Reusing Desulfurization Slag in Cement Clinker Production and the Influence on the Formation of Clinker Phases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Preparation of Cement Raw Mixes and Clinkers
2.3. Quantitative X-ray Diffraction Analysis
2.4. Other Analyses
3. Results and Discussion
3.1. Characterization of De-S Slag
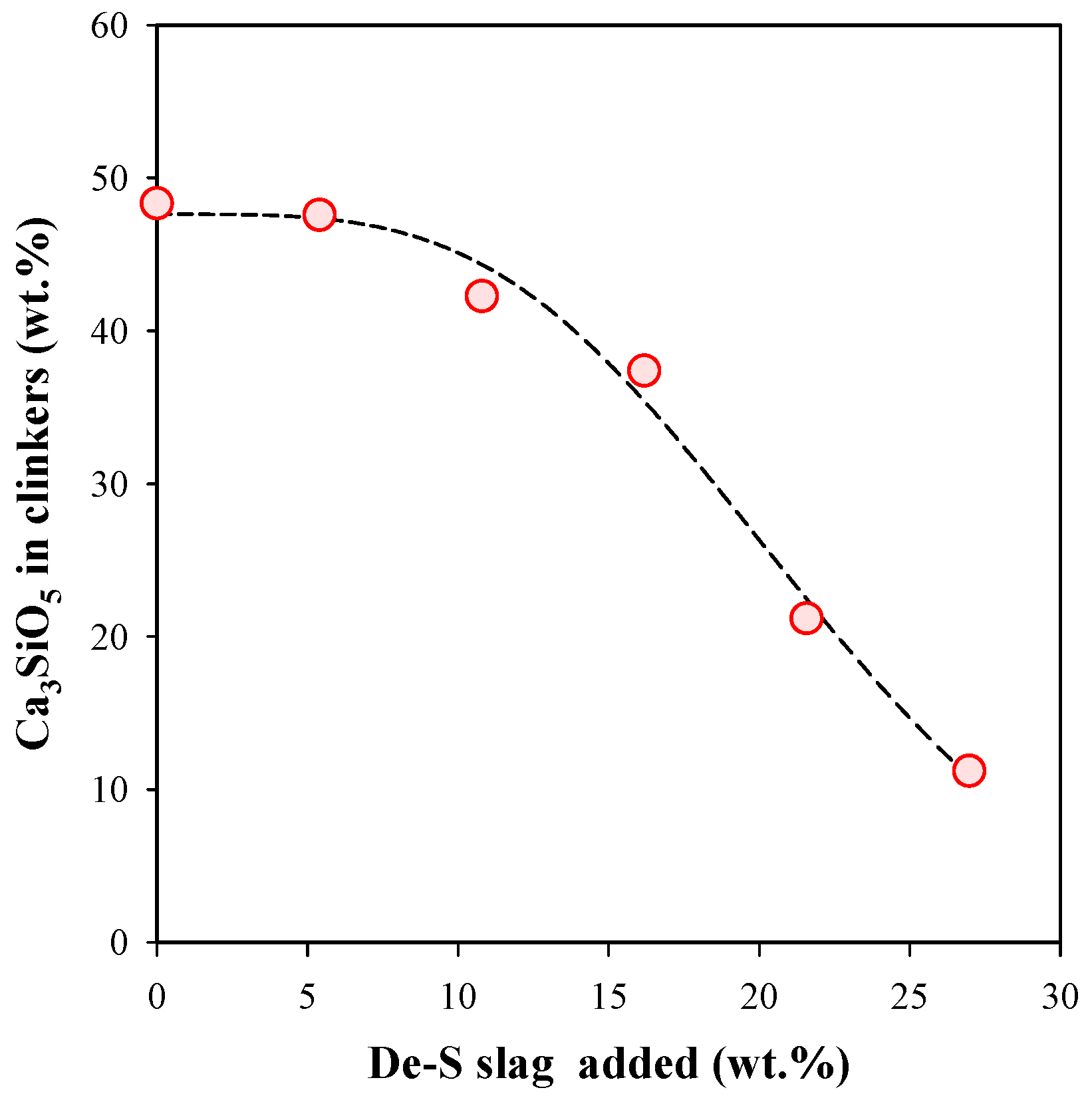
3.2. Influence of De-S slag Addition
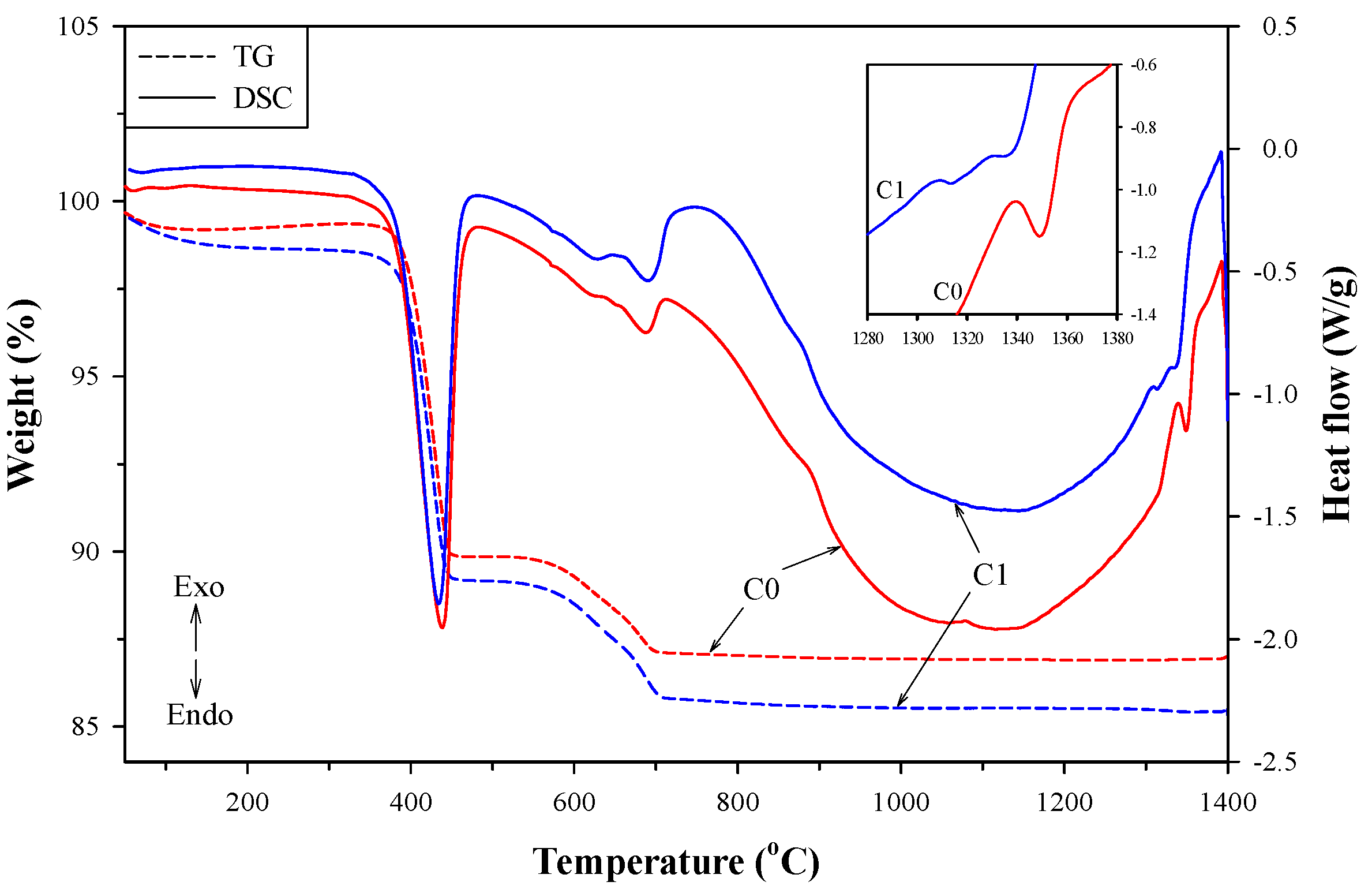
3.3. Mineralogical Composition of Clinkers at Different Temperatures
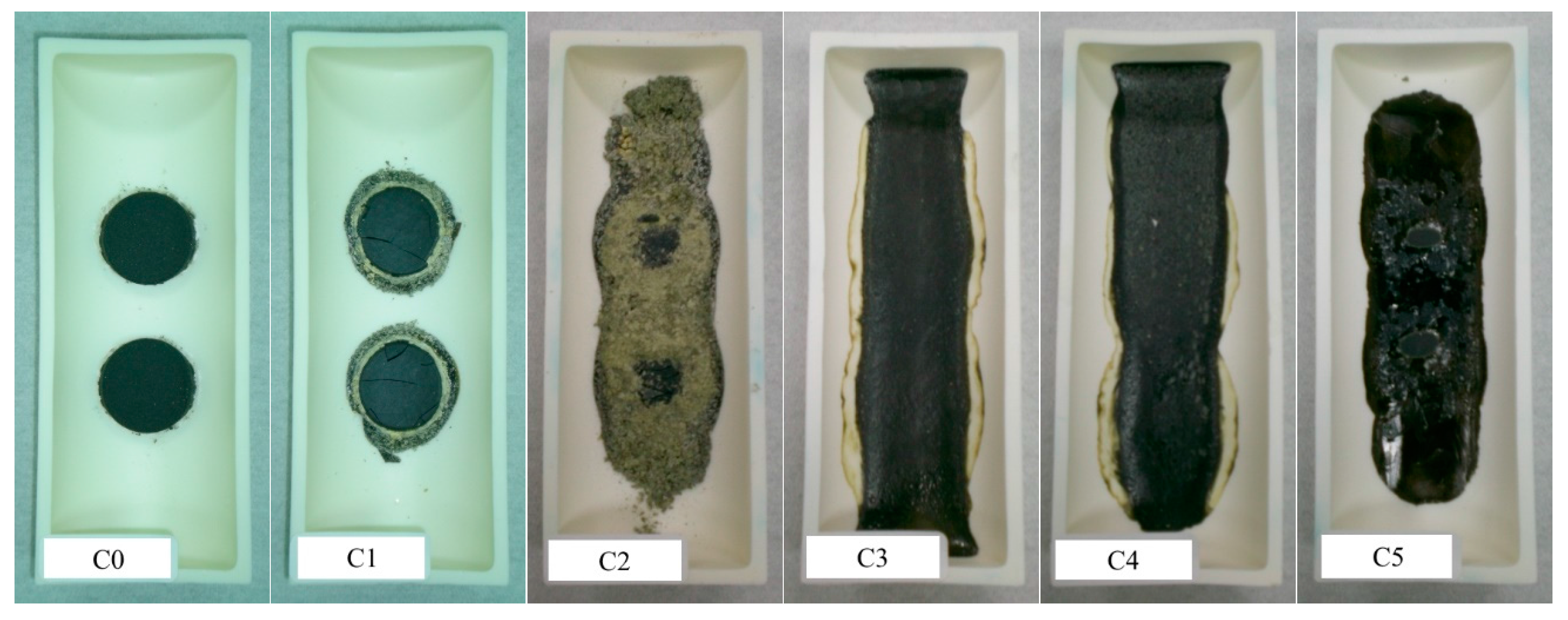
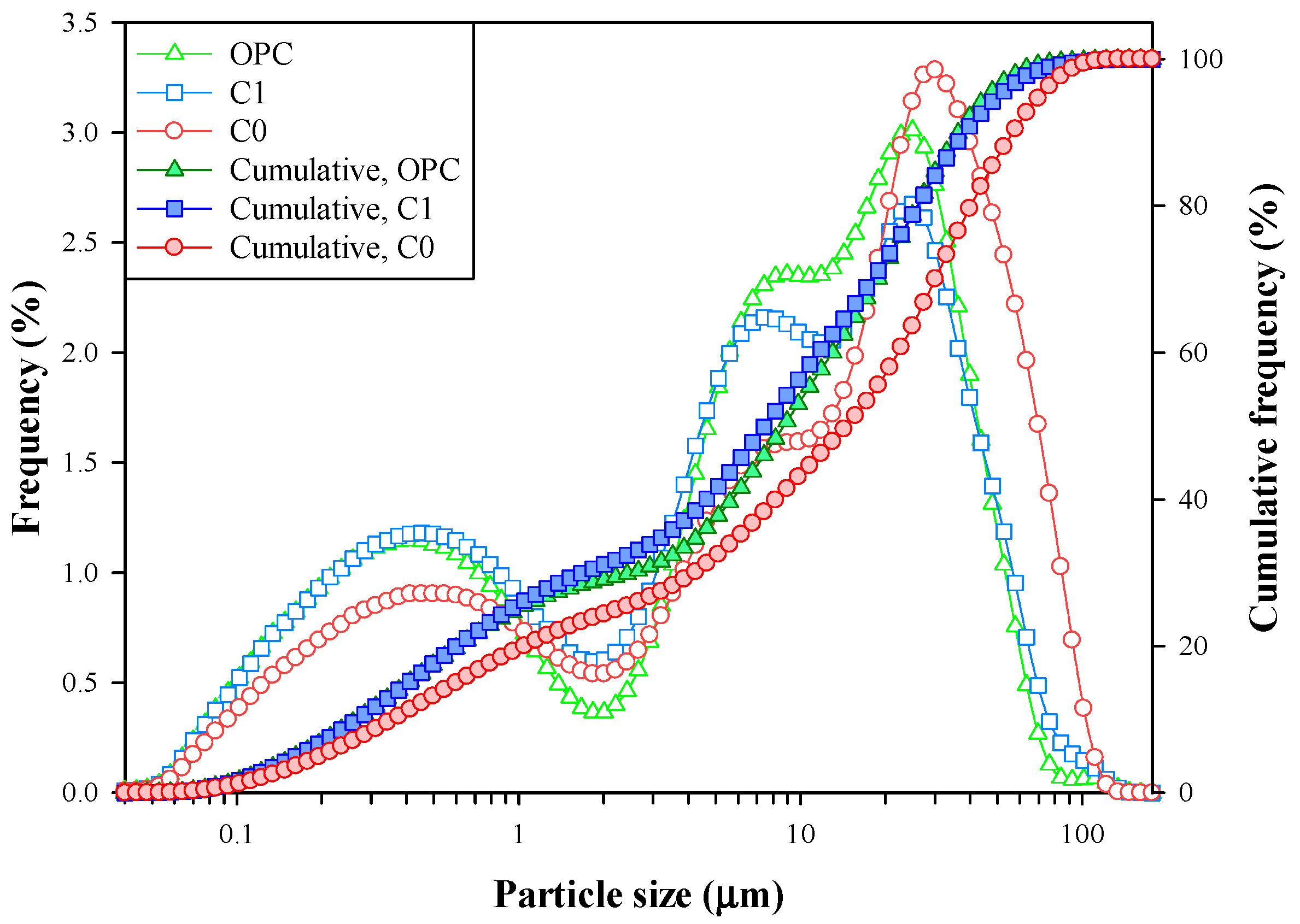
3.4. Description of Clinkerization and Grindability
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Van Oss, H.G.; Padovani, A.C. Cement Manufacture and the Environment: Part I: Chemistry and Technology. J. Ind. Ecol. 2002, 6, 89–105. [Google Scholar]
- European Cement Association (CEMBUREAU). Activity Report 2016; European Cement Association: Brussels, Belgium, 2016. [Google Scholar]
- Mikulčić, H.; Klemeš, J.J.; Vujanović, M.; Urbaniec, K.; Duić, N. Reducing greenhouse gasses emissions by fostering the deployment of alternative raw materials and energy sources in the cleaner cement manufacturing process. J. Clean. Prod. 2016, 136, 119–132. [Google Scholar] [CrossRef]
- Telschow, S.; Frandsen, F.; Theisen, K.; Dam-Johansen, K. Cement formation—A success story in a black box: High temperature phase formation of Portland cement clinker. Ind. Eng. Chem. Res. 2012, 51, 10983–11004. [Google Scholar] [CrossRef]
- Aruntaş, H.Y.; Gürü, M.; Dayı, M.; Tekin, İ. Utilization of waste marble dust as an additive in cement production. Mater. Des. 2010, 31, 4039–4042. [Google Scholar] [CrossRef]
- Chen, H.; Ma, X.; Dai, H. Reuse of water purification sludge as raw material in cement production. Cem. Concr. Compos. 2010, 32, 436–439. [Google Scholar] [CrossRef]
- Husillos Rodríguez, N.; Martínez-Ramírez, S.; Blanco-Varela, M.T.; Donatello, S.; Guillem, M.; Puig, J.; Fos, C.; Larrotcha, E.; Flores, J. The effect of using thermally dried sewage sludge as an alternative fuel on Portland cement clinker production. J. Clean. Prod. 2013, 52, 94–102. [Google Scholar] [CrossRef]
- Tsakiridis, P.E.; Agatzini-Leonardou, S.; Oustadakis, P. Red mud addition in the raw meal for the production of Portland cement clinker. J. Hazard. Mater. 2004, 116, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Shi, H.; Guo, X. Utilization of municipal solid waste incineration fly ash for sulfoaluminate cement clinker production. Waste Manag. 2011, 31, 2001–2008. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Romer, M.; Tschudin, M.; Bolio, H. Sustainable cement production—Present and future. Cem. Concr. Res. 2011, 41, 642–650. [Google Scholar] [CrossRef]
- Xu, W.; Xu, J.; Liu, J.; Li, H.; Cao, B.; Huang, X.; Li, G. The utilization of lime-dried sludge as resource for producing cement. J. Clean. Prod. 2014, 83, 286–293. [Google Scholar] [CrossRef]
- Monshi, A.; Asgarani, M.K. Producing Portland cement from iron and steel slags and limestone. Cem. Concr. Res. 1999, 29, 1373–1377. [Google Scholar] [CrossRef]
- Bernardo, G.; Marroccoli, M.; Nobili, M.; Telesca, A.; Valenti, G.L. The use of oil well-derived drilling waste and electric arc furnace slag as alternative raw materials in clinker production. Resour. Conserv. Recycl. 2007, 52, 95–102. [Google Scholar] [CrossRef]
- Tsakiridis, P.E.; Papadimitriou, G.D.; Tsivilis, S.; Koroneos, C. Utilization of steel slag for Portland cement clinker production. J. Hazard. Mater. 2008, 152, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.-J.; Wang, H.-Y.; Wei, C.-T. Engineering properties of controlled low strength desulfurization slags (CLSDS). Constr. Build. Mater. 2016, 115, 6–12. [Google Scholar] [CrossRef]
- Wu, Q.; You, R.; Clark, M.; Yu, Y. Pb(II) removal from aqueous solution by a low-cost adsorbent dry desulfurization slag. Appl. Surf. Sci. 2014, 314, 129–137. [Google Scholar] [CrossRef]
- Sheng, G.; Huang, P.; Wang, S.; Chen, G. Potential reuse of slag from the Kambara reactor desulfurization process of iron in an acidic mine drainage treatment. J. Environ. Eng. 2014, 140, 04014023. [Google Scholar] [CrossRef]
- Kolovos, K.; Loutsi, P.; Tsivilis, S.; Kakali, G. The effect of foreign ions on the reactivity of the CaO–SiO2–Al2O3–Fe2O3 system: Part I. Anions. Cem. Concr. Res. 2001, 31, 425–429. [Google Scholar] [CrossRef]
- Kolovos, K.; Tsivilis, S.; Kakali, G. The effect of foreign ions on the reactivity of the CaO–SiO2–Al2O3–Fe2O3 system: Part II: Cations. Cem. Concr. Res. 2002, 32, 463–469. [Google Scholar] [CrossRef]
- Kolovos, K.; Tsivilis, S.; Kakali, G. Study of clinker dopped with P and S compounds. J. Therm. Anal. Calorim. 2004, 77, 759–766. [Google Scholar] [CrossRef]
- Kacimi, L.; Simon-Masseron, A.; Ghomari, A.; Derriche, Z. Influence of NaF, KF and CaF2 addition on the clinker burning temperature and its properties. C. R. Chim. 2006, 9, 154–163. [Google Scholar] [CrossRef]
- Camargo, J.A. Fluoride toxicity to aquatic organisms: A review. Chemosphere 2003, 50, 251–264. [Google Scholar] [CrossRef]
- US Environmental Protection Agency (USEPA). Methods for Chemical Analysis of Water and Wastes, Method 340.2: Fluoride; US Environmental Protection Agency (USEPA): Cincinnati, OH, USA, 1991.
- USEPA. SW-846 Method 1311: Toxicity Characteristic Leaching Procedure; US Environmental Protection Agency (USEPA): Washington, DC, USA, 1992.
- Hillier, S. Use of an air brush to spray dry samples for X-ray powder diffraction. Clay Miner. 1999, 34, 127–135. [Google Scholar] [CrossRef]
- Cullity, B.D.; Stock, S.R. Elements of X-ray Diffraction; Prentice Hall: Upper Saddle River, NJ, USA, 2001. [Google Scholar]
- Jenkins, R.; Snyder, R.L. Introduction to X-ray Powder Diffractometry; John Wiley & Sons: New York, NY, USA, 1996. [Google Scholar]
- Snyder, R.L. The use of reference intensity ratios in X-ray quantitative analysis. Powder Diffr. 1992, 7, 186–193. [Google Scholar] [CrossRef]
- American Society for Testing and Materials (ASTM). D1857/D1857M-17 Standard Test Method for Fusibility of Coal and Coke Ash; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- Jeon, I.-Y.; Shin, S.-H.; Jung, S.-M.; Choi, H.-J.; Xu, J.; Baek, J.-B. One-pot purification and iodination of waste Kish graphite into high-quality electrocatalyst. Part. Part. Syst. Charact. 2017. [Google Scholar] [CrossRef]
- McKeown, D.A.; Muller, I.S.; Gan, H.; Pegg, I.L.; Stolte, W.C. Determination of sulfur environments in borosilicate waste glasses using X-ray absorption near-edge spectroscopy. J. Non-Cryst. Solids 2004, 333, 74–84. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Cement Chemistry, 2nd ed.; Thomas Telford: London, UK, 1997. [Google Scholar]
- Dominguez, O.; Torres-Castillo, A.; Flores-Velez, L.M.; Torres, R. Characterization using thermomechanical and differential thermal analysis of the sinterization of Portland clinker doped with CaF2. Mater. Charact. 2010, 61, 459–466. [Google Scholar] [CrossRef]
- Bădănoiu, A.; Paceagiu, J.; Voicu, G. Hydration and hardening processes of Portland cements obtained from clinkers mineralized with fluoride and oxides. J. Therm. Anal. Calorim. 2011, 103, 879–888. [Google Scholar] [CrossRef]
- Chan, C.J.; Kriven, W.M.; Young, J.F. Physical stabilization of the β→γ transformation in dicalcium silicate. J. Am. Ceram. Soc. 1992, 75, 1621–1627. [Google Scholar] [CrossRef]
- Yamashita, M.; Tanaka, H. Low-temperature burnt Portland cement clinker using mineralizer. Cem. Sci. Concr. Technol. 2011, 65, 82–87. [Google Scholar] [CrossRef]
- Zhou, Y.; Robl, T.; Henke, K. Synthesis of pure tricalcium silicate with calcium sulfate and calcium fluoride as mineralizers. J. Chin. Ceram. Soc. 2014, 42, 601–606. [Google Scholar]




| Phase | Chemical Formula | RIRcor | Peak | ||
|---|---|---|---|---|---|
| d-Spacing (Å) | h k l | Irel | |||
| Tricalcium silicate | Ca3SiO5 | 1.28 | 1.76 | 2 2 0 | 66.5 |
| β-dicalcium silicate | Ca2SiO4 | 0.76 | 2.88 | 1 2 0 | 27.8 |
| γ-dicalcium silicate | Ca2SiO4 | 1.30 | 4.32 | 0 2 1 | 33.9 |
| Corundum 1 | Al2O3 | 1.00 | 3.48 | 0 1 2 | 75.0 |
| Element | Weight Percentage (wt %) | Concentration in TCLP Extract (mg/L) |
|---|---|---|
| Ca | 48.92 ± 2.85 | 2980 ± 52 |
| Si | 17.83 ± 1.67 | 0.58 ± 0.13 |
| Al | 2.15 ± 0.29 | 0.07 ± 0.01 |
| Fe | 2.89 ± 0.24 | ND 1 |
| Mg | 0.37 ± 0.11 | ND 1 |
| Na | 2.63 ± 0.02 | 1.57 ± 0.01 |
| K | 0.61 ± 0.01 | 1.01 ± 0.02 |
| Mn | 0.12 ± 0.02 | ND 1 |
| Cu | 0.03 ± 0.01 | ND 1 |
| Zn | 0.22 ± 0.01 | ND 1 |
| Cr | ND 1 | ND 1 |
| Ni | ND 1 | ND 1 |
| Pb | ND 1 | ND 1 |
| Cd | ND 1 | ND 1 |
| S | 1.62 ± 0.19 | NA 2 |
| F | 0.39 ± 0.03 | NA 2 |
| Raw Mix | ST (°C) | FT (°C) |
|---|---|---|
| C0 | – 1 | – 1 |
| C1 | – 1 | – 1 |
| C2 | 1390 | – 1 |
| C3 | 1365 | 1390 |
| C4 | 1370 | 1395 |
| C5 | 1370 | 1390 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-L.; Chang, J.-E.; Ko, M.-S. Reusing Desulfurization Slag in Cement Clinker Production and the Influence on the Formation of Clinker Phases. Sustainability 2017, 9, 1585. https://doi.org/10.3390/su9091585
Chen Y-L, Chang J-E, Ko M-S. Reusing Desulfurization Slag in Cement Clinker Production and the Influence on the Formation of Clinker Phases. Sustainability. 2017; 9(9):1585. https://doi.org/10.3390/su9091585
Chicago/Turabian StyleChen, Ying-Liang, Juu-En Chang, and Ming-Sheng Ko. 2017. "Reusing Desulfurization Slag in Cement Clinker Production and the Influence on the Formation of Clinker Phases" Sustainability 9, no. 9: 1585. https://doi.org/10.3390/su9091585





