Scholar] [CrossRef]
- Romano, T.A.; Keogh, M.J.; Kelly, C.; Feng, P.; Berk, L.; Schlundt, C.E.; Carder, D.A.; Finneran, J.J. Anthropogenic sound and marine mammal health: Measures of the nervous and immune systems before and after intense sound exposure. Can. J. Fish. Aquat. Sci. 2004, 61, 1124–1134. [Google Scholar] [CrossRef]
- Morton, A. Displacement of Orcinus orca (L.) by high amplitude sound in British Columbia, Canada. ICES J. Mar. Sci. 2002, 59, 71–80. [Google Scholar] [CrossRef]
- Wysocki, L.E.; Dittami, J.P.; Ladich, F. Ship noise and cortisol secretion in European freshwater fishes. Biol. Conserv. 2006, 128, 501–508. [Google Scholar] [CrossRef]
- Sarà, G.; Dean, J.; D’Amato, D.; Buscaino, G.; Oliveri, A.; Genovese, S.; Ferro, S.; Buffa, G.; Martire, M.; Mazzola, S. Effect of boat noise on the behaviour of bluefin tuna Thunnus thynnus in the Mediterranean Sea. Mar. Ecol. Prog. Ser. 2007, 331, 243–253. [Google Scholar] [CrossRef]
- Parente, C.L.; de Araújo, J.P.; de Araújo, M.E. Diversity of cetaceans as tool in monitoring environmental impacts of seismic surveys. Biot. Neotrop. 2007, 7. [Google Scholar] [CrossRef]
- Fernández, A.; Edwards, J.F.; Rodríguez, F.; Espinosa de los Monteros, A.; Herráez, P.; Castro, P.; Jaber, J.R.; Martín, V.; Arbelo, M. ‘Gas and fat embolic syndrome’ involving a mass stranding of beaked whales (family Ziphiidae) exposed to anthropogenic sonar signals. Vet. Pathol. 2005, 42, 446–457. [Google Scholar] [CrossRef] [PubMed]
- International Whaling Commission. Report of the Scientific Committee. J. Cetacean Res. Manag. 2015, 16. Available online: https://archive.iwc.int/?r=3436&k=4173fd68bc (accessed on 27 June 2017).
- International Whaling Commission. Report of the Scientific Committee. J. Cetacean Res. Manag. 2012, 13. Available online: https://archive.iwc.int/?r=2126&k=e5974c39c4 (accessed on 27 June 2017).
- Hellweg, S.; Milà i Canals, L. Emerging approaches, challenges and opportunities in life cycle assessment. Science 2014, 344, 1109–1113. [Google Scholar] [CrossRef] [PubMed]
- ISO 14044. Environmental Management—Life Cycle Assessment—Requirements and Guidelines (ISO14044:2006); British Standards Institute: London, UK, 2006. [Google Scholar]
- Pennington, D.W.; Potting, J.; Finnveden, G.; Lindeijer, E.; Jolliet, O.; Rydberg, T.; Rebitzer, G. Life cycle assessment Part 2: Current impact assessment practice. Environ. Int. 2004, 30, 721–739. [Google Scholar] [CrossRef] [PubMed]
- Jolliet, O.; Müller-Wenk, R.; Bare, J.; Brent, A.; Goedkoop, M.; Heijungs, R.; Itsubo, N.; Peña, C.; Pennington, D.; Potting, J.; et al. The LCIA midpoint-damage framework of the UNEP/SETAC life cycle initiative. Int. J. Life Cycle Assess. 2004, 9, 394–404. [Google Scholar] [CrossRef]
- Hauschild, M.Z.; Huijbregts, M.A.J. Life Cycle Impact Assessment; Springer: Dordrecht, The Netherlands, 2015. [Google Scholar]
- Cucurachi, S.; Heijungs, R.; Ohlau, K. Towards a general framework for including noise impacts in LCA. Int. J. Life Cycle Assess. 2012, 17, 471–487. [Google Scholar] [CrossRef] [PubMed]
- Hollander, A.E.; Melse, J.M.; Kramers, P.G. An aggregate public health indicator to represent the impact of multiple environmental exposures. Epidemiol. Baltim. 1999, 10, 606–617. [Google Scholar] [CrossRef]
- Müller-Wenk, R. A method to include in LCA road traffic noise and its health effects. Int. J. Life Cycle Assess. 2004, 9, 76–85. [Google Scholar] [CrossRef]
- Peng, C.; Zhao, X.; Liu, G. Noise in the Sea and Its Impacts on Marine Organisms. Int. J. Environ. Res. Public Health 2015, 12, 12304–12323. [Google Scholar] [CrossRef] [PubMed]
- Tyack, P.L. Implications for marine mammals of large-scale changes in the marine acoustic environment. J. Mamm. 2008, 83, 549–558. [Google Scholar] [CrossRef]
- Richardson, W.J.; Greene, C.R.; Malme, C.I.; Thomson, D.H. Marine Mammals and Noise; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Nowacek, D.P.; Thorne, L.H.; Johnston, D.W.; Tyack, P.L. Responses of cetaceans to anthropogenic noise. Mamm. Rev. 2007, 37, 81–115. [Google Scholar] [CrossRef]
- NRC. Ocean Noise and Marine Mammals; National Academies Press: Washington, DC, USA, 2003. [Google Scholar]
- Erbe, C. Underwater Acoustics: Noise and the Effects on Marine Mammals, a Pocket Handbook; Jasco Applied Sciences: Halifax, NS, Canada, 2011. [Google Scholar]
- Cox, T.M.; Ragen, T.J.; Read, A.J.; Vos, E.; Baird, R.W.; Balcomb, K.; Barlow, J.; Caldwell, J.; Cranford, T.; Crum, L.; et al. Understanding the impacts of anthropogenic sound on beacked whales. J. Cetacean Res. Manag. 2006, 7, 177–187. [Google Scholar]
- Weilgart, L. The impacts of anthropogenic ocean noise on cetaceans and implications for management. Can. J. Zool. 2007, 85, 1091–1116. [Google Scholar] [CrossRef]
- Heinis, F.; de Jong, C.A.F. Cumulative Effects of Impulsive Underwater Sound on Marine Mammals; TNO Report; TNO: The Hague, The Netherlands, 2015. [Google Scholar]
- King, S.L.; Schick, R.S.; Donovan, C.; Booth, C.G.; Burgman, M.; Thomas, L.; Harwood, J. An interim framework for assessing the population consequences of disturbance. Methods Ecol. Evol. 2015, 6, 1150–11585. [Google Scholar] [CrossRef]
- Brandt, M.J.; Diederichs, A.; Betke, K.; Nehls, G. Responses of harbour porpoises to pile driving at the Horns Rev II offshore wind farm in the Danish North Sea. Mar. Ecol. Prog. Ser. 2011, 421, 205–216. [Google Scholar] [CrossRef]
- Dähne, M.; Gilles, A.; Lucke, K.; Peschko, V.; Adler, S.; Krügel, K.; Sundermeyer, J.; Siebert, U. Effects of pile-driving on harbour porpoises (Phocoena phocoena) at the first offshore wind farm in Germany. Environ. Res. Lett. 2013, 8, 1–16. [Google Scholar] [CrossRef]
- Tougaard, J.; Carstensen, J.; Teilmann, J.; Skov, H.; Rasmussen, P. Pile driving zone of responsiveness extends beyond 20 km for harbor porpoises (Phocoena phocoena (L.)). J. Acoust. Soc. Am. 2013, 126, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Tougaard, J.; Kyhn, L.A.; Amundin, M.; Wennerberg, D.; Bordin, C. Behavioral Reactions of Harbor Porpoise to Pile-Driving Noise. In The Effects of Noise on Aquatic Life; Popper, A.N., Hawkins, A., Eds.; Springer: New York, NY, USA, 2012; pp. 277–280. [Google Scholar]
- Tougaard, J.; Wright, A.J.; Madsen, P.T. Cetacean noise criteria revisited in the light of proposed exposure limits for harbour porpoises. Mar. Pollut. Bull. 2015, 90, 196–208. [Google Scholar] [CrossRef] [PubMed]
- New, L.F.; Clark, J.S.; Costa, D.P.; Fleishman, E.; Hindell, M.A.; Klanjcek, T.; Lusseau, D.; Kraus, S.; McMahon, C.R.; Robinson, P.W.; et al. Using short-term measures of behaviour to estimate long-term fitness of southern elephant seals. Mar. Ecol. Prog. Ser. 2013, 496, 99–108. [Google Scholar] [CrossRef]
- Harwood, J.; King, S.L. The Sensitivity of UK Marine Mammal Populations to Marine Renewables Developments; Natural Environment Research Council (NERC): Swindon, UK, 2014. [Google Scholar]
- National Research Council. Marine Mammal Populations and Ocean Noise: Determining When Noise Causes Biologically Significant Effects; The National Academy Press: Washington, DC, USA, 2005.
- Matthews, M.-N.R.; Zykov, M. Underwater Acoustic Modeling of Construction Activities: Marine Commerce South Terminal in New Bedford, MA; LCC: Boston, MA, USA, 2012. [Google Scholar]
- Ainslie, M.A.; de Jong, C.A.F.; Dol, H.S.; Blacquière, G.; Marasini, C. Assessment of Natural and Anthropogenic Sound Sources and Acoustic Propagation in the North Sea; TNO: The Hague, The Netherlands, 2009. [Google Scholar]
- De Jong, C.A.F.; Ainslie, M.A. Underwater Sound due to Piling Activities for Prinses Amaliawindpark; TNO: The Hague, The Netherlands, 2012. [Google Scholar]
- Huijbregts, M.A.J.; Hellweg, S.; Hertwich, E. Do We Need a Paradigm Shift in Life Cycle Impact Assessment? Environ. Sci. Technol. 2011, 45, 3833–3834. [Google Scholar] [CrossRef] [PubMed]
- U.S. Navy. Atlantic Fleet Active Sonar Traning Environmental Impact Statement; Naval Facilities Engineering Command: Atlantic, NJ, USA, 2008.
- Parsons, E.C.M.; Dolman, S.J.; Wright, A.J.; Rose, N.A.; Burns, W.C.G. Navy sonar and cetaceans: Just how much does the gun need to smoke before we act? Mar. Pollut. Bull. 2008, 56, 1248–1257. [Google Scholar] [CrossRef] [PubMed]
- Bronštejn, I.N.; Semendjaev, K.A.; Musiol, G.; Mühlig, H. Taschenbuch der Mathematik, 1. Auflage; Verlag Harri Deutsch: Frankfurt, Germany, 1993. [Google Scholar]
- Geelhoed, S.; Scheidat, M.; Aarts, G.; van Bemmelen, R.; Janinhoff, N.; Verdaat, H.; Witte, R. Shortlist Masterplan Wind Aerial Surveys of Harbour Porpoises on the Dutch Continental Shelf; Institute for Marine Resources and Ecosystem Studies: Wageningen, The Netherlands, 2011; Available online: https://tethys.pnnl.gov/publications/shortlist-masterplan-wind-aerial-surveys-harbour-porpoises-dutch-continental-shelf (accessed on 27 June 2017).
- Goedkoop, M.; Spriensma, R. The Eco-Indicator 99: A Damage Oriented Method for Life Cycle Impact Assessment—Methodology Report and Annex; Pré Consultants B.V.: Amersfoort, The Netherlands, 1999. [Google Scholar]
- Goedkoop, M.; Heijungs, R.; Huijbregts, M.A.J.; De Schryver, A.; Struijs, J.; Van Zelm, R. ReCiPe 2008: A Life Cycle Impact Assessment Method Which Comprises Harmonised Category Indicators at the Midpoint and Endpoint Level, 1st ed.; Ruimte en Milieu, Ministerie van Volkshuisvesting, Ruimtelijke Ordening en Milieubeheer, TNO: The Hague, The Netherlands, 2009. [Google Scholar]
- Verones, F.; Hellweg, S.; Azevedo, L.B.; Chaudhary, A.; Cosme, N.; Fantke, P.; Goedkoop, M.; Hauschild, M.Z.; Laurent, A.; Mutel, C.L.; et al. LC-IMPACT Version 0.5: A Spatially Differentiated Life Cycle Impact Assessment Approach. 2016. Available online: http://www.lc-impact.eu/downloads/documents/LC-Impact_report_SEPT2016_20160927.pdf (accessed on 28 April 2017).
- Verones, F.; Huijbregts, M.A.J.; Chaudhary, A.; de Baan, L.; Koellner, T.; Hellweg, S. Harmonizing the Assessment of Biodiversity Effects from Land and Water Use within LCA. Environ. Sci. Technol. 2015, 49, 3584–3592. [Google Scholar] [CrossRef] [PubMed]
- Hammond, P.S.; Macleod, K.; Berggren, P.; Leopold, M.F.; Scheidat, M. Cetacean abundance and distribution in European Atlantic shelf waters to inform conservation and management. Biol. Conserv. 2013, 164, 107–122. [Google Scholar] [CrossRef]
- Arvesen, A.; Birkeland, C.; Hertwich, E.G. The Importance of Ships and Spare Parts in LCAs of Offshore Wind Power. Environ. Sci. Technol. 2013, 47, 2948–2956. [Google Scholar] [CrossRef] [PubMed]
- Papathanasopoulou, E.; Beaumont, N.; Hooper, T.; Nunes, J.; Queirós, A.M. Energy systems and their impacts on marine ecosystem services. Renew. Sustain. Energy Rev. 2015, 52, 917–926. [Google Scholar] [CrossRef]
- Dähne, M.; Peschko, V.; Gilles, A.; Lucke, K.; Adler, S.; Ronnenberg, K.; Siebert, U. Marine mammals and windfarms: Effects of alpha ventus on harbour porpoises. In Ecological Research at the Offshore Windfarm Alpha Ventus; Federal Maritime and Hydrographic Agency, Federal Ministry for the Environment, Nature Conservation and Nuclear Safety, Eds.; Springer Fachmedien Wiesbaden: Wiesbaden, Germany, 2014. [Google Scholar]
- Wright, A.J. Reducing Impacts of Human Ocean Noise on Cetaceans: Knowledge Gap Analysis and Recommendations; WWF Global Arctic Programme: Ottawa, ON, Canada, 2014. [Google Scholar]
- Kaiser, M.J.; Attrill, M.J. Marine Ecology: Processes, Systems, and Impacts, 2nd ed.; Oxford University Press: New York, NY, USA, 2011. [Google Scholar]
- NOAA. The Large Marine Ecosystem Approach to the Assessment and Management of Coastal Ocean Waters. Large Marine Ecosystems of the World, 2016. Available online: http://www.lme.noaa.gov/ (accessed on 5 September 2016).
- Cosme, N.; Jones, M.C.; Cheung, W.W.L.; Larsen, H.F. Spatial differentiation of marine eutrophication damage indicators based on species density. Ecol. Indic. 2017, 73, 676–685. [Google Scholar] [CrossRef]
- De Baan, L.; Alkemade, R.; Koellner, T. Land use impacts on biodiversity in LCA: A global approach. Int. J. Life Cycle Assess. 2013, 18, 1216–1230. [Google Scholar] [CrossRef]
- Curran, M.; de Baan, L.; De Schryver, A.; Van Zelm, R.; Hellweg, S.; Koellner, T.; Sonnemann, G.; Huijbregts, M.A.J. Toward Meaningful End Points of Biodiversity in Life Cycle Assessment. Environ. Sci. Technol. 2011, 45, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Donovan, C.; Harwood, J.; King, S.; Booth, C.; Caneco, B.; Walker, C. Expert Elicitation Methods in Quantifying the Consequences of Acoustic Disturbance from Offshore Renewable Energy Developments. In The Effects of Noise on Aquatic Life II; Popper, A.N., Hawkins, A., Eds.; Springer: New York, NY, USA, 2016. [Google Scholar]
- Harwood, J.; King, S.; Schick, R.; Donovan, C.; Booth, C. A Protocol for Implementing the Interim Population Consequences of Disturbance (PCOD) Approach: Quantifying and Assessing the Effects of UK Offshore Renewable energy Developments on Marine Mammal Populations. Report Number SMRUL-TCE-2013-014. Available online: http://www.gov.scot/Resource/0044/00443360.pdf (accessed on 28 April 2017).
- Wisniewska, D.M.; Johnson, M.; Teilmann, J.; Rojano-Doñate, L.; Shearer, J.; Sveegaard, S.; Miller, L.A.; Siebert, U.; Madsen, P.T. Ultra-High Foraging Rates of Harbor Porpoises Make Them Vulnerable to Anthropogenic Disturbance. Curr. Biol. 2016, 26, 1441–1446. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Value | Unit | References |
|---|---|---|---|
| Wind farm capacity | 350 | MW | [42,65] |
| Lifetime | 20 | Years | [65] |
| Full load hours | 3000 | Hours | [65] |
| Total lifetime production | 2.10 × 1010 | kWh | Calculated |
| Disturbance days per year | 58 | Days | [42] |
| Construction time | 5 | Years | [42] |
| Functional Hearing Group | Midpoint Local [ind.yr] | Midpoint Regional [ind.yr] | Endpoint Local [PDF.yr/kWh] | Endpoint Regional [PDF.yr/kWh] |
|---|---|---|---|---|
| Low-frequency cetaceans | ||||
| Minke whale (B. acutorostrata) | 49.964 | 80.661 | 9.93 × 10−13 | 1.60 × 10−12 |
| Mid-frequency cetaceans | ||||
| Bottlenose dolphin (T. truncatus) | 0.063 | 0.030 | 1.35 × 10−14 | 6.39 × 10−15 |
| Whitebeaked dolphin (L. albirostris) | 0.000 | 0.284 | 0.00 × 10 | 6.39 × 10−15 |
| Short-beaked common dolphin (D. delphis) | 0.793 | 0.133 | 3.84 × 10−14 | 6.39 × 10−15 |
| High-frequency cetaceans | ||||
| Harbour porpoise (Phocoena phocoena) | 280.038 | 288.518 | 2.65 × 10−13 | 2.73 × 10−13 |
| Total | 2.62 × 10−13 | 3.79 × 10−13 | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Middel, H.; Verones, F. Making Marine Noise Pollution Impacts Heard: The Case of Cetaceans in the North Sea within Life Cycle Impact Assessment. Sustainability 2017, 9, 1138. https://doi.org/10.3390/su9071138
Middel H, Verones F. Making Marine Noise Pollution Impacts Heard: The Case of Cetaceans in the North Sea within Life Cycle Impact Assessment. Sustainability. 2017; 9(7):1138. https://doi.org/10.3390/su9071138
Chicago/Turabian StyleMiddel, Heleen, and Francesca Verones. 2017. "Making Marine Noise Pollution Impacts Heard: The Case of Cetaceans in the North Sea within Life Cycle Impact Assessment" Sustainability 9, no. 7: 1138. https://doi.org/10.3390/su9071138




