Adsorption Kinetics and Breakthrough of Carbon Dioxide for the Chemical Modified Activated Carbon Filter Used in the Building
Abstract
:1. Introduction
2. Experimental Method
3. Results and Discussion
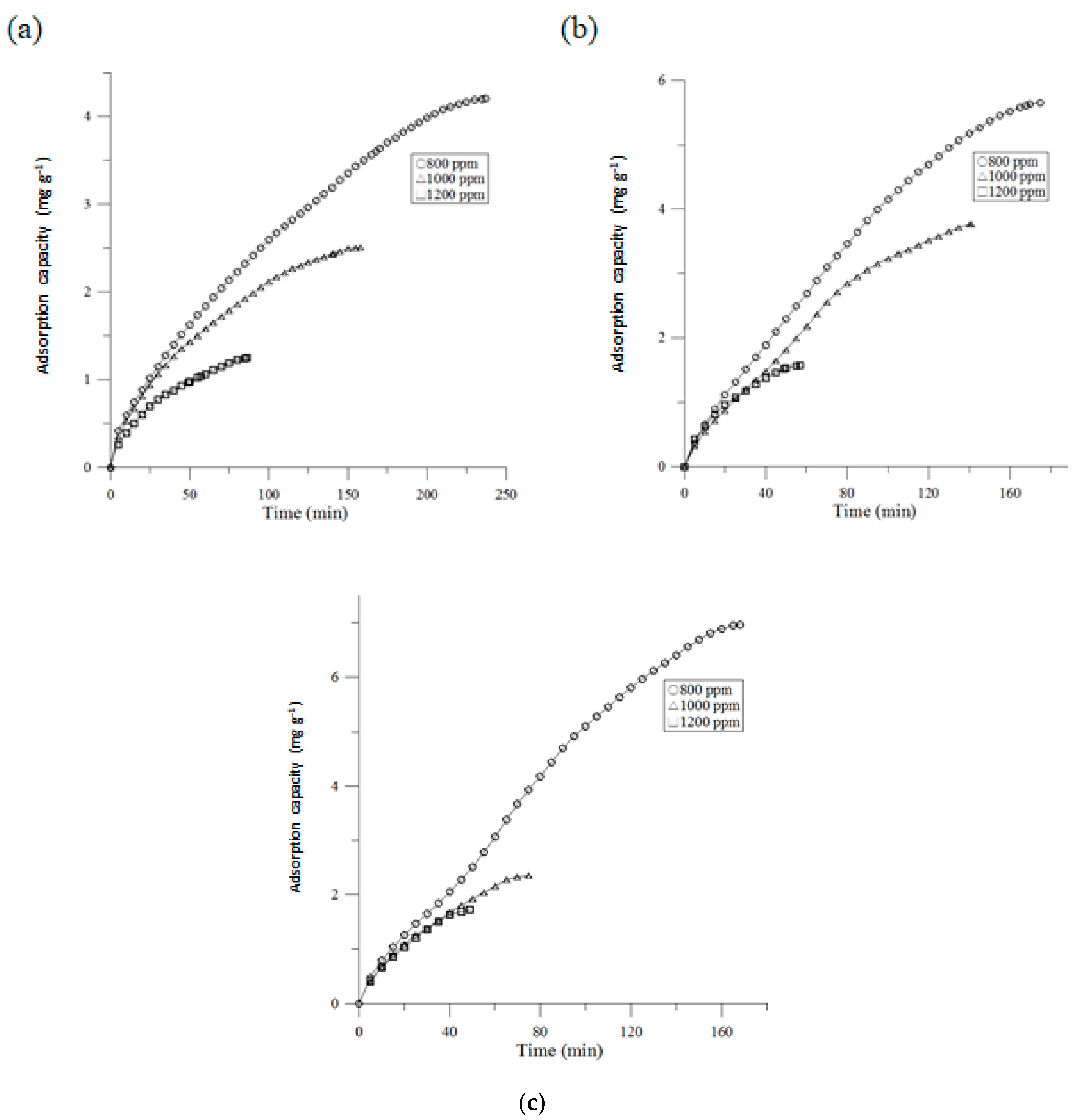
3.1. Adsorption Capacity
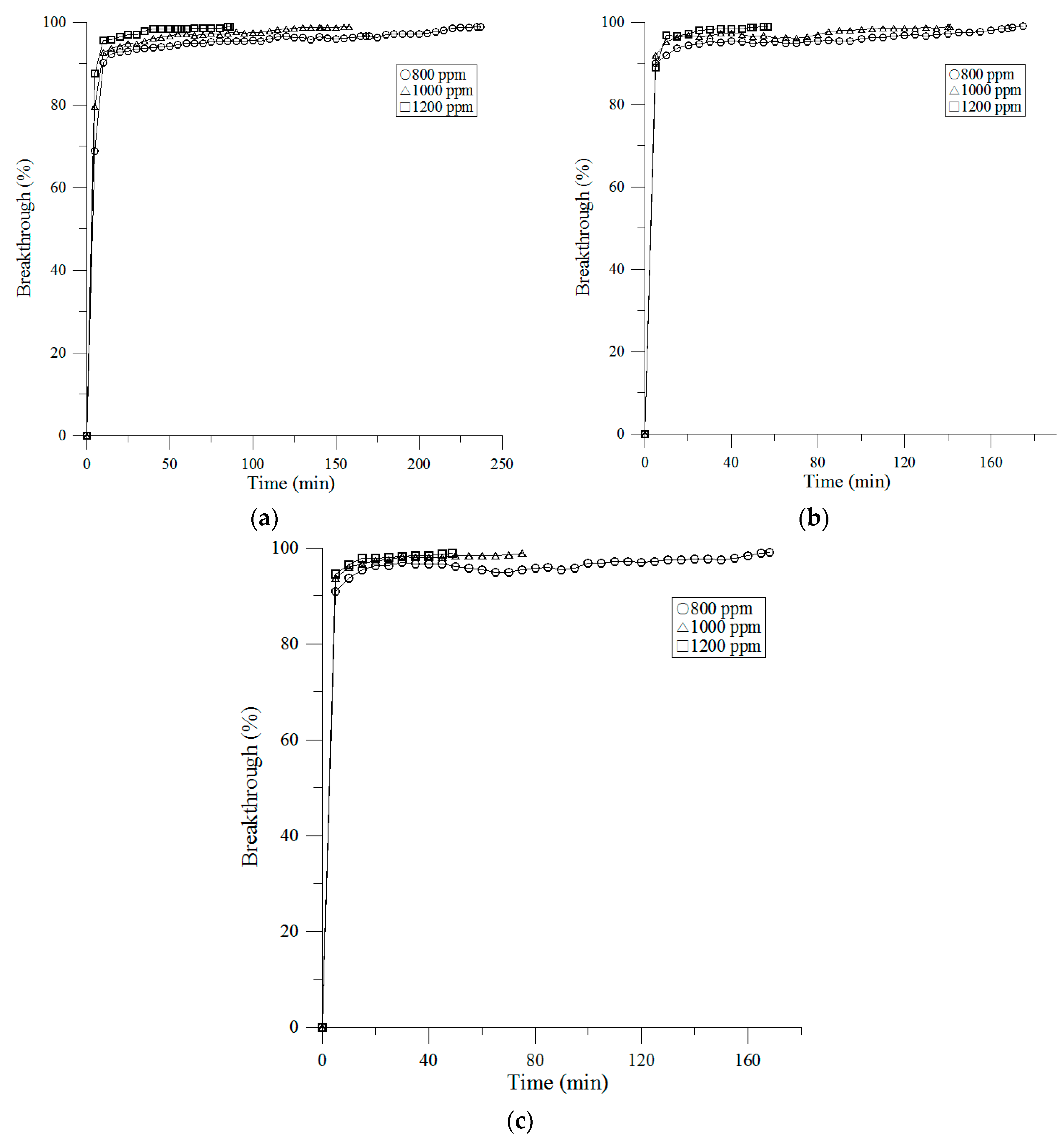
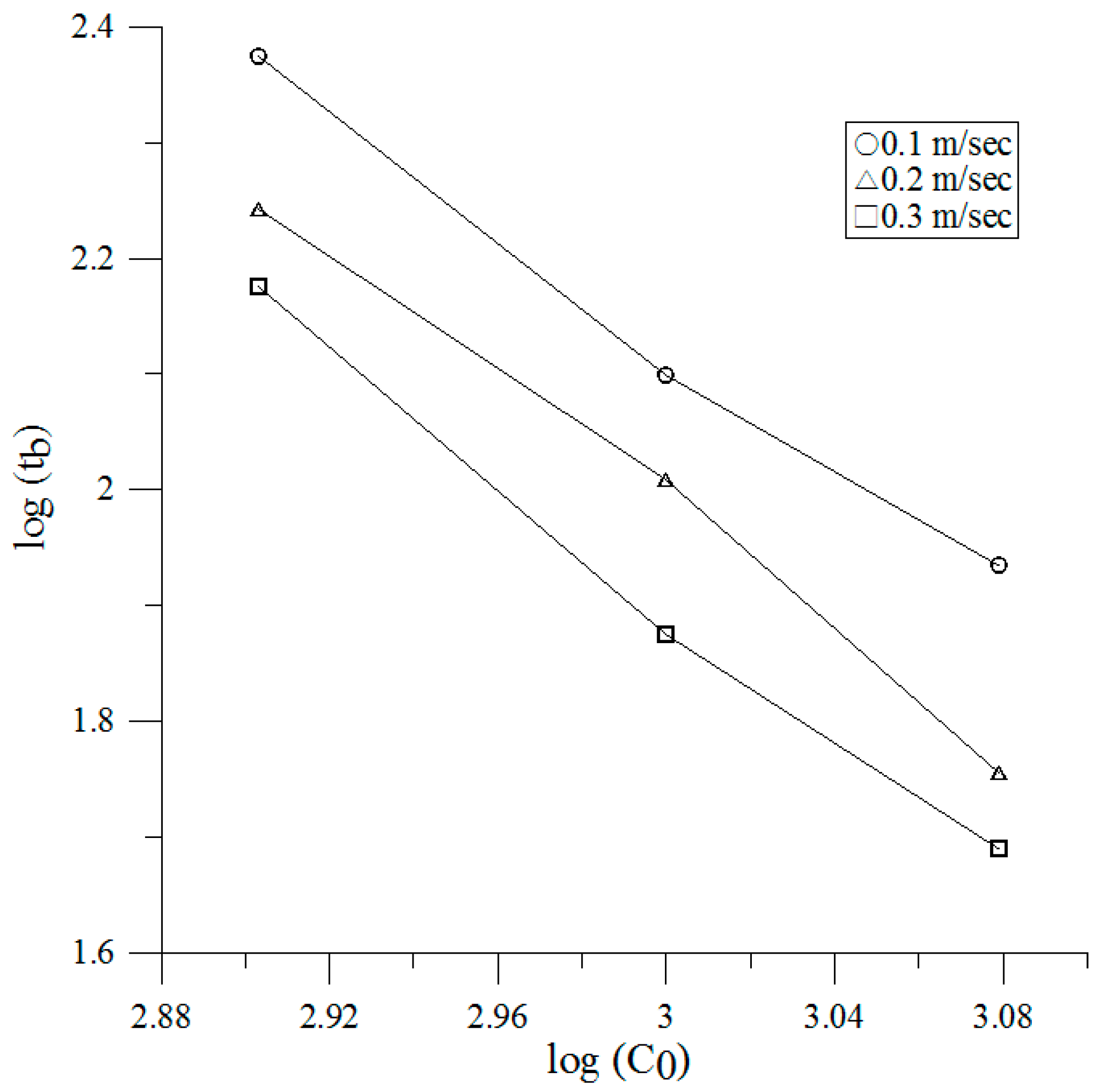
3.2. Breakthrough
3.3. Adsorption Kinetics
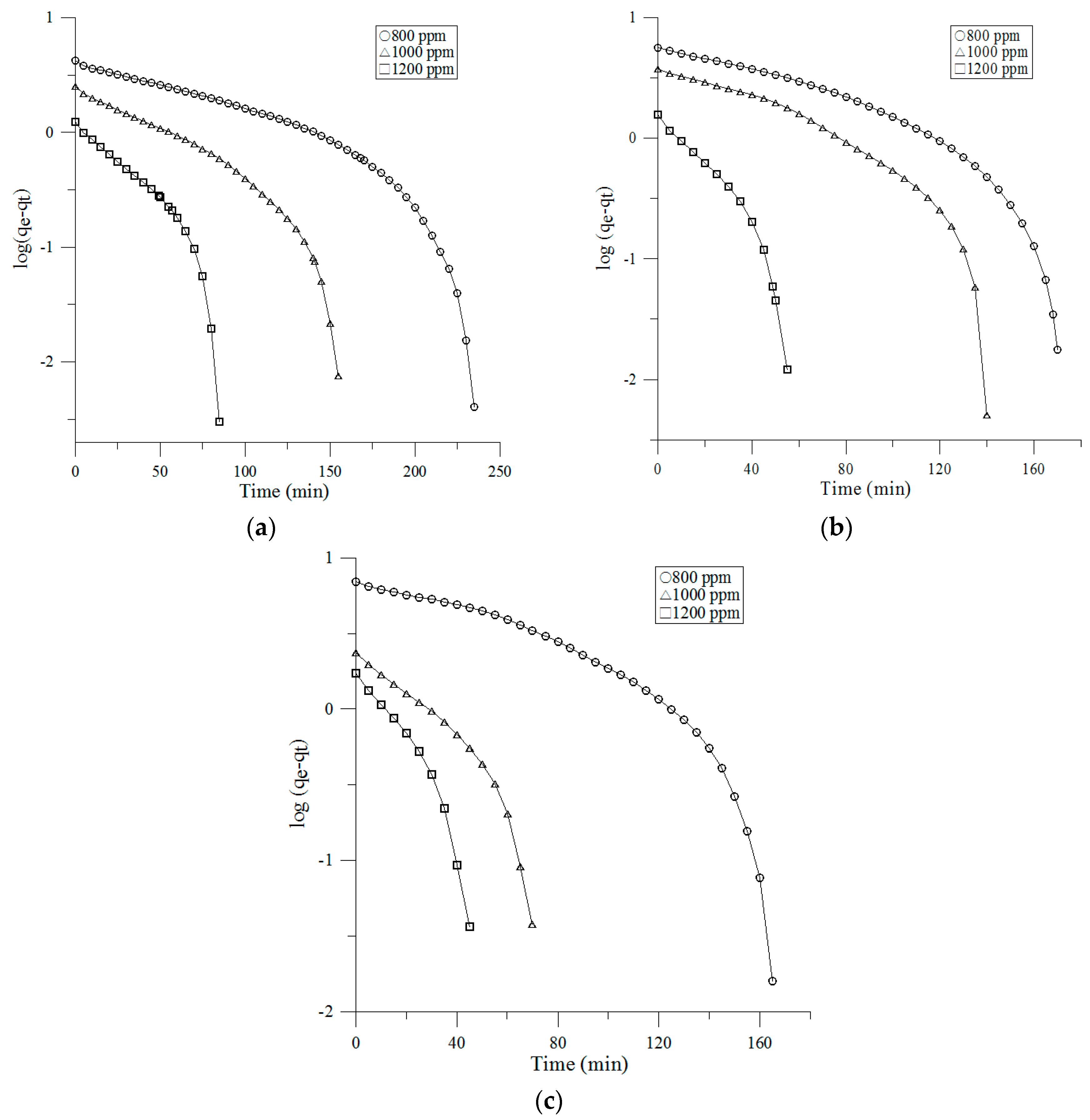
3.3.1. Pseudo-First-Order Model
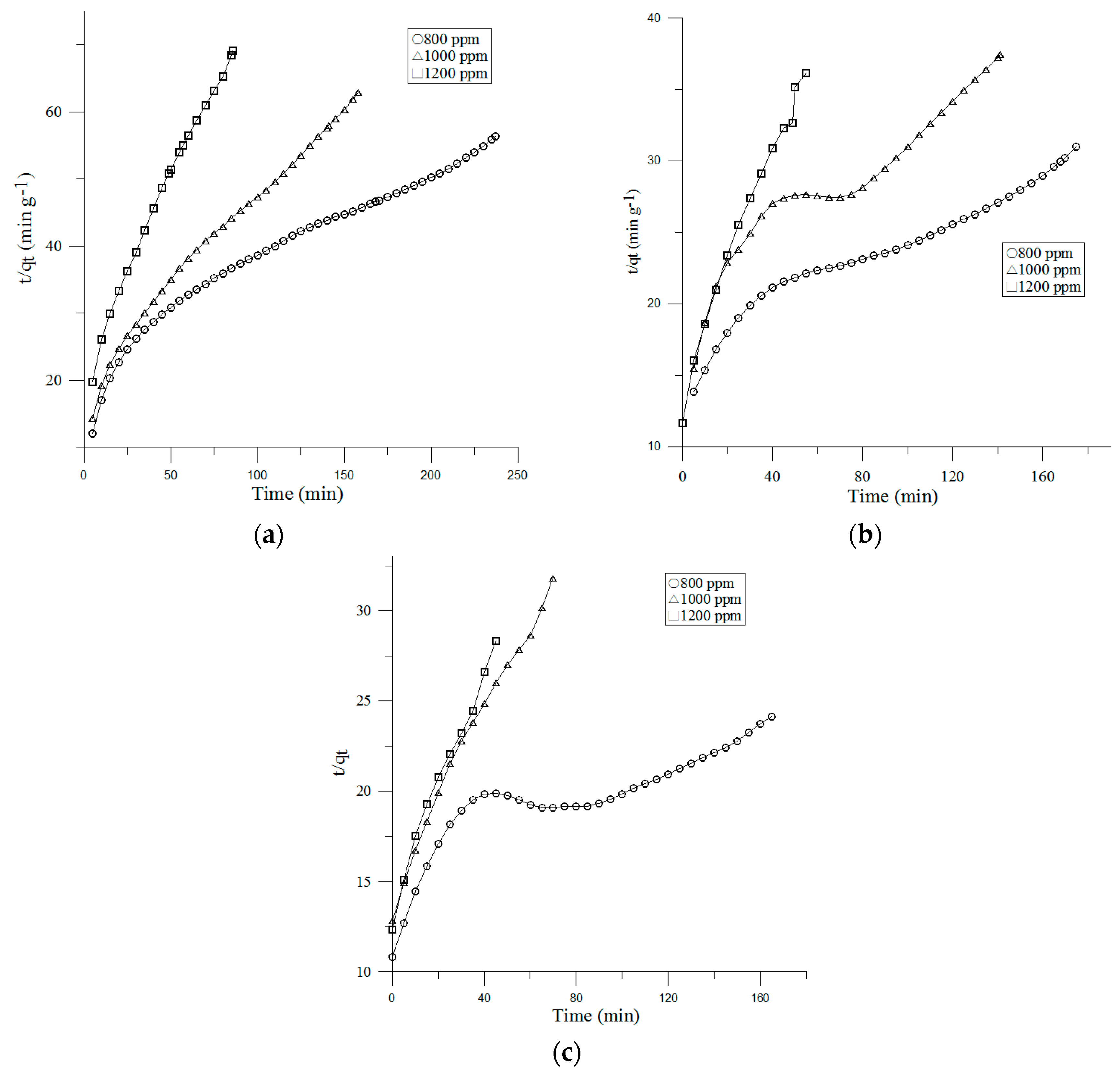
3.3.2. Pseudo-Second-Order Model
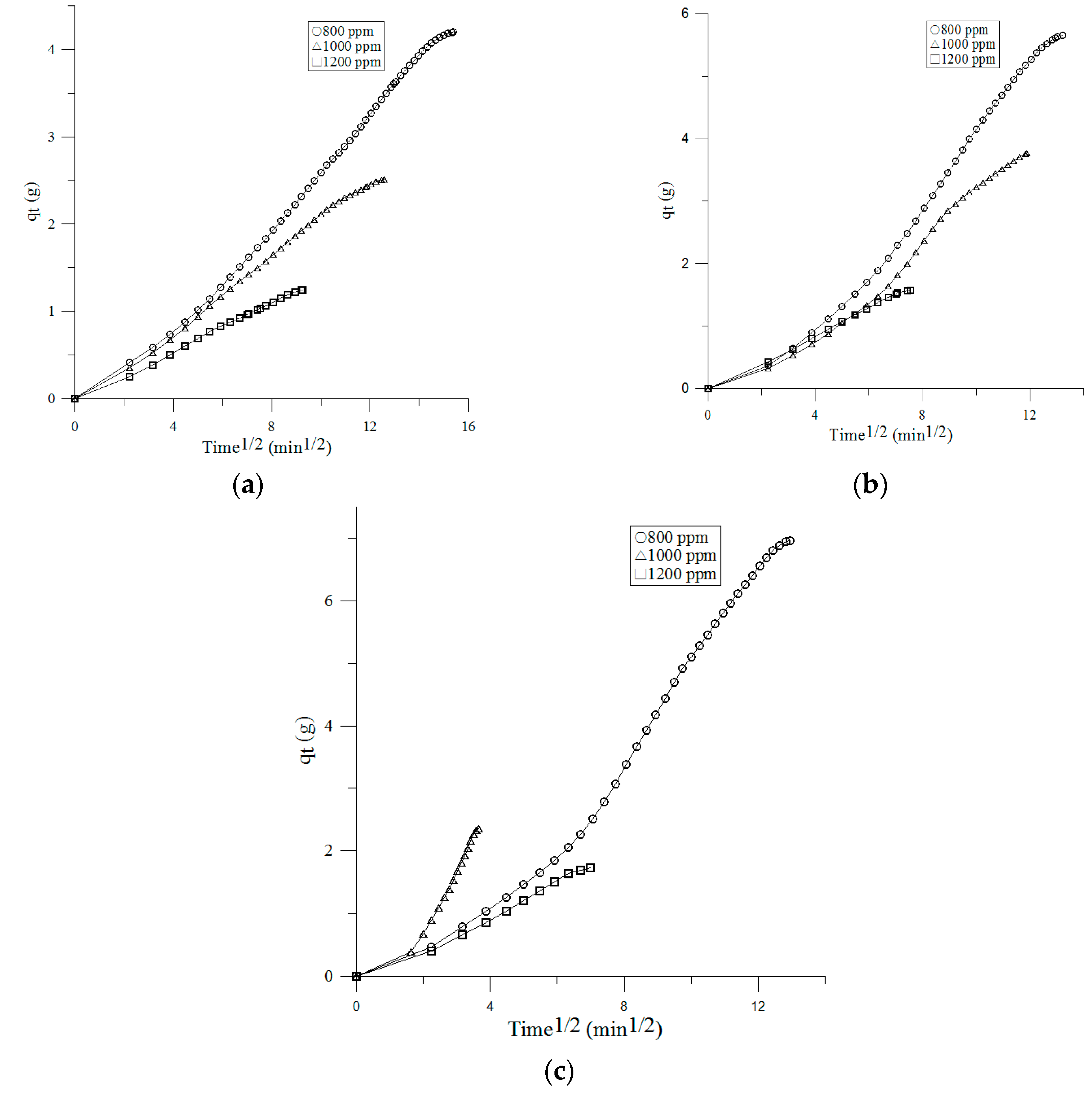
3.3.3. Intraparticle Diffusion Model
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Nomenclature
| C | intercept of intraparticle diffusion model, mg kg−1 |
| Ce | concentration of free formaldehyde in air, mg L−1 |
| C0 | the inlet concentration, ppm |
| k1 | the pseudo-first-order rate coefficient, min−1 |
| k2 | the pseudo-second-order rate coefficient, g mg−1 min−1 |
| ki | the intraparticle diffusion rate constant, mg g−1 min−1/2 |
| M | testing gas molecular weight, g mole−1 |
| Q | adsorption capacity, mg g−1 |
| qe | the amount of adsorbed CO2, mg g−1 |
| qt | the amount of adsorbate adsorbed at time t, mg g−1 |
| t | testing time, min |
| V | the airflow rate, L min−1 |
References
- Brunsgaard, C.; Heiselberg, P.; Knudstrup, M.A.; Larsen, T.S. Evaluation of the Indoor Environment of Comfort Houses: Qualitative and Quantitative Approaches. Indoor Built Environ. 2012, 21, 432–451. [Google Scholar] [CrossRef]
- Davis, I.; Harvey, V. Zero Carbon: What Does It Mean to Homeowners and House Builders? NHBC Foundation Report, NF9; London IHS BRE Press: London, UK, 2008. [Google Scholar]
- Morrell, P. HM Government, Innovation & Growth Team. 2010. Available online: http://www.bis.gov.uk/assets/biscore/business-sectors/docs/l/10-1266-low-carbon-construction-igtfinal-report.pdf (accessed on 4 November 2011).
- Yu, C.W.F.; Kim, J.T. Building Pathology, Investigation of Sick Buildings—VOC Emissions. Indoor Built Environ. 2010, 19, 30–39. [Google Scholar] [CrossRef]
- Chuck, W.F.; Kim, J.T. Photocatalytic Oxidation for Maintenance of Indoor Environmental Quality. Indoor Built Environ. 2013, 22, 39–51. [Google Scholar]
- Yao, R.; Yu, C.W.F. Towards “Zero-Carbon Homes”—Issues of Thermal Comfort. Indoor Built Environ. 2012, 21, 483–485. [Google Scholar] [CrossRef]
- Lee, S.C.; Hsieh, C.C.; Chen, C.H.; Chen, Y.S. CO2 Adsorption by Y-Type Zeolite Impregnated with Amines in Indoor Air. Aerosol Air Q. Res. 2013, 13, 360–366. [Google Scholar] [CrossRef]
- American Society of Testing and Materials (ASTM). Standard Guide for Using Indoor Carbon Dioxide Concentrations to Evaluate Indoor Air Quality and Ventilation; American Society for Testing and Materials International: West Conshohocken, PA, USA, 2012. [Google Scholar]
- Occupational Safety and Health Administration, Department of Labor. Indoor Air Quality in Commercial and Institutional Buildings; OSHA: Department of Labor: Washington, DC, USA, 2011.
- Ventilation for Acceptable Indoor Air Quality; ASHRAE Standard 62.1; American Society of Heating, Refrigerating and Air-Conditioning: Atlanta, GA, USA, 2016.
- What Is the Allowable Level of Carbon Dioxide in an Occupied Space? ASHRAE TC-04.03-FAQ-35. 2015. Available online: https://www.ashrae.org/FileLibrary/docLib/.../TC-04-03-FAQ-35.pd (accessed on 24 July 2017).
- Ogawa, M.; Nakano, Y. Separation of CO2/CH4 mixture through carbonized membrane prepared by gel modification. J. Membr. Sci. 2000, 173, 123–132. [Google Scholar] [CrossRef]
- Liao, C.H.; Li, M.H. Kinetics of absorption of carbon dioxide into aqueous solutions of monoethanolamine + N-methyldiethanolamine. Chem. Eng. Sci. 2002, 57, 4569–4582. [Google Scholar] [CrossRef]
- Cornelissen, R.L.; Hirs, G.G. Energy analysis of cryogenic air separation. Energy Convers. Manag. 1998, 39, 1821–1826. [Google Scholar] [CrossRef]
- Sarkar, S.C.; Bose, A. Role of activated carbon pellets in carbon dioxide removal. Energy Convers. Manag. 1997, 38, S105–S110. [Google Scholar] [CrossRef]
- Song, H.K.; Won, C.K.; Lee, K.H. Adsorption of carbon dioxide on the chemically modified silica adsorbents. J. Non-Cryst. Solids 1998, 242, 69–80. [Google Scholar] [CrossRef]
- Shin, D.H.; Cheigh, H.S.; Lee, D.S. The use of Na2CO3-based CO2 absorbent systems to alleviate pressure buildup and volume expansion of kimchi packages. J. Food Eng. 2002, 53, 229–235. [Google Scholar] [CrossRef]
- Ranjani, V.S.; Shen, M.S.; Edward, P.F. Adsorption of CO2 zeolites at moderate temperatures. Energy Fuel. 2005, 19, 1153–1159. [Google Scholar]
- Liu, B.T.; Shui, Y.H.; Zhu, G.Y. Adsorption of a Non-Woven Fabric with Activated Carbon for CO2. Adv. Mater. Res. 2012, 518, 683–686. [Google Scholar]
- Jensen, M.B.; Pettersson, L.G.M.; Swang, O.; Olsbye, U. CO2 Sorption on MgO and CaO Surfaces: A Comparative Quantum Chemical Cluster Study. J. Phys. Chem. B 2005, 109, 16774–16781. [Google Scholar] [CrossRef] [PubMed]
- Lahuri, A.H.; Yarmo, M.A.; Marliza, T.S.; Tahariri, M.N.A.; Samad, W.Z.; Yusop, N.D.M.R. Carbon Dioxide Adsorption and Desorption Study Using Bimetallic Calcium Oxide Impregnated on Iron (III) Oxide. Mater. Sci. Forum 2017, 888, 479–484. [Google Scholar]
- Hu, S.C.; Chang, A.; Angus Shiue, A.; Lin, T.; Song-Dun Liao, S.D. Adsorption characteristics and kinetics of organic airborne contamination for the chemical filters used in the fan-filter unit (FFU) of a cleanroom. J. Taiwan Inst. Chem. Eng. 2017, 75, 87–96. [Google Scholar] [CrossRef]
- American Society of Heating, Refrigerating and Air-Conditioning Engineers (ASHRAE). Laboratory Test Method for Assessing the Performance of Gas-Phase Air-Cleaning Systems: Air-Cleaning Devices; American Society of Heating, Refrigerating and Air-Conditioning Engineers: Atlanta, GA, USA, 2011. [Google Scholar]
- VanOsdell, D.W.; Owen, M.K.; Jaffe, L.B.; Sparks, L.E. VOC removal at low contaminant concentrations using granular activated carbon. J. Air Waste Manag. Assoc. 1996, 46, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Zhang, B.; Jianshun, S.; Nair, S.; Chen, W.; Smith, J. VOC removal performance of pellet/granular-type sorbent media-experimental results. ASHRAE Trans. 2006, 112, 430–440. [Google Scholar]
- Shiue, A.; Kang, Y.H.; Hu, S.C.; Jou, G.T.; Lin, C.H.; Hu, M.C.; Lin, S.I. Vapor adsorption characteristics of toluene in an activated carbon adsorbent-loaded nonwoven fabric media for chemical filters applied to cleanrooms. Build. Environ. 2010, 45, 2123–2131. [Google Scholar] [CrossRef]
- Nelson, G.O.; Harder, C.A. Respirator cartridge efficiency studies: VI. Effect of concentration. Am. Ind. Hyg. Assoc. J. 1976, 37, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Lagergren, S. Zur Theorie der Sogenannten Adsorption Gelöster Stoffe, Kungliga Svenska Vetenskapsakademiens. Handlingar 1898, 24, 1–39. [Google Scholar]
- Shafeeyan, M.S.; Ashri, W.M.; Daud, W.; Shamiri, A.; Aghamohammadi, N. Modeling of Carbon Dioxide Adsorption onto Ammonia-Modified Activated Carbon: Kinetic Analysis and Breakthrough Behavior. Energy Fuels 2015, 29, 6565–6577. [Google Scholar] [CrossRef]
- Singh, V.K.; Kumar, E.A. Comparative Studies on CO2 Adsorption Kinetics by Solid Adsorbents. Energy Procedia 2016, 90, 316–325. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. The kinetics of sorption of basic dyes from aqueous solution by sphagnum moss peat. Can. J. Chem. Eng. 1998, 76, 822–827. [Google Scholar] [CrossRef]
- Guibal, E. Metal-Anion Sorption by Chitosan Beads: Equilibrium and Kinetic Studies. Ind. Eng. Chem. Res. 1998, 37, 1454–1463. [Google Scholar] [CrossRef]
- Fungaro, D.A.; Borrely, S.I.; Carvalho, T.E.T. Surfactant Modified Zeolite from Cyclone Ash as Adsorbent for Removal of Reactive Orange 16 from Aqueous Solution. Am. J. Environ. Prot. 2013, 1, 1–9. [Google Scholar] [CrossRef]
- Shiue, A.; Hu, S.C. Adsorption Kinetics for the Chemical Filters Used in the Make-Up Air Unit (MAU) of a Cleanroom. Sep. Sci. Technol. 2012, 47, 577–583. [Google Scholar] [CrossRef]
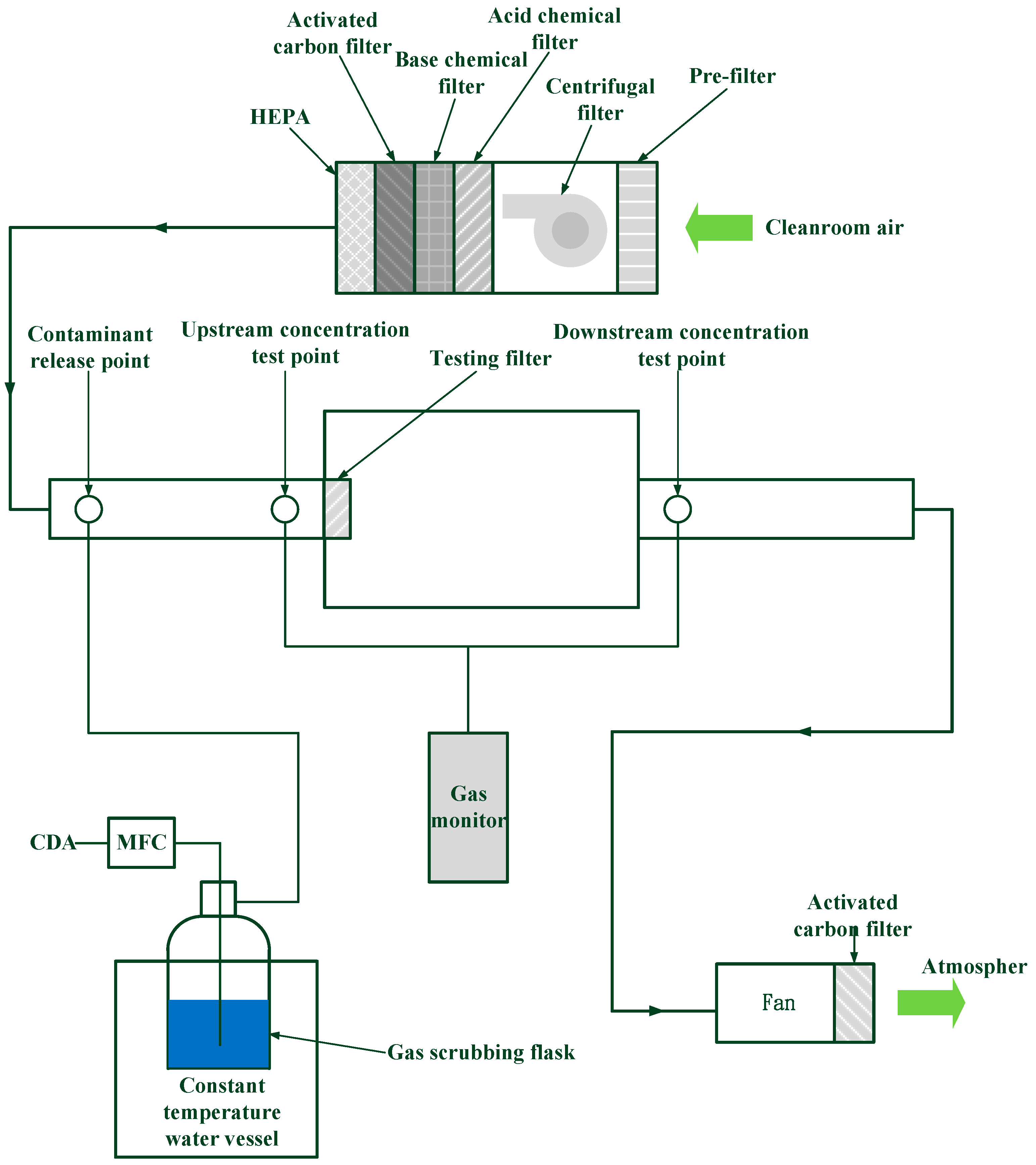
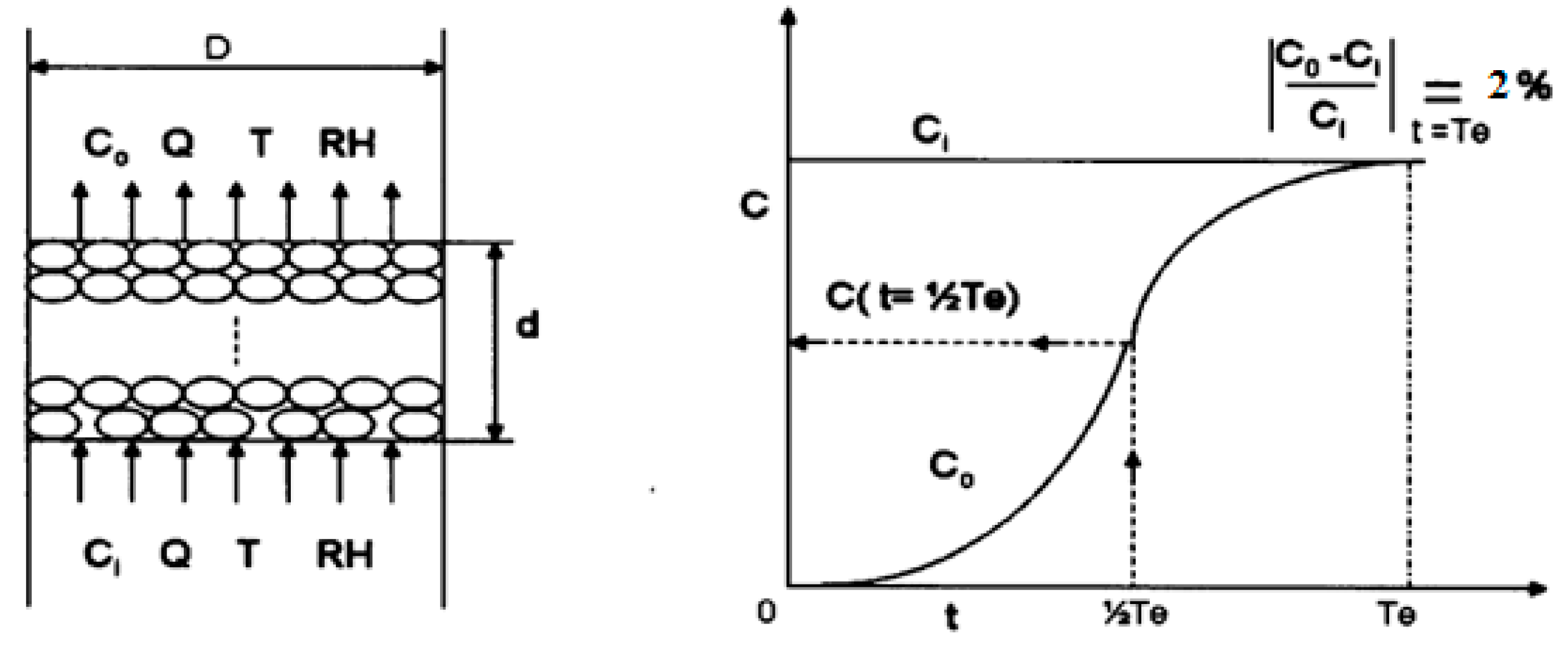
| Reagent and Amount | Solution (wt %) | DI Water (mL) | AC (g) |
|---|---|---|---|
| 10 g Ca(CH3CO2)2·H2O | 4.80% | 200 | 10 |
| Face Velocity (m/s) | Breakthrough Time vs. Initial Concentration |
|---|---|
| 0.1 | , R2 = 0.9982 |
| 0.2 | , R2 = 0.9963 |
| 0.3 | , R2 = 0.9909 |
| Face Velocity | Inlet Concentration | Pseudo-First Order | Pseudo-Second Order | Intra-Particle Diffusion | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| qe (exp, g) | k1 (min−1) | qe,1 (g) | r12 | k2 (g−1 min−1) | qe,2 (g) | r22 | ki (g min−1/2) | C | ri2 | ||
| 0.1 m/s | 800 ppm | 2.77 | 0.0082 | 2.73 | 0.7815 | 0.149 | 4.2 | 0.9519 | 0.3076 | −0.4487 | 0.9935 |
| 1000 ppm | 3.66 | 0.0119 | 3.64 | 0.866 | 0.2783 | 2.51 | 0.9844 | 0.2153 | −0.0979 | 0.9958 | |
| 1200 ppm | 4.54 | 0.0217 | 4.51 | 0.7843 | 0.5714 | 1.24 | 0.9888 | 0.1397 | −0.0266 | 0.9472 | |
| 0.2 m/s | 800 ppm | 2.28 | 0.011 | 2.24 | 0.8213 | 0.0777 | 5.65 | 0.9504 | 0.5096 | −1.0158 | 0.9811 |
| 1000 ppm | 3.01 | 0.0132 | 2.99 | 0.7827 | 0.1214 | 3.77 | 0.921 | 0.3727 | −0.6231 | 0.9771 | |
| 1200 ppm | 3.21 | 0.0324 | 3.16 | 0.8884 | 0.4141 | 1.58 | 0.9806 | 0.2182 | −0.0286 | 0.9975 | |
| 0.3 m/s | 800 ppm | 2.10 | 0.0108 | 2.10 | 0.7874 | 0.0496 | 6.96 | 0.9565 | 0.6473 | −1.4821 | 0.9671 |
| 1000 ppm | 2.42 | 0.0214 | 2.41 | 0.8874 | 0.253 | 2.36 | 0.9857 | 0.7353 | −0.5122 | 0.905 | |
| 1200 ppm | 2.77 | 0.0337 | 2.75 | 0.9074 | 0.33 | 1.73 | 0.9847 | 0.2657 | −0.1127 | 0.9888 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shiue, A.; Hu, S.-C.; Chang, S.-M.; Ko, T.-Y.; Hsieh, A.; Chan, A. Adsorption Kinetics and Breakthrough of Carbon Dioxide for the Chemical Modified Activated Carbon Filter Used in the Building. Sustainability 2017, 9, 1533. https://doi.org/10.3390/su9091533
Shiue A, Hu S-C, Chang S-M, Ko T-Y, Hsieh A, Chan A. Adsorption Kinetics and Breakthrough of Carbon Dioxide for the Chemical Modified Activated Carbon Filter Used in the Building. Sustainability. 2017; 9(9):1533. https://doi.org/10.3390/su9091533
Chicago/Turabian StyleShiue, Angus, Shih-Cheng Hu, Shu-Mei Chang, Tzu-Yu Ko, Arson Hsieh, and Andrew Chan. 2017. "Adsorption Kinetics and Breakthrough of Carbon Dioxide for the Chemical Modified Activated Carbon Filter Used in the Building" Sustainability 9, no. 9: 1533. https://doi.org/10.3390/su9091533




