Effects of Wheat-Maize Intercropping on Population Dynamics of Wheat Aphids and Their Natural Enemies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Area and Experimental Design
2.2. Sampling
2.3. Statistical Methods
3. Results
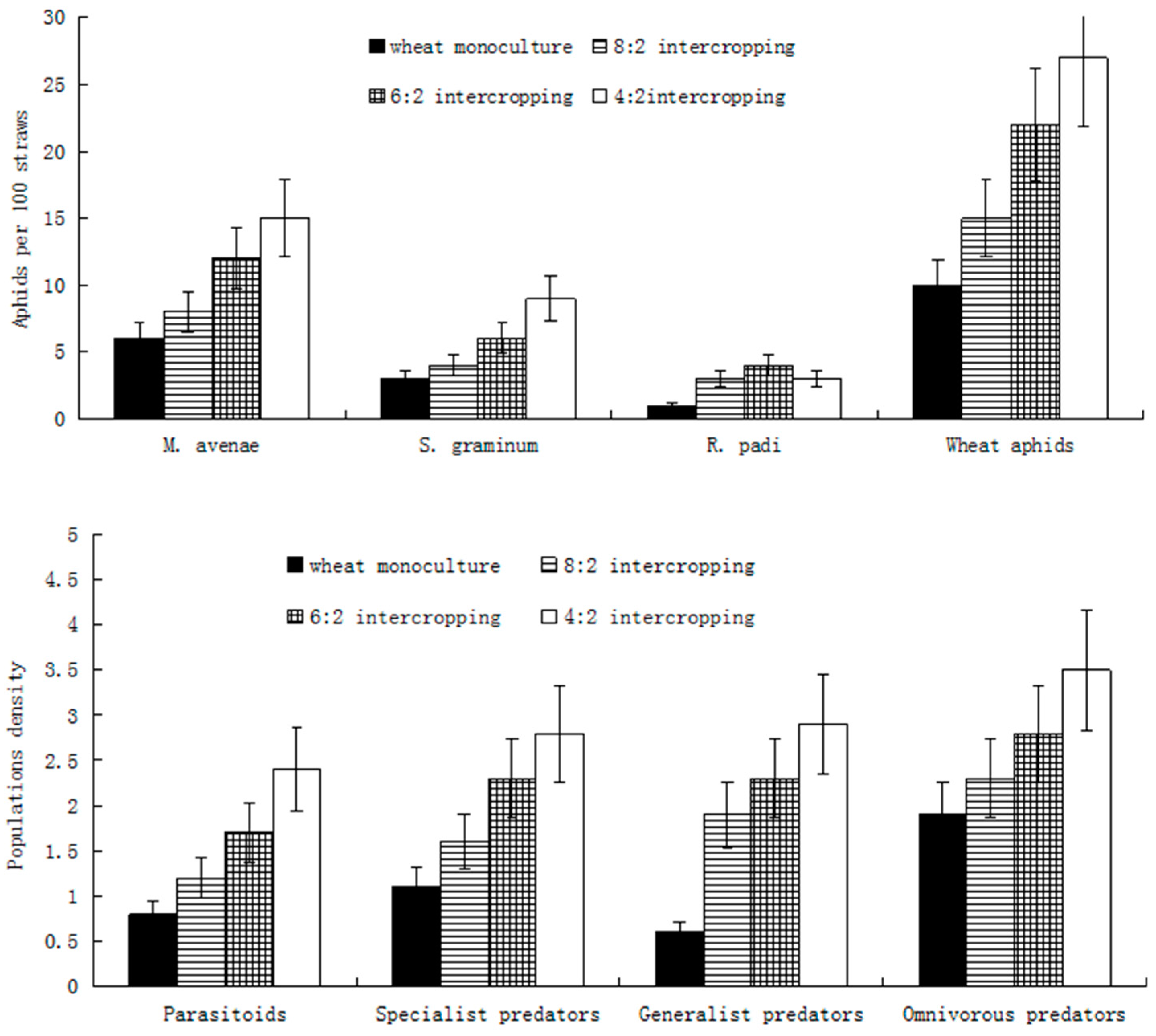
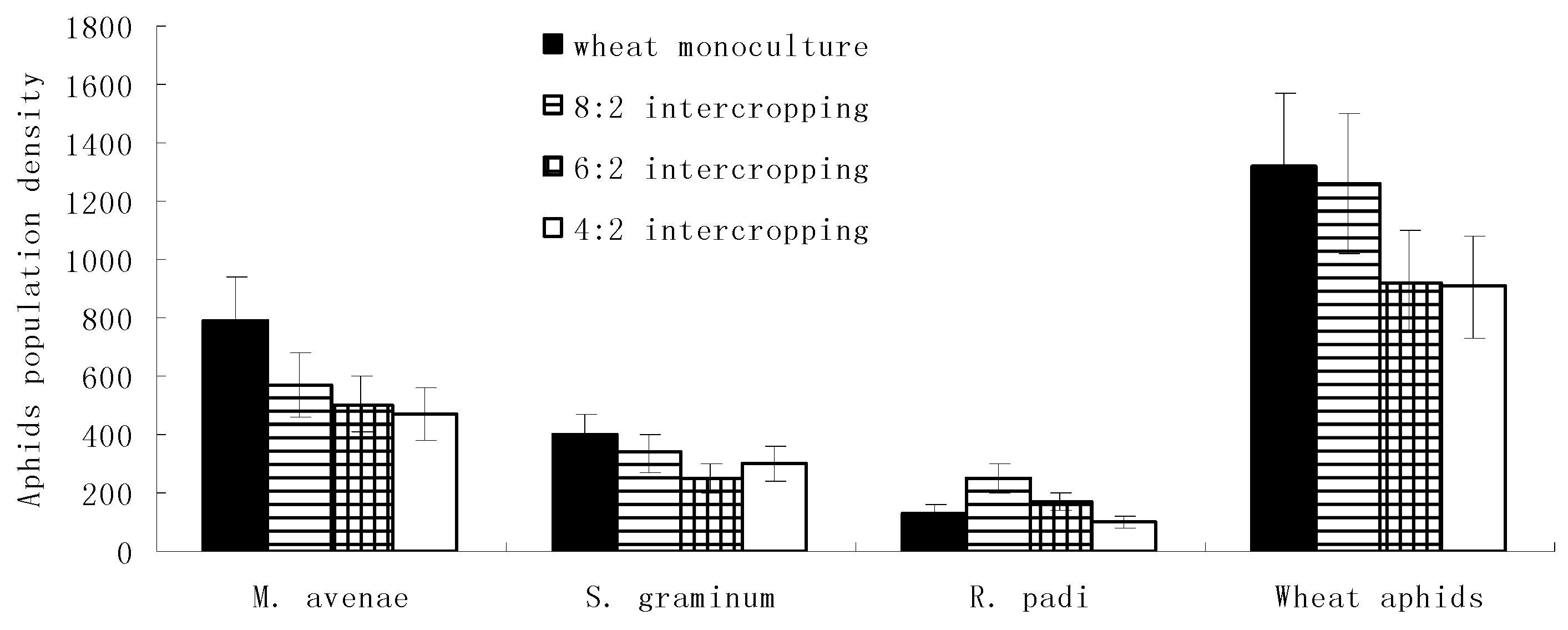
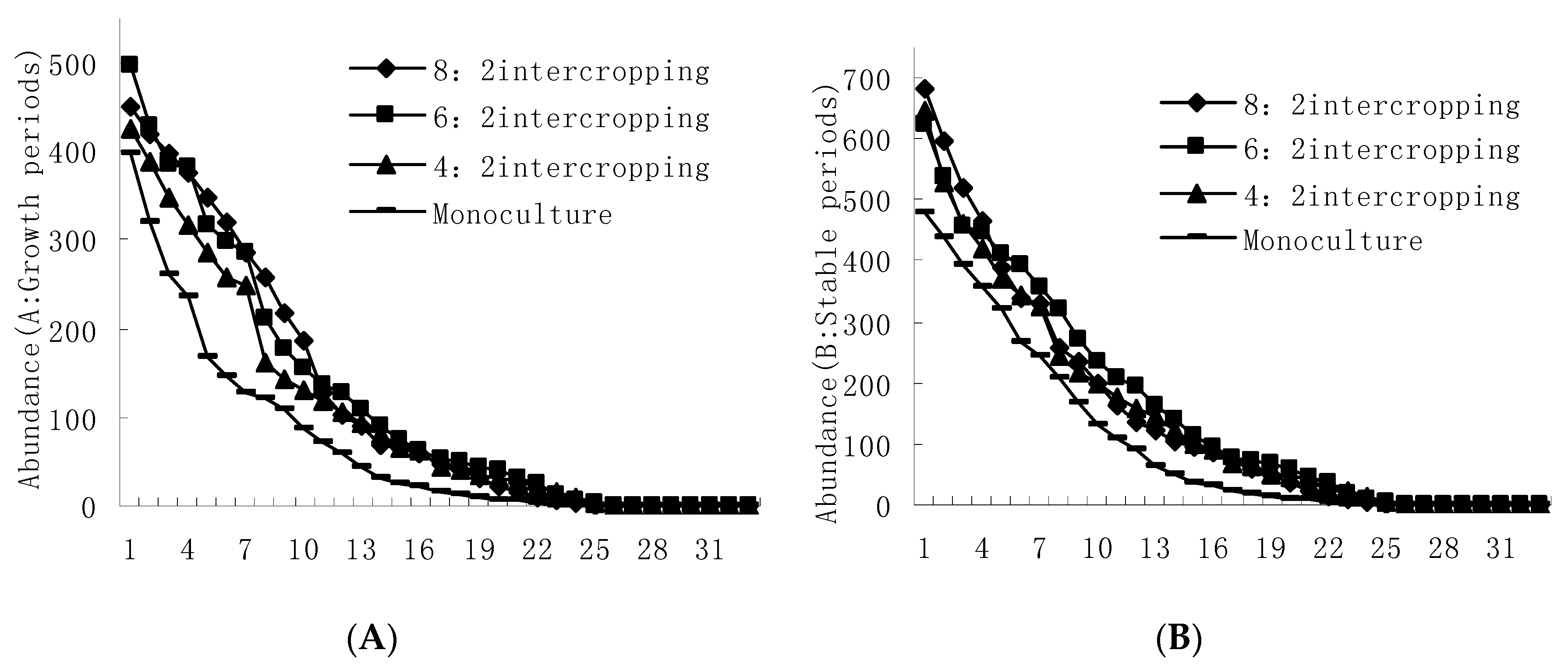
3.1. The Immigration Period
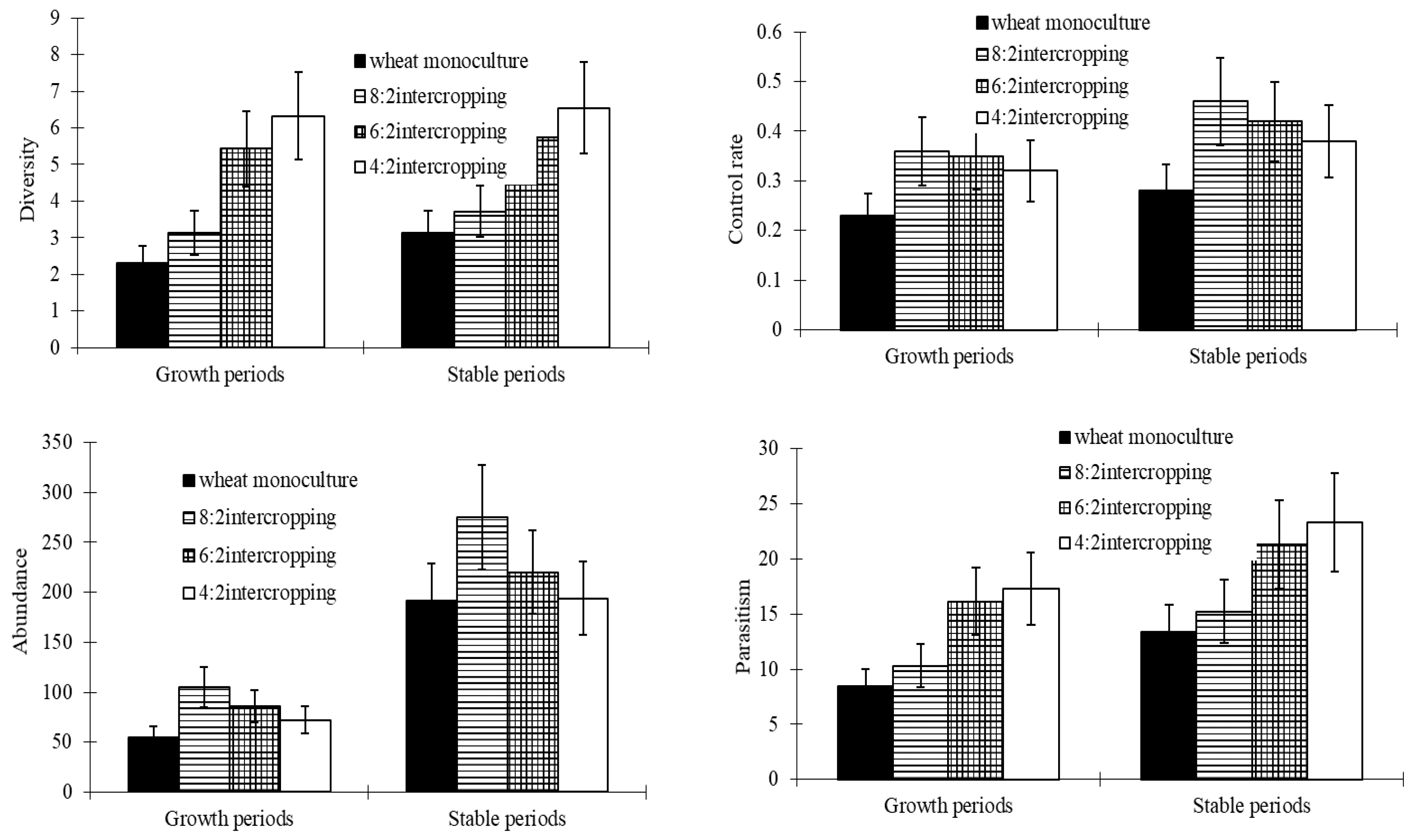
3.2. The Growth Period
3.3. The Stable Period
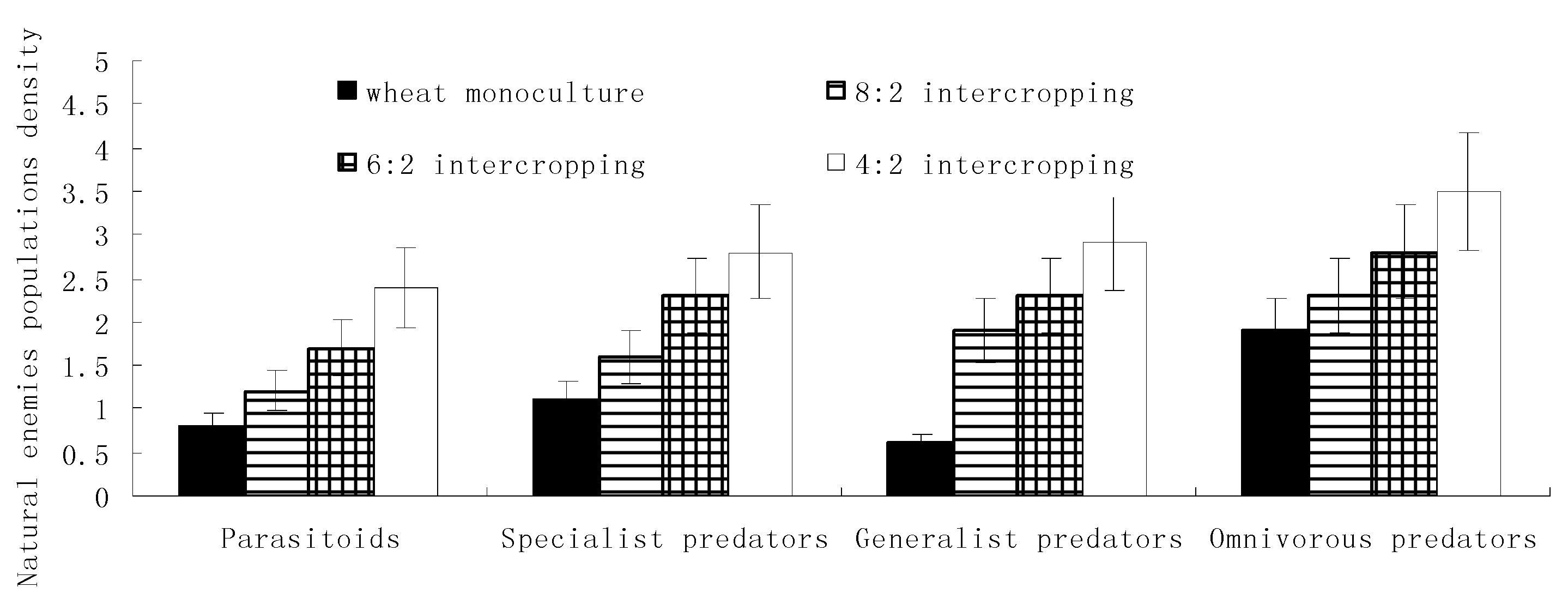
3.4. Control of Wheat Aphids’ Natural Enemies
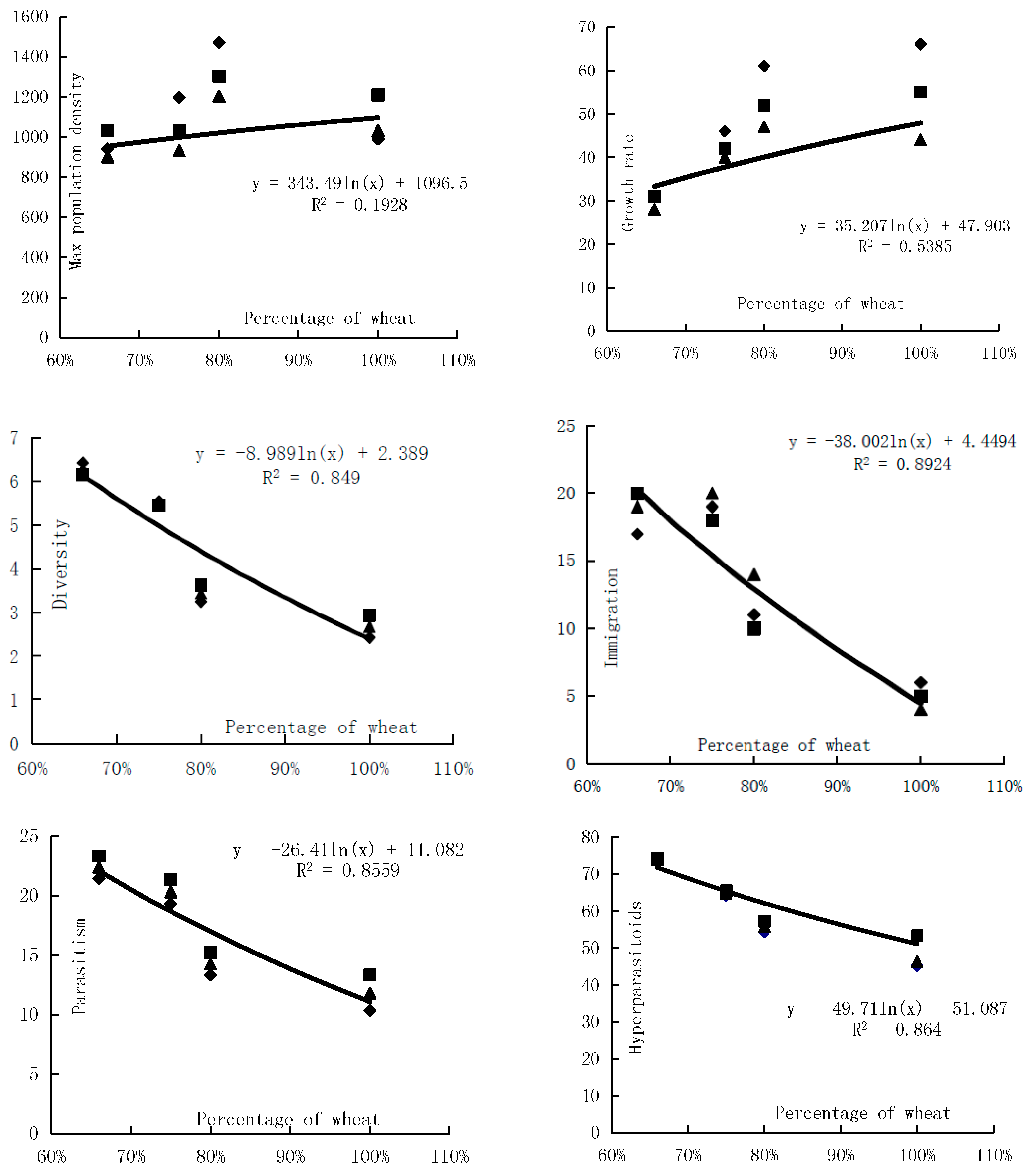
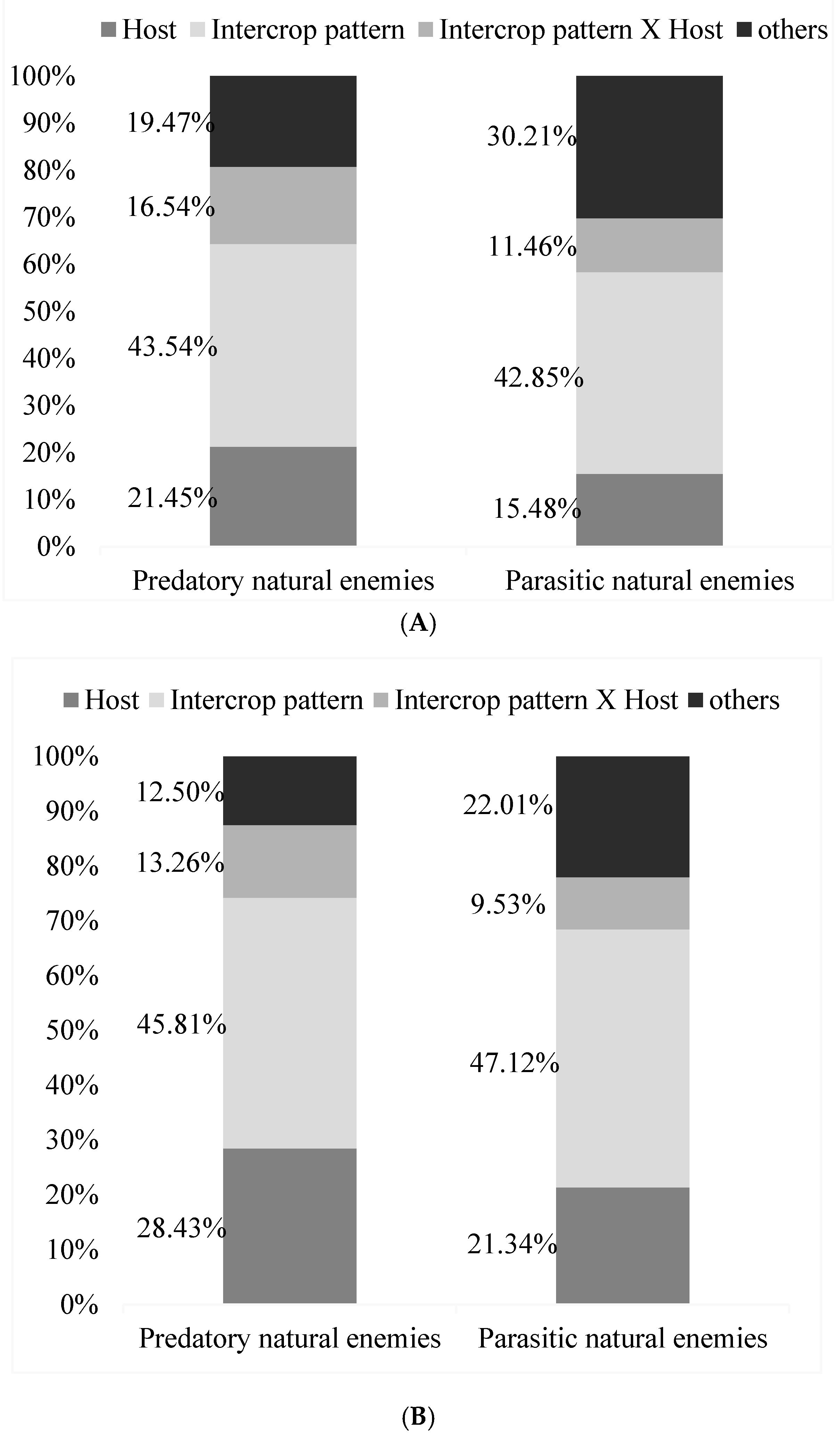
3.5. Effects of Intercropping and Host Density on Wheat Aphids’ Natural Enemies
3.6. Effect of Intercropping on Wheat Aphids’ Natural Enemies
3.7. Interactions between Host Density and Intercropping on Wheat Aphids’ Natural Enemies
4. Discussion
4.1. Effects of Intercropping on Wheat Aphid Populations
4.2. Effects of Intercropping on Populations of Wheat Aphids’ Natural Enemies
5. Summary
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, S.-J.; Liu, A.-Z.; Ru, T.-Q.; Wu, Y.-Q.; Han, S. Effects of different intercropping models on wheat aphids and their predator communities. Acta Agric. North China 2007, 22, 141–144. [Google Scholar]
- Clough, Y.; Kruss, A.; Tscharnthe, T. Local and landscape factors in differently managed arable fields affect the insect herbivore community of a non-crop plant species. J. Appl. Ecol. 2007, 44, 22–28. [Google Scholar] [CrossRef]
- Ferguson, A.W.; Barari, H.; Warner, D.J.; Campbell, J.M.; Smith, E.T.; Watts, N.P.; Williams, I.H. Distributions and interactions of the stem miners Psylliodes chrysocephala and Ceutorhynchus pallidactylus and their parasitoids in a crop of winter oilseed rape (Brassica napus). Entomol. Exp. Appl. 2006, 119, 81–92. [Google Scholar] [CrossRef]
- Elliott, N.C.; Kieckhefer, R.W.; Lee, J.H.; French, B.W. Influence if within-field and landscape factors on aphid predator populations in wheat. Landsc. Ecol. 1998, 14, 239–252. [Google Scholar] [CrossRef]
- Elliott, N.C.; Kieckhefer, R.W.; Michels, G.J.; Giles, K.L. Predator abundance in alfalfa fields in relation to aphids, within-field vegetation, and landscape matrix. Environ. Entomol. 2002, 31, 253–260. [Google Scholar] [CrossRef]
- Elliott, N.C.; Tao, F.L.; Giles, K.L.; Kindler, S.D.; French, B.W.; Greenstone, M.H.; Shufran, K.A. Ground beetle density in Oklahoma winter wheat fields. Southwest. Entomol. 2006, 31, 121–128. [Google Scholar]
- Macfadyen, S.; Gibson, R.; Raso, L.; Sint, D.; Traugott, M.; Memmott, J. Parasitoids control of aphids in organic and conventional farming systems. Agric. Ecosyst. Environ. 2009, 133, 14–18. [Google Scholar] [CrossRef]
- Maes, D.; Dyck, H.V. Habitat quality and biodiversity indicator performances of a threatened butterfly versus a multi-species group for wet heathlands in Belgium. Biol. Conserv. 2005, 123, 177–187. [Google Scholar] [CrossRef]
- Qiu, S.-B.; Yang, H.-W. Biological control, an important technology in integrated pest management. Plant Prot. 2007, 33, 1–6. [Google Scholar]
- He, D.-H.; Zhao, Z.-H.; Zhang, D.-H. Responses of insect communities and populations on habitat fragmentation in grassland landscapes. Acta Pratacult. Sin. 2009, 18, 235–241. [Google Scholar]
- Muthukumar, M.; Sharma, R.K. Eco-friendly management of insect pests of cauliflower (Brassica oleracea var. botrytis) with intercropping and botanicals. Indian J. Agric. Sci. 2009, 9, 135–137. [Google Scholar]
- Ma, K.Z.; Hao, S.G.; Zhao, H.Y.; Kang, L. Strip cropping wheat and alfalfa to improve the biological control of the wheat aphid Macrosiphum avenae by the mite Allothrom biumovatum. Agric. Ecosyst. Environ. 2007, 119, 49–52. [Google Scholar] [CrossRef]
- Zhou, H.-B. Study on the Effect Using Wheat-Pea Intercropping and Varieties Diversity Ecological Regulation on Sitobion avenae and Its Mechanism. Master’s Thesis, Shandong Agricultural University, Tai’an, China, 2009. [Google Scholar]
- Zhou, H.-B.; Chen, J.-L.; Liu, Y.; Chen, D.-F.; Chen, L.; Sun, J.-R. Using genetic diversity of wheat varieties for ecological regulation on Sitobion avenae. Acta Phytophylacica Sin. 2009, 36, 151–156. [Google Scholar]
- Zhou, H.-B.; Chen, L.; Chen, J.-L.; Chen, D.-F.; Liu, Y.; Sun, J.-R. Effect of intercropping between wheat and pea on spatial distribution of Sitobion avenae based on GIS. Sci. Agric. Sin. 2009, 42, 3904–3913. [Google Scholar]
- Zhou, H.-B.; Chen, J.-L.; Chen, D.-F.; Liu, Y.; Sun, J.-R. Effects of wheat-pea intercropping on the population dynamics of Sitobion avenae (Homoptera, Aphididae) and its main natural enemies. Acta Entomol. Sin. 2009, 52, 775–782. [Google Scholar]
- Huang, D.-C.; Zhang, R.-Z.; Dong, Z.-K.; Gao, F.-J.; Pan, G.-Y.; Ouyang, Z. Multi-population dynamics of aphids and natural enemies on winter wheat and forages and their relationships. J. Environ. Entomol. 2008, 30, 325–330. [Google Scholar]
- Wang, W.-L. The Control Effect of Wheat Biodiversity on Wheat Filed. Master’s Thesis, Shandong Agricultural University, Tai’an, China, 2008. [Google Scholar]
- Wang, W.-L.; Liu, Y.; Ji, X.-L.; Zhou, H.-B. Effects of wheat-oilseed rape or wheat-garlic intercropping on the population dynamics of Sitobion avenae and its main natural enemies. Chin. J. Appl. Ecol. 2008, 19, 1331–1336. [Google Scholar]
- Zhao, Z.-H.; He, D.-H.; Hui, C. From the inverse density-area relationship to the minimum patch size of a host-parasitoid system. Ecol. Res. 2012, 27, 303–309. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, T.-W.; Liu, C.-Z. Effect of Inter-cropping of Wheat with different crops on occurrence quantity of wheat aphid. Guizhou Agric. Sci. 2010, 38, 148–149. [Google Scholar]
- Finch, S.; Collier, R.H. Host plant selection by insects, a theory based on ‘appropriate/inappropriate landings’ by pest insects of cruciferous plants. Entomol. Exp. Appl. 2000, 96, 91–102. [Google Scholar] [CrossRef]
- Shen, J.-H.; Nie, Q.; Huang, D.-R.; Liu, G.-J.; Tao, L.-X. Recent advances in controlling plant diseases and insect pests by mixture planting ang inter-panting of crops. Acta Phytophylacica Sin. 2007, 34, 209–216. [Google Scholar]
- Liu, J.-H.; Yu, M.-F.; Abid, A.; Liu, J.-Y.; Li, K.M.; Niaz, H.K. Density estimation of ground dwelling predators in wheat fields of northwest China. Pak. J. Zool. 2016, 47, 21–29. [Google Scholar]
- Gagic, V.; Tscharntke, T.; Dormann, C.F.; Gruber, B.; Wilstermann, A.; Thies, C. Food web structure and biocontrol in a four trophic level system across a landscape complexity gradient. Proc. Biol. Sci. 2011, 278, 2946–2953. [Google Scholar] [CrossRef] [PubMed]
- Lohaus, K.; Vidal, S.; Thies, C. Farming practices change food web structures in cereal aphids-parasitoid-hyperparasitoid communities. Oecologia 2013, 171, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Brewer, M.J.; Elliott, N.C. Biological control of cereal aphids in North America and mediating effects of host plant and habitat manipulation. Annu. Rev. Entomol. 2004, 49, 19–242. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.H.; Roschewitz, I.; Thies, C.; Tscharntke, T. Differential effects of landscape and management on diversity and density of ground-dwelling farmland spiders. J. Appl. Ecol. 2005, 42, 281–287. [Google Scholar] [CrossRef]
- Pertz, O.; Hodgson, L.; Klemke, R.L.; Hahn, K.M. Spatiotemporal dynamics of RhoA activity in migrating cells. Nature 2006, 440, 1069–1072. [Google Scholar] [CrossRef] [PubMed]


| Wheat:maize = 8:2 | Wheat:maize = 6:2 | Wheat:maize = 4:2 | Monoculture wheat |
| Wheat:maize = 6:2 | Wheat:maize = 4:2 | Monoculture wheat | Wheat:maize = 8:2 |
| Wheat:maize = 4:2 | Monoculture wheat | Wheat:maize = 8:2 | Wheat:maize = 6:2 |
| Monoculture wheat | Wheat:maize = 8:2 | Wheat:maize = 6:2 | Wheat:maize = 4:2 |
| Family | Species | Low Host Density | High Host Density | ||
|---|---|---|---|---|---|
| Intercrop | Monoculture | Intercrop | Monoculture | ||
| Aphidiidae | Aphidius avenae | 257 | 198 | 425 | 309 |
| A. gifuensis | 64 | 31 | 92 | 45 | |
| A. sichuanensis | 5 | 8 | 7 | 11 | |
| Lysiphlebus confusus | 5 | 2 | 7 | 4 | |
| Praon volucre | 4 | 1 | 7 | 2 | |
| P. orientale | 2 | 0 | 4 | 1 | |
| P. rhopalosiphum | 3 | 0 | 6 | 2 | |
| Trioxys asiaticus | 0 | 0 | 4 | 1 | |
| T. sp. | 0 | 0 | 2 | 0 | |
| Toxares sp. | 0 | 0 | 2 | 0 | |
| Aphelinidae | Phelinus sp. | 5 | 1 | 11 | 2 |
| Family | Species | Low Host Density | High Host Density | ||
|---|---|---|---|---|---|
| Intercrop | Monoculture | Intercrop | Monoculture | ||
| Coccinellidae | H. tredecimpunctata | 8 | 7 | 15 | 11 |
| C. septempunctata | 7 | 6 | 13 | 11 | |
| H. variegata | 9 | 5 | 19 | 14 | |
| Syphidae | Syrphus nitens | 9 | 6 | 12 | 9 |
| S. cylindrica | 7 | 5 | 9 | 6 | |
| Chrysopidae | Chrysopa sinica | 8 | 7 | 11 | 6 |
| S. Croceolum | 5 | 2 | 7 | 3 | |
| Carabidae | C. maderae | 4 | 6 | 5 | 8 |
| S. terricola | 5 | 7 | 4 | 7 | |
| Chlaenius pallipes | 11 | 15 | 13 | 16 | |
| Lycosidae | Lycisa coelestris | 7 | 5 | 6 | 8 |
| Pardosa astrigera | 14 | 12 | 16 | 14 | |
| Linypiidae | E. graminicolum | 11 | 15 | 19 | 16 |
| Family | Species | Low Host Density | High Host Density | ||
|---|---|---|---|---|---|
| Intercrop | Monoculture | Intercrop | Monoculture | ||
| Charipidae | Alloxysta sp. 1 | 59 | 48 | 142 | 101 |
| A. sp. 2 | 29 | 17 | 58 | 34 | |
| Pteromalidae | Pachyneuron aphidis | 83 | 62 | 146 | 119 |
| Asaphes suspensus | 69 | 46 | 113 | 89 | |
| Asaphes vulgaris | 37 | 26 | 64 | 51 | |
| Pteromalidae sp. | 4 | 1 | 6 | 3 | |
| Encyrtidae | Aphide–ncyrtus aphidivorus | 7 | 3 | 11 | 3 |
| EuloPhidae | Tetrastichus sp. | 4 | 2 | 6 | 4 |
| Megaspilidae | Dendrocerus carpenteri | 7 | 2 | 8 | 3 |
| Monoculture vs. Intercropping | ||
| Primary parasitoids | Low host density | F = 13.92, df = 1, 7, p = 0.01 |
| High host density | F = 15.32, df = 1, 7, p = 0.01 | |
| Hyperparasitoids | Low host density | F = 3.72, df = 1, 7, p = 0.31 |
| High host density | F = 3.72, df = 1, 14, p = 0.35 | |
| Predators | Low host density | F = 11.89, df = 1, 7, p = 0.01 |
| High host density | F = 9.84, df = 1, 7, p = 0.01 | |
| Low Host Density vs. High Host Density | ||
| Primary parasitoids | Monoculture | F = 24.12, df = 1, 7, p = 0.01 |
| Intercropping | F = 48.32, df = 1, 7, p = 0.01 | |
| Hyperparasitoids | Monoculture | F = 43.23, df = 1, 7, p = 0.01 |
| Intercropping | F = 38.32, df = 1, 7, p = 0.01 | |
| Predators | Monoculture | F = 21.87, df = 1, 7, p = 0.01 |
| Intercropping | F = 17.09, df = 1, 7, p = 0.01 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Yan, Y.; Ali, A.; Wang, N.; Zhao, Z.; Yu, M. Effects of Wheat-Maize Intercropping on Population Dynamics of Wheat Aphids and Their Natural Enemies. Sustainability 2017, 9, 1390. https://doi.org/10.3390/su9081390
Liu J, Yan Y, Ali A, Wang N, Zhao Z, Yu M. Effects of Wheat-Maize Intercropping on Population Dynamics of Wheat Aphids and Their Natural Enemies. Sustainability. 2017; 9(8):1390. https://doi.org/10.3390/su9081390
Chicago/Turabian StyleLiu, Junhe, Yan Yan, Abid Ali, Ningtao Wang, Zihua Zhao, and Mingfu Yu. 2017. "Effects of Wheat-Maize Intercropping on Population Dynamics of Wheat Aphids and Their Natural Enemies" Sustainability 9, no. 8: 1390. https://doi.org/10.3390/su9081390




