Estimating Net Primary Productivity (NPP) and Debris-Fall in Forests Using Lidar Time Series
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Lidar
2.3. GAM Predictors of Net Change in Canopy Carbon,
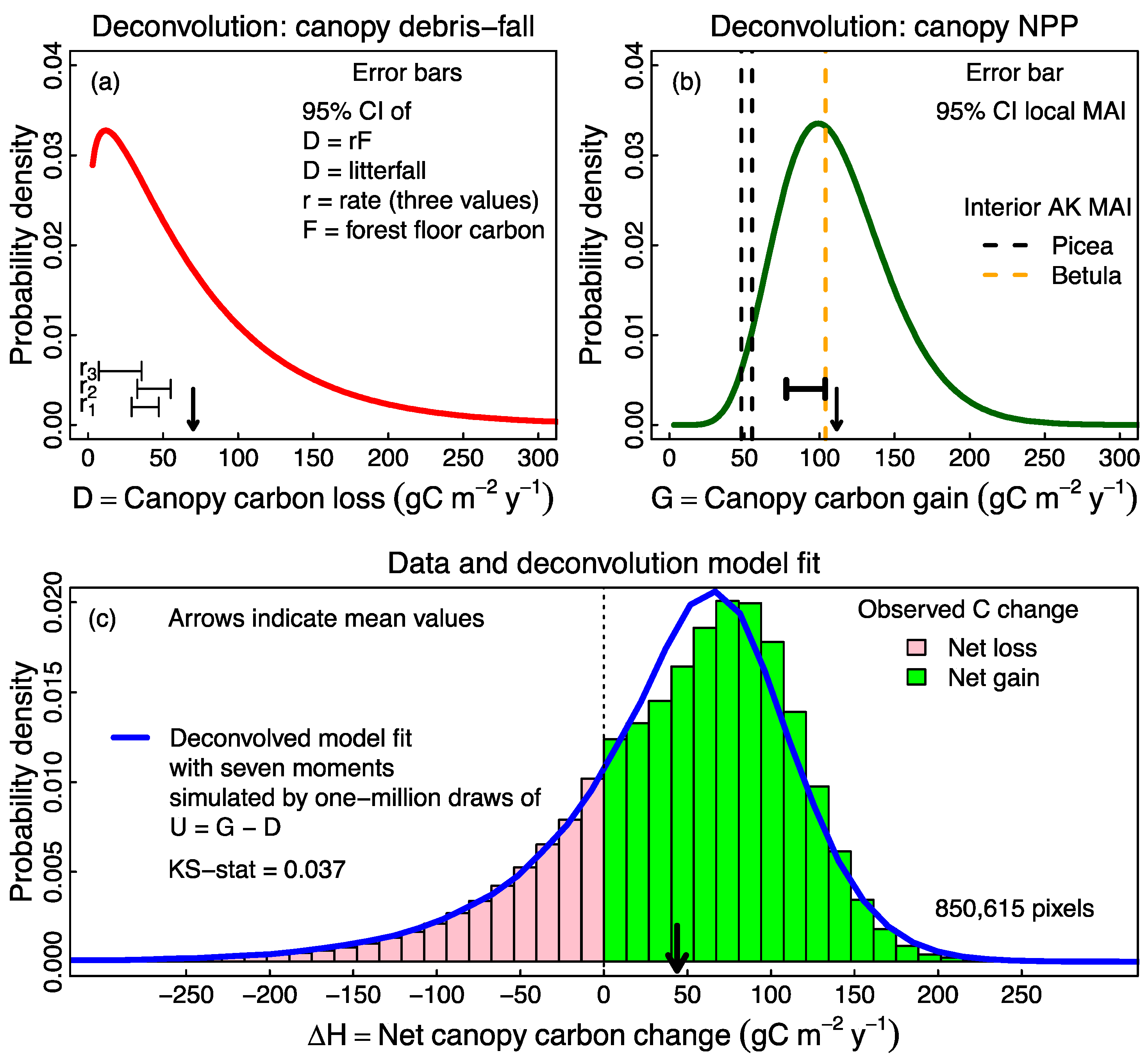
2.4. Probabilistic Deconvolution
3. Results
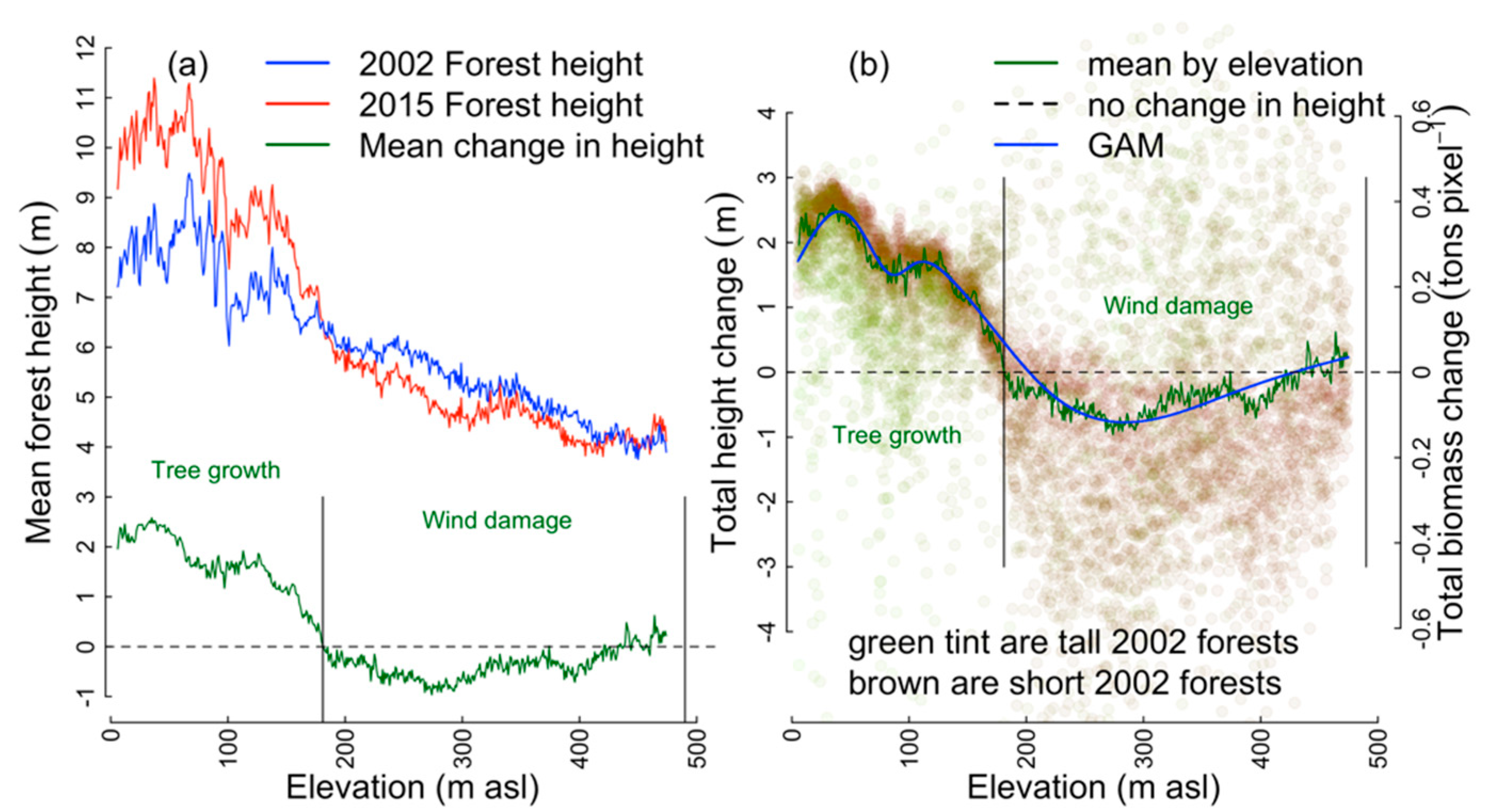
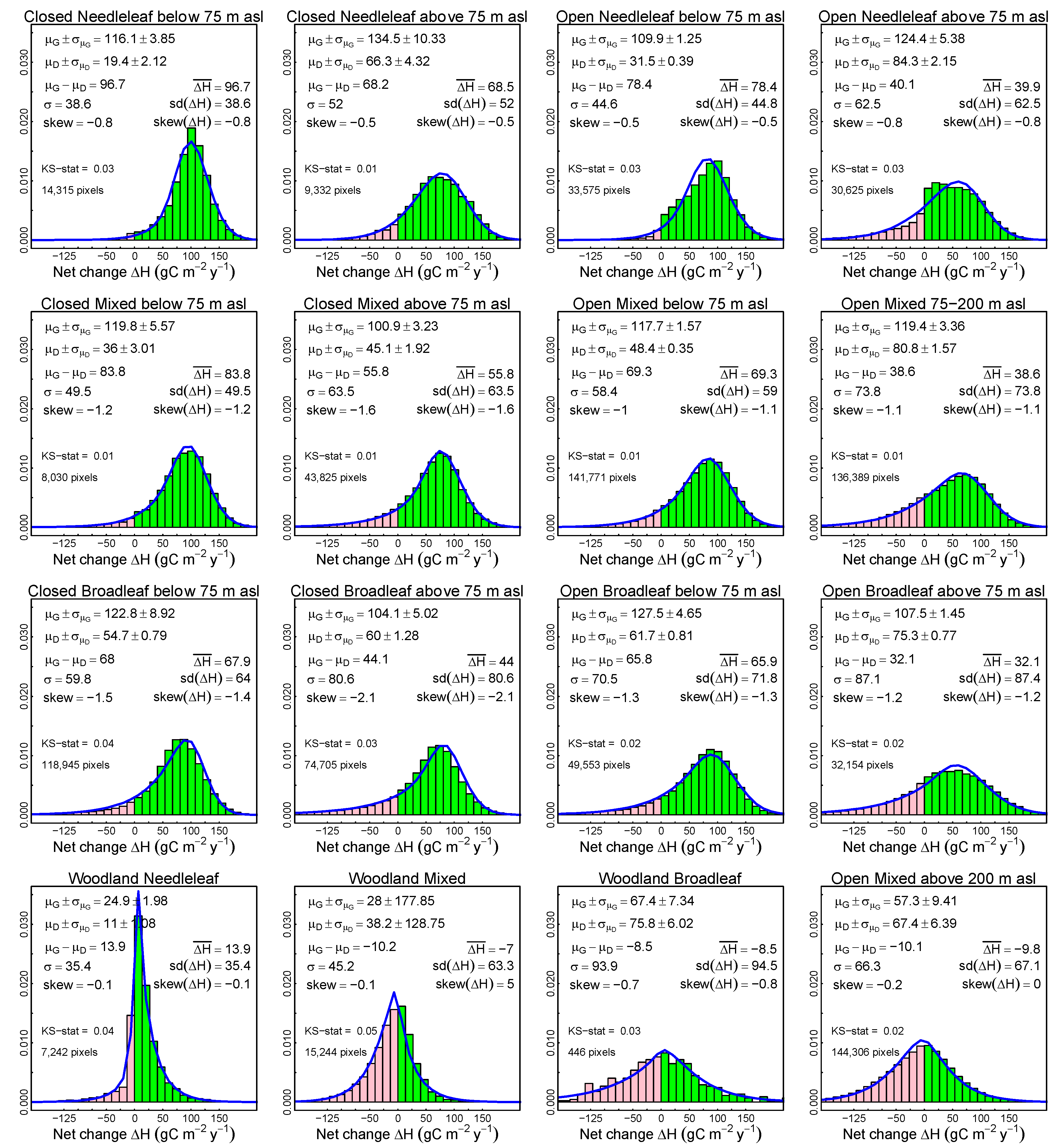
3.1. GAM Predictors of Net Change in Aboveground Tree Carbon,
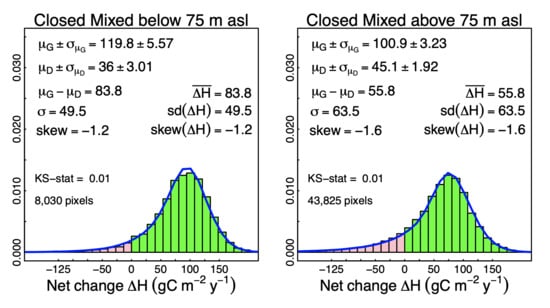
3.2. Probabilistic Deconvolution
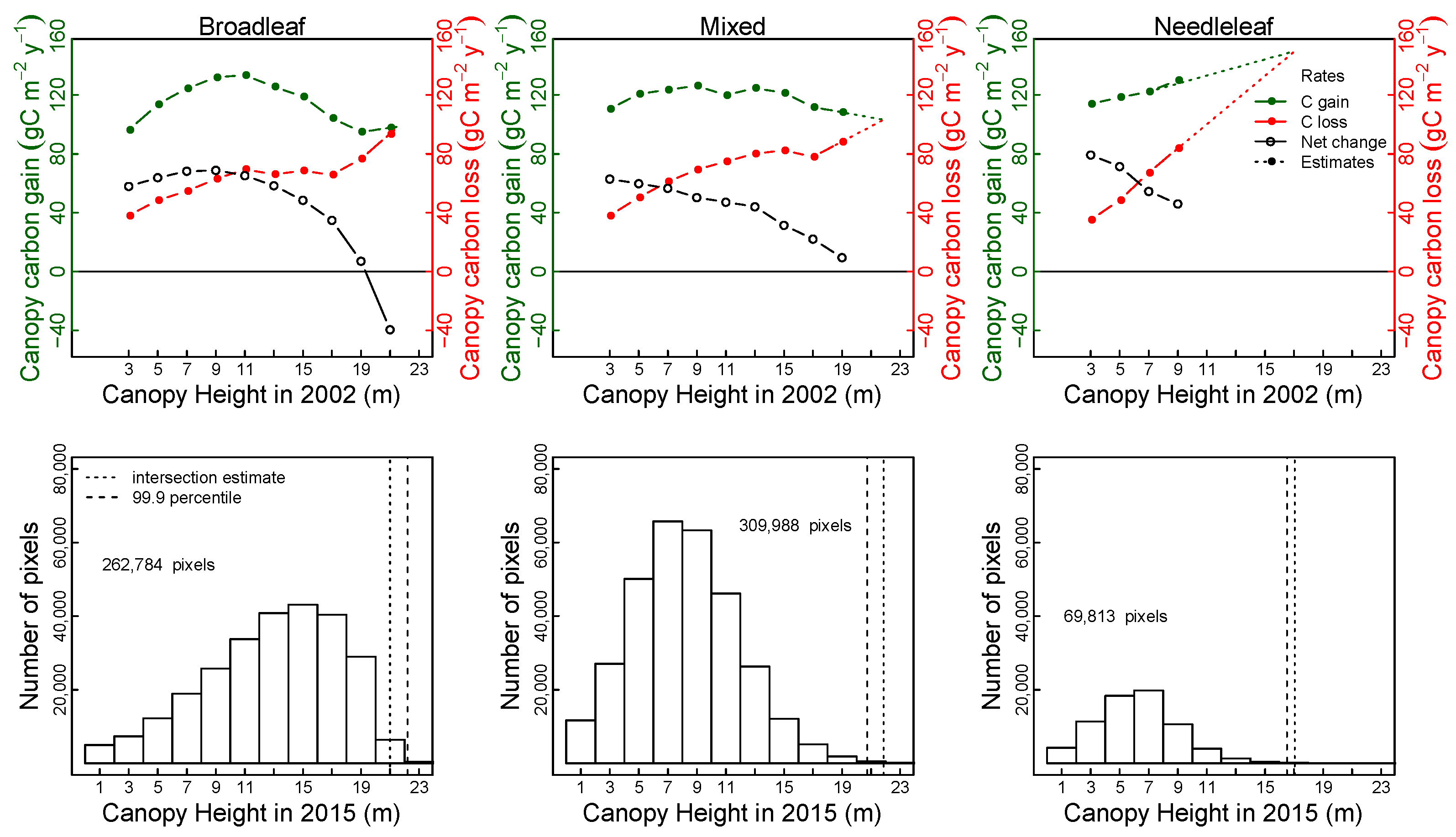
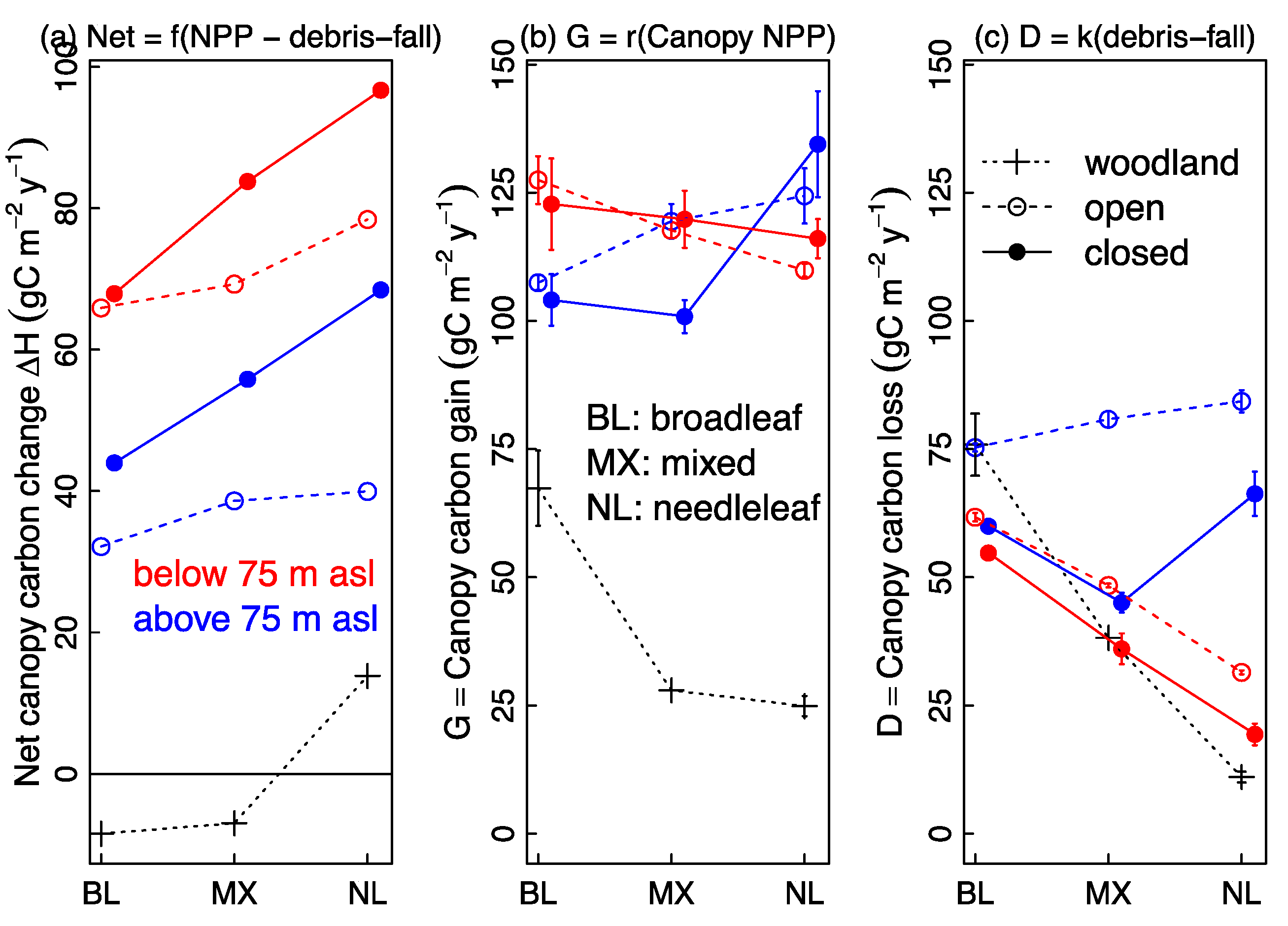
3.3. Predictors of Carbon Gain and Canopy Loss: Canopy Height and Taxonomic Composition
3.4. Predictors of C Gain and C Loss: Canopy Structure, Taxonomic Composition and Elevation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
Field Validation
References
- Vanderwel, M.C.; Zeng, H.; Caspersen, J.P.; Kunstler, G.; Lichstein, J.W. Demographic Controls of Aboveground Forest Biomass across North America. Ecol. Lett. 2016, 19, 414–423. [Google Scholar] [CrossRef]
- Foster, J.R.; Finley, A.O.; D’Amato, A.W.; Bradford, J.B.; Banerjee, S. Predicting Tree Biomass Growth in the Temperate-Boreal Ecotone: Is Tree Size, Age, Competition, or Climate Response Most Important? Glob. Chang. Biol. 2016, 22, 2138–2151. [Google Scholar] [CrossRef]
- Mencuccini, M.; Martínez-Vilalta, J.; Vanderklein, D.; Hamid, H.A.; Korakaki, E.; Lee, S.; Michiels, B. Size-Mediated Ageing Reduces Vigour in Trees: Size Reduces Vigour in Tall Trees. Ecol. Lett. 2005, 8, 1183–1190. [Google Scholar] [CrossRef]
- Gower, S.T.; McMurtrie, R.E.; Murty, D. Aboveground Net Primary Production Decline with Stand Age: Potential Causes. Trends Ecol. Evol. 1996, 11, 378–382. [Google Scholar] [CrossRef]
- Goetz, S.; Dubayah, R. Advances in Remote Sensing Technology and Implications for Measuring and Monitoring Forest Carbon Stocks and Change. Carbon Manag. 2011, 2, 231–244. [Google Scholar] [CrossRef]
- McRoberts, R.E.; Næsset, E.; Gobakken, T. Estimation for Inaccessible and Non-Sampled Forest Areas Using Model-Based Inference and Remotely Sensed Auxiliary Information. Remote Sens. Environ. 2014, 154, 226–233. [Google Scholar] [CrossRef]
- Nasset, E.; Gobakken, T. Estimating Forest Growth Using Canopy Metrics Derived from Airborne Laser Scanner Data. Remote Sens. Environ. 2005, 96, 453–465. [Google Scholar] [CrossRef]
- Koenderink, J.J.; van Doorn, A.J. Affine Structure from Motion. J. Opt. Soc. Am. A 1991, 8, 377. [Google Scholar] [CrossRef]
- Hyyppä, J.; Hyyppä, H.; Leckie, D.; Gougeon, F.; Yu, X.; Maltamo, M. Review of Methods of Small-footprint Airborne Laser Scanning for Extracting Forest Inventory Data in Boreal Forests. Int. J. Remote Sens. 2008, 29, 1339–1366. [Google Scholar] [CrossRef]
- Eitel, J.U.H.; Höfle, B.; Vierling, L.A.; Abellán, A.; Asner, G.P.; Deems, J.S.; Glennie, C.L.; Joerg, P.C.; LeWinter, A.L.; Magney, T.S.; et al. Beyond 3-D: The New Spectrum of Lidar Applications for Earth and Ecological Sciences. Remote Sens. Environ. 2016, 186, 372–392. [Google Scholar] [CrossRef] [Green Version]
- White, J.C.; Coops, N.C.; Wulder, M.A.; Vastaranta, M.; Hilker, T.; Tompalski, P. Remote Sensing Technologies for Enhancing Forest Inventories: A Review. Can. J. Remote Sens. 2016, 42, 619–641. [Google Scholar] [CrossRef] [Green Version]
- Hansen, L.P. Large Sample Properties of Generalized Method of Moments Estimators. Econometrica 1982, 50, 1029. [Google Scholar] [CrossRef]
- Chaussé, P. Computing Generalized Method of Moments and Generalized Empirical Likelihood with R. J. Stat. Softw. 2010, 34. [Google Scholar] [CrossRef] [Green Version]
- Cao, L.; Coops, N.C.; Innes, J.L.; Sheppard, S.R.J.; Fu, L.; Ruan, H.; She, G. Estimation of Forest Biomass Dynamics in Subtropical Forests Using Multi-Temporal Airborne LiDAR Data. Remote Sens. Environ. 2016, 178, 158–171. [Google Scholar] [CrossRef]
- Knapp, N.; Huth, A.; Kugler, F.; Papathanassiou, K.; Condit, R.; Hubbell, S.; Fischer, R. Model-Assisted Estimation of Tropical Forest Biomass Change: A Comparison of Approaches. Remote Sens. 2018, 10, 731. [Google Scholar] [CrossRef] [Green Version]
- Zhao, K.; Suarez, J.C.; Garcia, M.; Hu, T.; Wang, C.; Londo, A. Utility of Multitemporal Lidar for Forest and Carbon Monitoring: Tree Growth, Biomass Dynamics, and Carbon Flux. Remote Sens. Environ. 2018, 204, 883–897. [Google Scholar] [CrossRef]
- Tompalski, P.; Coops, N.; Marshall, P.; White, J.; Wulder, M.; Bailey, T. Combining Multi-Date Airborne Laser Scanning and Digital Aerial Photogrammetric Data for Forest Growth and Yield Modelling. Remote Sens. 2018, 10, 347. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Keenan, T.F.; Smith, M. Predictability of the Terrestrial Carbon Cycle. Glob. Chang. Biol. 2015, 21, 1737–1751. [Google Scholar] [CrossRef]
- Jorgensen, M.T.; Roth, J.E.; Schlentner, S.F.; Pullman, E.R.; Macander, M.; Racine, C.H. An. Ecological Land Survey for Fort Richardson, Alaska; ERDC/CRREL Report TR-03-19; ABR Inc.: Fairbanks, AK, USA, 2003. [Google Scholar]
- Dial, R.J.; Smeltz, T.S.; Rinas, C.; Golden, T.; Barnett, K.; Walker, A.H.; Geck, J.E. Ecological, Climatic, and Land Cover Trends on Joint Base Elmendorf-Richardson, Alaska: 1950-2012; 673D Air Base Wing, Natural Resources Element, Joint Base Elmendorf-Richardson, Alaska Pacific University: Anchorage, AK, USA, 2015. [Google Scholar]
- Smeltz, T.S.; Dial, R.J.; Golden, T. Report on Timber Cruising across Joint Base Elmendorf-Richardson, Alaska, 2013; 673D Air Base Wing, Natural Resources Element, Joint Base Elmendorf-Richardson, Alaska Pacific University: Anchorage, AK, USA, 2015. [Google Scholar]
- Smeltz, T.S.; Rinas, C.; Golden, T.; Barnett, K.; Dial, R.J. Long-term Vegetation Monitoring on Joint Base Elmendorf-Richardson, Alaska, 2013. A Survey of Vegetation, Birds, and Small Mammals; 673D Air Base Wing, Natural Resources Element, Joint Base Elmendorf-Richardson, Alaska Pacific University: Anchorage, AK, USA, 2014. [Google Scholar]
- Nowacki, G.J.; Spencer, P.; Fleming, M.; Brock, T.; Jorgenson, T. Unified Ecoregions of Alaska: 2001; (No. 2002-297); Geological Survey, 2003. [Google Scholar]
- Berg, E.E.; David Henry, J.; Fastie, C.L.; De Volder, A.D.; Matsuoka, S.M. Spruce Beetle Outbreaks on the Kenai Peninsula, Alaska, and Kluane National Park and Reserve, Yukon Territory: Relationship to Summer Temperatures and Regional Differences in Disturbance Regimes. For. Ecol. Manag. 2006, 227, 219–232. [Google Scholar] [CrossRef]
- Berg, E.E.; Anderson, R.S. Fire History of White and Lutz Spruce Forests on the Kenai Peninsula, Alaska, over the Last Two Millennia as Determined from Soil Charcoal. For. Ecol. Manag. 2006, 227, 275–283. [Google Scholar] [CrossRef]
- Bechtold, W.A.; Scott, C.T. The Forest Inventory and Analysis Plot Design.; Gen. Tech. Rep. SRS-80; U.S. Department of Agriculture, Forest Service, Southern Research Station: Asheville, NC, USA, 2005; pp. 37–52. [Google Scholar]
- Harter, H.L. Order Statistics and Their Use in Testing and Estimation; US Government Printing Office: Washington, DC, USA, 1970; Volume II. [Google Scholar]
- Magnussen, S.; Næsset, E.; Gobakken, T. LiDAR-Supported Estimation of Change in Forest Biomass with Time-Invariant Regression Models. Can. J. For. Res. 2015, 45, 1514–1523. [Google Scholar] [CrossRef]
- Luo, S.; Chen, J.M.; Wang, C.; Xi, X.; Zeng, H.; Peng, D.; Li, D. Effects of LiDAR Point Density, Sampling Size and Height Threshold on Estimation Accuracy of Crop Biophysical Parameters. Opt. Express 2016, 24, 11578. [Google Scholar] [CrossRef]
- Wood, S.N. Generalized Additive Models: An. Introduction with R., 2nd ed.; Chapman & Hall/CRC: Boca Raton, FL, USA, 2017; ISBN 978-1-4987-2833-1. [Google Scholar]
- Klar, B. 31_ A Note on Gamma Difference Distributions. J. Stat. Comput. Simul. 2015, 85, 3708–3715. [Google Scholar] [CrossRef]
- Yatskov, M.A. The Impact of litterfall on C Stores and Dynamics in Forests of Coastal Alaska. Ph.D. Dissertation, Oregon State University, Corvalis, OR, USA, 2016. [Google Scholar]
- Gower, S.T.; Krankina, O.; Olson, R.J.; Apps, M.; Linder, S.; Wang, C. Net Primary Production and C Allocation Patterns of Boreal Forest Ecosystems. Ecol. Appl. 2001, 11, 1395–1411. [Google Scholar] [CrossRef]
- Soetaert, K.; Herman, P.M.J. A Practical Guide to Ecological Modelling; Springer: Dordrecht, The Netherlands, 2009; ISBN 978-1-4020-8623-6. [Google Scholar]
- Komsta, L.; Novomestky, F. Moments, Cumulants, Skewness, Kurtosis and Related Tests, R package version 0.14; 2015. [Google Scholar]
- Dial, R.J.; Allgeier, M.; Lewis-Clark, E.; Day, T. Joint Base Elmendorf-Richardson Forest Carbon Stock Inventory: Past, Present, and Future (FXSB61518216); 673D Air Base Wing, Natural Resources Element, Joint Base Elmendorf-Richardson, Alaska Pacific University: Anchorage, AK, USA, 2018. [Google Scholar]
- Clark, D.A.; Brown, S.; Kicklighter, D.W.; Chambers, J.Q.; Thomlinson, J.R.; Ni, J. Measuring Net Primary Production in Forests: Concepts and Field Methods. Ecol. Appl. 2001, 11, 356–370. [Google Scholar] [CrossRef]
- Knorr, W.; Prentice, I.C.; House, J.I.; Holland, E.A. Long-Term Sensitivity of Soil Carbon Turnover to Warming. Nature 2005, 433, 298–301. [Google Scholar] [CrossRef]
- Koch, G.W.; Sillett, S.C.; Jennings, G.M.; Davis, S.D. The Limits to Tree Height. Nature 2004, 428, 851–854. [Google Scholar] [CrossRef]
- Avery, T.E.; Burkhart, H.E. Forest Measurements, 5th ed.; McGraw-Hill: New York, NY, USA, 2002. [Google Scholar]
- Raile, G.K. Estimating Stump Volume; Res. Pap. NC-224; U.S. Department of Agriculture, Forest Service, North Central Forest Experiment Station: St. Paul, MN, USA, 1982. [Google Scholar]
- Larson, F.R.; Winterberger, K.C. Tables and Equations for Estimating Volumes of Trees in the Susitna River Basin, Alaska; US Forest Service Research Note PNW: Portland, OR, USA, 1988. [Google Scholar]
- Jenkins, J.C.; Chojnacky, D.C.; Heath, L.S.; Birdsey, R. A National scale biomass estimators for United States tree species. Forest Sci. 2003, 49, 12–35. [Google Scholar]
- Jenkins, J.C.; Chojnacky, D.C.; Heath, L.S.; Birdsey, R.A. Comprehensive Database of Diameter-Based Biomass Regressions for North. American Tree Species. Gen. Tech. 15 Rep. NE-319; U.S. Department of Agriculture, Forest Service, Northeastern Research Station: Newtown Square, PA, USA, 2004. [Google Scholar]
- Woodall, C.W.; Monleon, V.J. Sampling, Estimation, and Analysis Procedures for the Down Woody Materials Indicator; USDA Forest Service General Technical Report NRS-22; Northern Research Station: Newtown Square, PA, USA, 2008. [Google Scholar]
- Woodall, C.W.; Heath, L.S.; Domke, G.M.; Nichols, M.C. Methods and Equations for Estimating Aboveground Volume, Biomass, and C for Trees in the U.S. Forest Inventory, 2010; General Technical Report NRS-88; 2011. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dial, R.; Chaussé, P.; Allgeier, M.; Smeltz, T.S.; Golden, T.; Day, T.; Wong, R.; Andersen, H.-E. Estimating Net Primary Productivity (NPP) and Debris-Fall in Forests Using Lidar Time Series. Remote Sens. 2021, 13, 891. https://doi.org/10.3390/rs13050891
Dial R, Chaussé P, Allgeier M, Smeltz TS, Golden T, Day T, Wong R, Andersen H-E. Estimating Net Primary Productivity (NPP) and Debris-Fall in Forests Using Lidar Time Series. Remote Sensing. 2021; 13(5):891. https://doi.org/10.3390/rs13050891
Chicago/Turabian StyleDial, Roman, Pierre Chaussé, Mallory Allgeier, Tom Scott Smeltz, Trevor Golden, Thomas Day, Russell Wong, and Hans-Erik Andersen. 2021. "Estimating Net Primary Productivity (NPP) and Debris-Fall in Forests Using Lidar Time Series" Remote Sensing 13, no. 5: 891. https://doi.org/10.3390/rs13050891