Arctic Ecological Classifications Derived from Vegetation Community and Satellite Spectral Data
Abstract
:1. Introduction
2. Methods
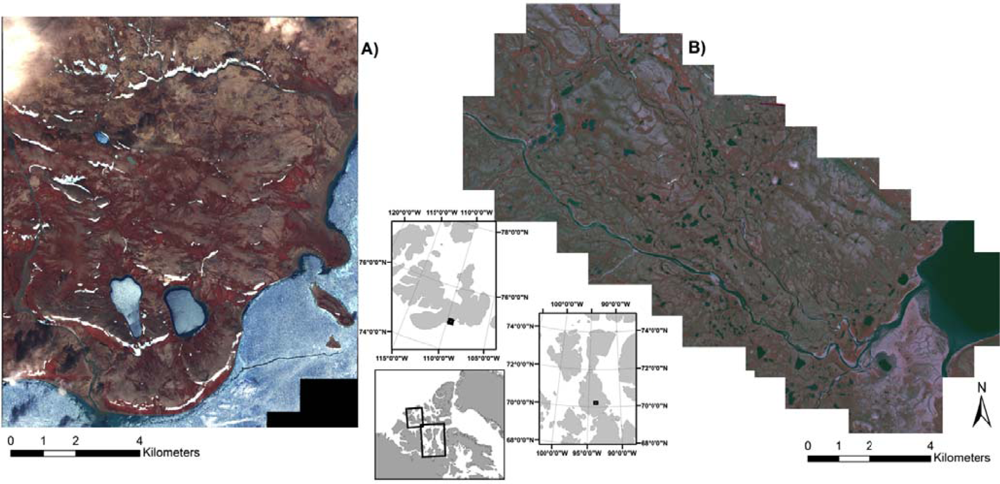
2.1. Study Area
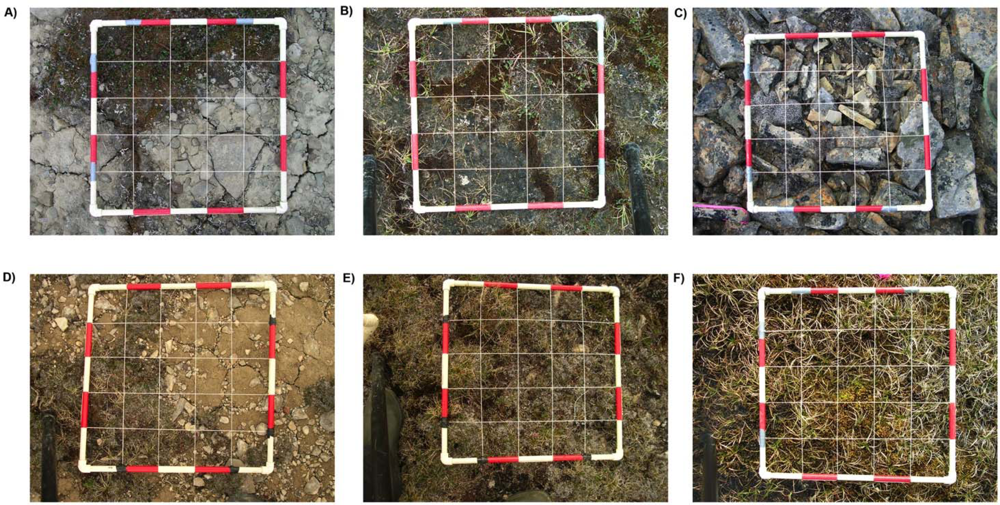
2.2. Data Collection
2.3. Multivariate Analysis Techniques
2.3.1. Correspondence Analysis (CA)
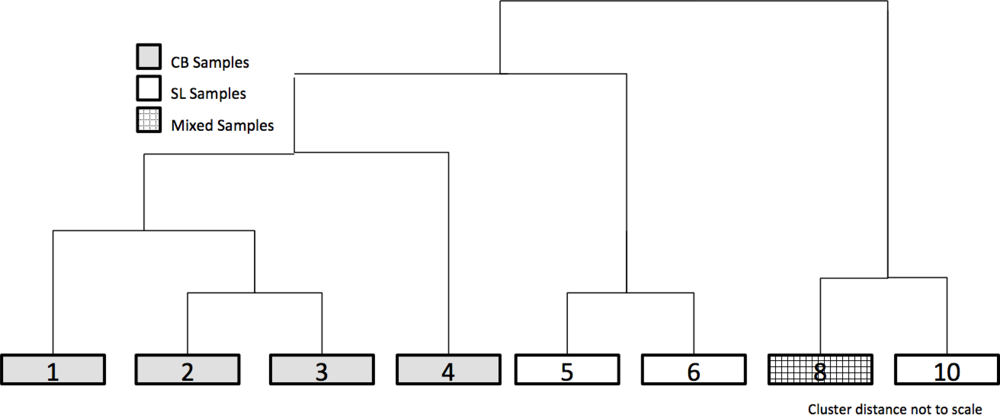
2.3.2. Clustering
2.3.3. Multi-Response Permutation Procedures (MRPP)
2.3.4. Spectral Separability Analysis (SSA)
- Clusters found to have good separability (i.e., J-M ≥ 1.9) from each other were not grouped together;
- Clusters that exhibited poor separability (i.e., J-M ≤ 1.0) with another cluster were grouped together; and
- Clusters with low separability (i.e., J-M > 1.0 and <1.9 were combined with the cluster for which they were most poorly separable if they had similar ecological characteristics (e.g., soil moisture, PVC, indicator species, etc.).
2.3.5. Indicator Species Analysis (ISA)
2.4. Ecological Classification and Error Assessment
3. Results
3.1. Correspondence Analysis (CA) for Community Delineation
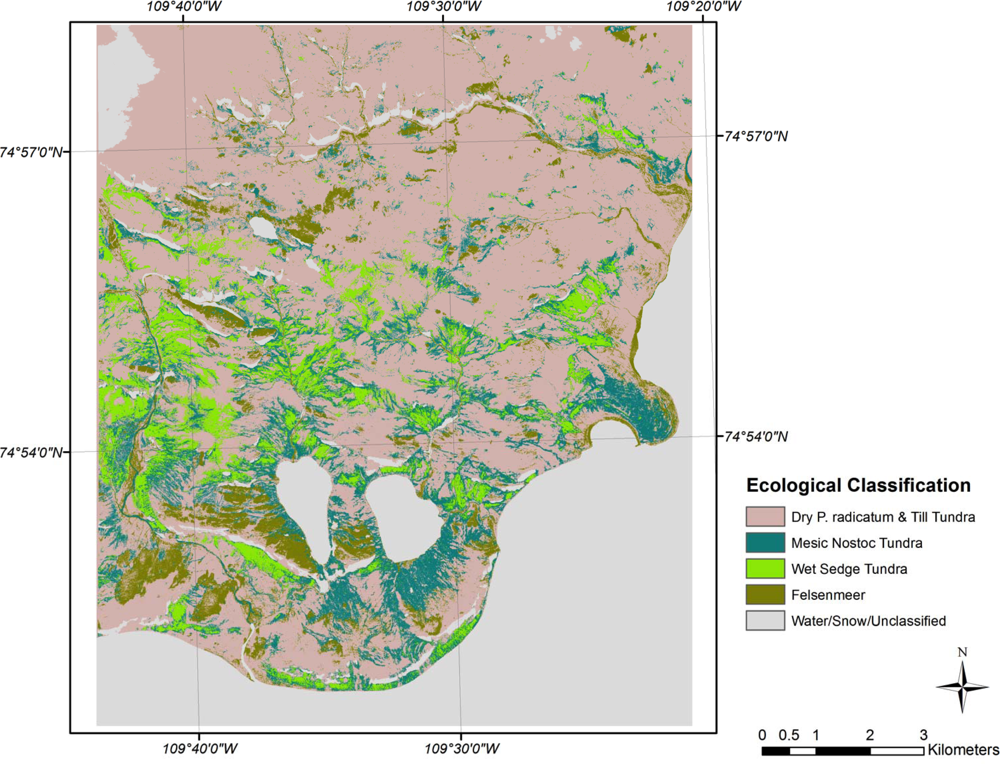
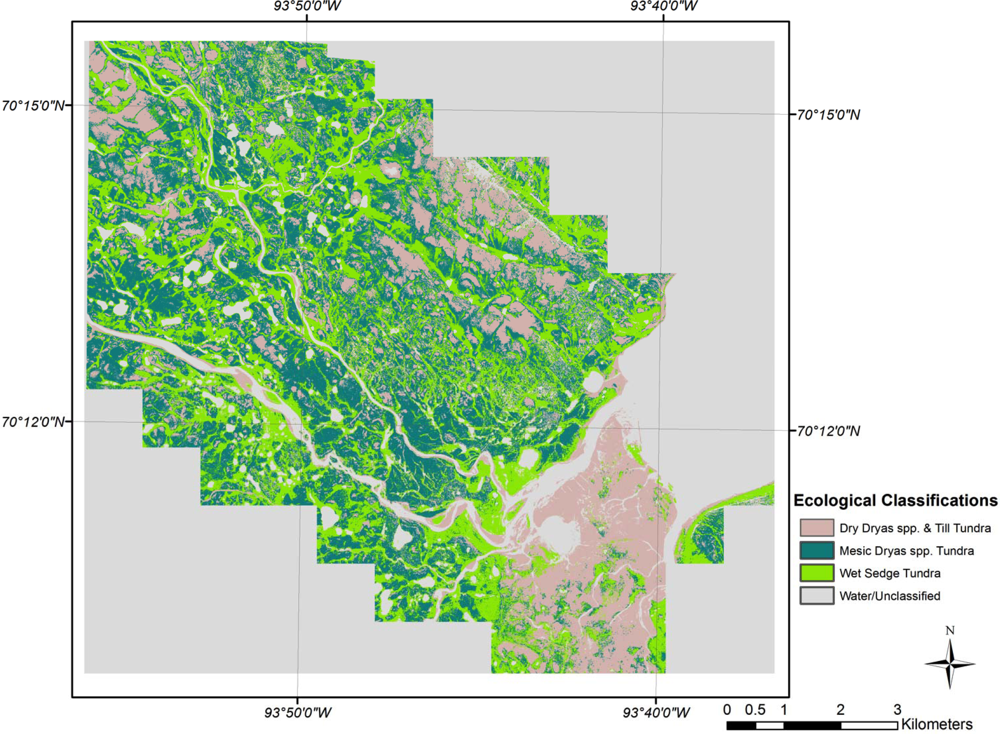
3.2. Ecological Classifications
4. Discussion
4.1. Ecological Classes
4.2. Suitability of the Techniques and Future Development
4. Conclusions
Acknowledgments
References
- Hope, A.S.; Kimball, J.S.; Stow, D.A. The relationship between tussock tundra spectral reflectance properties and biomass and vegetation composition. Int. J. Remote Sens 1993, 14, 1861–1874. [Google Scholar]
- Stow, D.A.; Hope, A.; McGuire, D.; Verbyla, D.; Gamon, J.; Huemmrich, F.; Houston, S.; Racine, C.; Sturm, M.; Tape, K.; et al. Remote sensing of vegetation and land-cover change in Arctic tundra ecosystems. Remote Sens. Environ 2004, 89, 281–308. [Google Scholar]
- Walker, D.A.; Raynolds, M.K.; Daniels, F.J.A.; Einarsson, E.; Elvebakk, A.; Gould, W.A.; Katenin, A.E.; Kholod, S.S.; Markon, C.J.; Melnikov, E.S.; et al. The circumpolar arctic vegetation map. J. Veg. Sci 2005, 16, 267–282. [Google Scholar]
- Intergovernmental Channel on Climate Change (IPCC). Working Group I: The Physical Science Basis of Climate Change. ARA 4 Report, Observations 4: Changes in Snow, Ice and Frozen Ground. 2007. Available online: http://ipcc-wg1.ucar.edu/wg1/wg1-report.html (accessed on 1 September 2012).
- ACIA. Arctic Climate Impact Assessment; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Shaver, G.R.; Street, L.E.; Rastetter, E.B.; Van Wijk, M.T.; Williams, M. Functional convergence in regulation of net CO2 in heterogeneous tundra landscapes in Alaska and Sweden. J. Ecol 2007, 95, 802–817. [Google Scholar]
- Dagg, J.; Lafleur, P. Vegetation community, foliar nitrogen, and temperature effects on Tundra CO2 exchange across a soil moisture gradient. Arct. Antarc. Alpine Res 2011, 43, 189–197. [Google Scholar]
- Ostendorf, B.; Reynolds, J.F. A model of arctic tundra vegetation derived from topographic gradients. Landscape Ecol 1998, 13, 187–201. [Google Scholar]
- Goetz, S.J.; Prince, S.D. Modeling terrestrial carbon exchange and storage: Evidence and implications of functional convergence in light-use efficiency. Adv. Ecol. Res 1999, 28, 57–92. [Google Scholar]
- McMichael, C.E.; Hope, A.S.; Stow, D.A.; Fleming, J.B.; Vourlitis, G.; Oechel, W. Estimating CO2 exchange at two sites in Arctic tundra ecosystems during the growing season using a spectral vegetation index. Int. J. Remote Sens 1999, 20, 683–698. [Google Scholar]
- Nobrega, S.; Grogan, P. Landscape and ecosystem-level controls on net carbon dioxide exchange along a natural moisture gradient in Canadian low arctic tundra. Ecosystems 2008, 11, 377–396. [Google Scholar]
- Braun-Blanquet, J. Plant Sociology: The Study of Plant Communities; Mcgraw-Hill: New York, NY, USA, 1965. [Google Scholar]
- Boelman, N.T.; Stieglitz, M.; Rueth, H.M.; Sommerkorn, M.; Griffin, K.L.; Shaver, G.R. Response of NDVI, biomass, and ecosystem gas exchange to long-term warming and fertilization in wet sedge tundra. Oecologia 2003, 135, 414–421. [Google Scholar]
- Williams, M.; Rastetter, E.B.; Shaver, G.R.; Hobbie, J.E.; Carpino, E.; Kwiatkowski, B.L. Primary production in an arctic watershed: An uncertainty analysis. Ecol. Appl 2001, 11, 1800–1816. [Google Scholar]
- Thomas, V.; Treitz, P.M.; Jelinski, D.; Miller, J.; Lafleur, P.; McCaughey, H. Image classification of a northern peatland complex using spectral and plant community data. Remote Sens. Environ 2002, 84, 83–99. [Google Scholar]
- Tieszen, L.L.; Reed, B.C.; Bliss, N.B.; Wylie, B.K.; Dejong, D.D. NDVI C3 and C4 production and distributions in Great Plains grassland land cover classes. Ecol. Appl 1997, 7, 59–78. [Google Scholar]
- Stow, D.A.; Hope, A.S.; Boynton, W.; Phinn, S.; Walker, D.; Auerbach, N.A. Satellite-derived vegetation index and cover type maps for estimating carbon dioxide flux for arctic tundra regions. Geomorphology 1998, 21, 313–327. [Google Scholar]
- Stow, D.A.; Daeschner, S.; Boynton, W.; Hope, A.S. Arctic tundra functional types by classification of single-date and AVHRR bi-weekly NDVI composite datasets. Int. J. Remote Sens 2000, 21, 1773–1779. [Google Scholar]
- Laidler, G.J.; Treitz, P. Biophysical remote sensing of arctic environments. Progr. Phys. Geogr 2003, 27, 44–68. [Google Scholar]
- Stow, D.A.; Burns, B.H.; Hope, A.S. Spectral spatial and temporal characteristics of arctic tundra reflectance. Int. J. Remote Sens 1993, 14, 2445–2462. [Google Scholar]
- Shippert, M.M.; Walker, D.A.; Auerbach, N.A.; Lewis, B.E. Biomass and leaf-area index maps derived from SPOT images for Toolik Lake and Imnavait Creek areas Alaska. Polar Rec 1995, 31, 147–154. [Google Scholar]
- Gould, W.A. Remote sensing of vegetation plant species richness and regional biodiversity hotspots. Ecol. Appl 2000, 10, 1861–1870. [Google Scholar]
- Lewis, M.M. Numeric classification as an aid to spectral mapping of vegetation communities. Plant Ecol 1998, 136, 133–149. [Google Scholar]
- Graetz, R.D. Remote Sensing of Terrestrial Ecosystem Structure: An Ecologist’s Pragmatic View. In Remote Sensing of Biosphere Function; Mooney, HA, Hobbs, RJ, Eds.; Springer-Verlag: New York, NY, USA, 1989; pp. 5–30. [Google Scholar]
- Roughgarden, J.; Running, S.W.; Matson, P.A. What does remote sensing do for Ecology? Ecology 1991, 72, 1918–1922. [Google Scholar]
- Wickland, D.E. Mission to planet earth: The ecological perspective. Ecology 1991, 72, 1923–1933. [Google Scholar]
- Ustin, S.L.; Adams, J.B.; Elvidge, C.D.; Rejmanek, M.; Rock, B.N.; Smith, M.O.; Thomas, R.W.; Woodward, R.A. Thematic mapper studies of semiarid shrub communities. BioScience 1986, 36, 446–452. [Google Scholar]
- Pando, M.; Lange, R.T.; Sparrow, A.D. Relations between reflectance in Landsat MSS wavebands and floristic composition of Australian chenopod rangelands. Int. J. Remote Sens 1992, 13, 1861–1867. [Google Scholar]
- Hobbs, R.J.; Wallace, J.F.; Campbell, N.A. Classification of vegetation in the Western Australian wheatbelt using Landsat MSS data. Vegetatio 1989, 80, 91–105. [Google Scholar]
- Ghitter, G.S.; Hall, R.J.; Franklin, S.E. Variability of Landsat Thematic Mapper data in boreal deciduous and mixedwood stands with conifer understorey. Int. J. Remote Sens 1995, 16, 2989–3002. [Google Scholar]
- Brook, R.K.; Kenkel, N.C. A multivariate approach to vegetation mapping of Manitoba’s Hudson Bay Lowlands. Int. J. Remote Sens 2002, 23, 4761–4776. [Google Scholar]
- Price, K.P.; Pyke, D.A.; Mendes, L. Shrub dieback in a semiarid ecosystem: The integration of remote sensing and geographic information systems for detecting vegetation change. Photogramm. Eng. Remote Sensing 1992, 58, 455–463. [Google Scholar]
- Catt, P.; Lange, R.T.; Sparrow, A.D. The Botanical and Spectral Separability of Arid Zone Rangeland Plant Communities on Landsat MSS Imagery: A Pilot Study in South Australia. Proceedings of Fourth Australasian Remote Sensing Conference, Adelaide, Australia, August 1987; 1, pp. 145–150.
- Toth, T.; Csillag, F.; Biehl, L.L.; Micheli, E. Characterisation of semi-vegetated salt-affected soils by means of field remote sensing. Remote Sens. Environ 1991, 37, 167–180. [Google Scholar]
- Lewis, M.M. Species composition related to spectral classification in an Australian hummock grassland. Int. J. Remote Sens 1994, 15, 3223–3239. [Google Scholar]
- Treitz, P.M.; Howarth, P.J.; Suffling, R.C.; Smith, P. Application of detailed ground information to vegetation mapping with high spatial resolution digital imagery. Remote Sens. Environ 1992, 42, 65–82. [Google Scholar]
- Jacobsen, A.; Nielson, A.A.; Ejrnaes, R.; Groom, G.B. Spectral identification of Danish grassland classes related to management and plant species composition. Proceedings of 4th International Airborne Remote Sensing Conference and Exhibition/21st Canadian Symposium On Remote Sensing, Ottawa, ON, Canada, 21–24 June 1999; 1, pp. 74–81.
- Anderson, M.J.; Clements, A. Resolving environmental disputes: a statistical method for choosing among competing cluster models. Ecol. Appl 2000, 10, 1341–1355. [Google Scholar]
- Hodgson, D.A.; Vincent, J.S.; Fyles, J.G. Quaternary Geology of Central Melville Island Northwest Territories; No 83–16; Geological Survey of Canada: Ottawa, ON, Canada, 1984. [Google Scholar]
- Billings, W.D. Arctic and alpine vegetation: similarities differences and susceptibility to disturbances. BioScience 1973, 23, 685–696. [Google Scholar]
- Walker, D.A.; Gould, W.A.; Maier, H.A.; Raynolds, M.K. The circumpolar arctic vegetation map: AVHRR-derived base maps environmental controls and integrated mapping procedures. Int. J. Remote Sens 2002, 23, 4551–4570. [Google Scholar]
- Laidler, G.J.; Treitz, P.M.; Atkinson, D.M. Remote Sensing of Arctic Vegetation: The relations between NDVI, spatial resolution, and vegetation cover on Boothia Peninsula, Nunavut. Arctic 2008, 61, 1–13. [Google Scholar]
- Wein, R.C.; Rencz, A.N. Plant cover and standing crop-sampling procedures for the Canadian high arctic. Arct. Alp. Res 1976, 8, 139–150. [Google Scholar]
- Chapin, F.S.; Bret-Harte, M.S.; Hobbie, S.E.; Zhong, H. Plant functional types as predictors of transient responses of arctic vegetation to global change. J. Veg. Sci 1996, 7, 347–358. [Google Scholar]
- Norum, R.A.; Miller, M. Gen. Tech. Rep. PNW-171: Measuring Fuel Moisture Content in Alaska: Standard Methods and Procedures; US Department of Agriculture, Forest Service Pacific Northwest Forest and Range Experiment Station: Portland, OR, USA, 1984. [Google Scholar]
- NASA (National Aeronautics and Space Administration). Landsat 7 Science Data User’s Handbook. 2002. Available online: http://landsathandbook.gsfc.nasa.gov (accessed on 31 August 2012).
- Taylor, M. IKONOS Planetary Reflectance and Mean Solar Exo-Atmospheric Irradiance. In IKONOS Planetary Reflectance QSOL Rev.1; Space Imaging Inc. (now GEOEYE Inc.): Herndon, VA, USA, 2005. [Google Scholar]
- Jongman, R.H.G.; ter Braak, C.J.F.; van Tongeren, O.F.R. (Eds.) Data Analysis in Community and Landscape Ecology; Cambridge University Press: Cambridge, UK, 1995.
- McCune, B.; Grace, J.B. Analysis of Ecological Communities; MJM Software Design: Gleneden Beach, OR, USA, 2002. [Google Scholar]
- Cumming, B. Personal Communication. Department of Biology, Queen’s University, Kingston, ON, Canada,. 2007.
- Equihua, M. Fuzzy clustering of ecological data. J. Ecol 1990, 78, 519–534. [Google Scholar]
- Nekola, J. Vascular plant compositional gradients within and between Iowa fens. J. Veg. Sci 2004, 15, 771–780. [Google Scholar]
- Ward, J.H. Hierarchical grouping to optimise an objective function. J. Amer. Stat Assoc 1963, 58, 236–244. [Google Scholar]
- Causton, D.R. An Introduction to Vegetation Analysis: Principles Practice and Interpretation; Unwin Hyman: Boston, MA, USA, 1988. [Google Scholar]
- Jensen, J.R. Introductory Digital Image Processing: A Remote Sensing Perspective, 3rd ed; Prentice Hall: Englewood Cliffs, NJ, USA, 2005. [Google Scholar]
- Bruzzone, L.; Roli, F.; Serpico, B. An extension of the Jeffreys-Matusita Distance to Multiclass Cases for feature selection. IEEE Trans. Geosci. Remote Sens 1995, 6, 1318–1321. [Google Scholar]
- Schott, J.R. Remote Sensing: The Image Chain Approach; Oxford Univ Press: Toronto, ON, Canada, 1997. [Google Scholar]
- Dufrene, M.; Legendre, P. Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol. Monogr 1997, 67, 345–366. [Google Scholar]
- Lepš, J.; Šmilauer, P. Multivariate Analysis of Ecological Data Using CANOCO; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Liengen, T.; Olsen, R. Nitrogen fixation by free-living cyanobacteria from different coastal sites in a high arctic tundra, Spitsbergen. Arct. Alp. Res 1997, 29, 470–477. [Google Scholar]
- Dickson, L.G. Constraints to nitrogen fixation by cryptogamic crusts in a polar desert ecosystem, Devon Island, NWT, Canada. Arct. Antarct. Alpine Res 2000, 32, 40–45. [Google Scholar]
- Shaver, G.R.; Chapin, F.S. Response to fertilization by various plant growth forms in an Alaskan tundra. Ecology 1980, 61, 662–675. [Google Scholar]
- Nadelhoffer, K.J.; Giblin, A.E.; Shaver, G.R.; Lirikins, A.E. Microbial Processes and Plant Nutrient Availability in Arctic Soils. In Arctic Ecosystems in a Changing Climate: An Ecophysiological Perspective; Chapin, F.S., III, Jefferies, R.L., Reynolds, J.F., Shaver, G.R., Svoboda, J., Eds.; Academic Press Inc: San Diego, CA, USA, 1992; pp. 281–300. [Google Scholar]
- Atkin, O.K. Reassessing the nitrogen relations of Arctic plants: A mini-review. Plant Cell Environ 1996, 19, 695–704. [Google Scholar]
- Alexander, V. A Synthesis of the IBP Tundra Biome Study of Nitrogen Fixation. In Soil Organisms and Decomposition in Tundra; Holding, A.J., Heal, O.W., MacLean, S.F., Flanagan, P.W., Eds.; Tundra Biome Steering Committee: Stockholm, Sweden, 1974; pp. 109–121. [Google Scholar]
- Lennihan, R.; Chapin, D.M.; Dickson, L.G. Nitrogen fixation and photosynthesis in high arctic forms of Nostoc commune. Can. J. Bot 1994, 72, 940–945. [Google Scholar]
- Solheim, B.; Endal, A.; Vigstad, H. Nitrogen fixation in Arctic vegetation and soils from Svalbard, Norway. Polar Biol 1996, 16, 35–40. [Google Scholar]
- Oberbauer, S.F.; Dawson, T.E. Water-Relations of Arctic Vascular Plants. In Arctic Ecosystems in a Changing Climate—An Ecophysiological Perspective; Chapin, S.F., III, Jefferies, R.L., Reynolds, J.F., Shaver, G.R., Svoboda, J., Chu, E.W., Eds.; Academic Press Inc.: San Diego, CA, USA, 1992; pp. 259–279. [Google Scholar]
- Bliss, L.C.; Matveyeva, N.V. Circumpolar Arctic Vegetation. In Arctic Ecosystems in a Changing Climate—An Ecophysiological Perspective; Chapin, S.F., III, Jefferies, R.L., Reynolds, J.F., Shaver, G.R., Svoboda, J., Chu, E.W., Eds.; Academic Press Inc.: San Diego, CA, USA, 1992; pp. 59–89. [Google Scholar]
- Walker, D.A. Hierarchical subdivision of arctic tundra based on vegetation response to climate, parent material, and topography. Global Change Biol 2000, 6, 9–34. [Google Scholar]
- Foody, G.M. Approaches for the production and evaluation of fuzzy land cover classifications from remotely sensed data. Int. J. Remote Sens 1996, 17, 1317–1340. [Google Scholar]
- Walker, D.A.; Epstein, H.E.; Jia, G.J.; Balser, A.; Copass, C.; Edwards, E.J.; Gould, W.A.; Hollingsworth, J.; Knudson, J.; Maier, H.A.; et al. Phytomass, LAI, and NDVI in northern Alaska: Relationships to summer warmth, soil pH, plant function types, and extrapolation to the circumpolar arctic. J. Geophys. Res 2003, 108, D2. [Google Scholar]
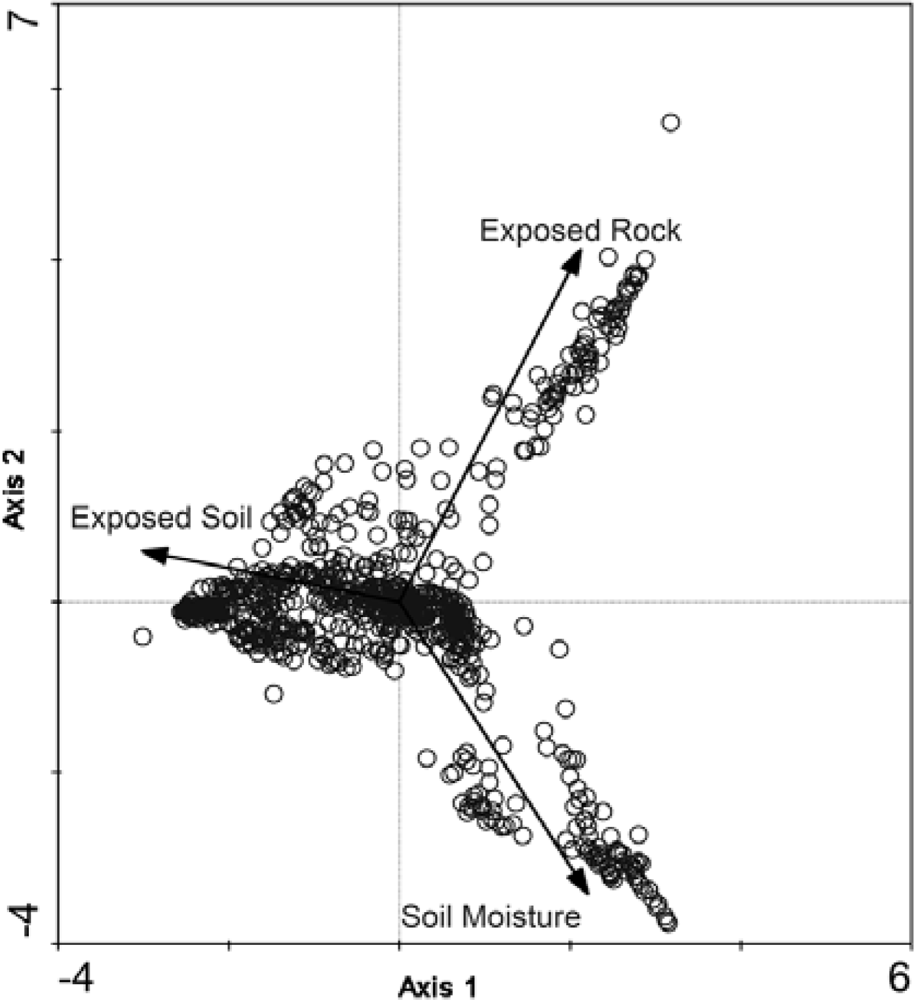
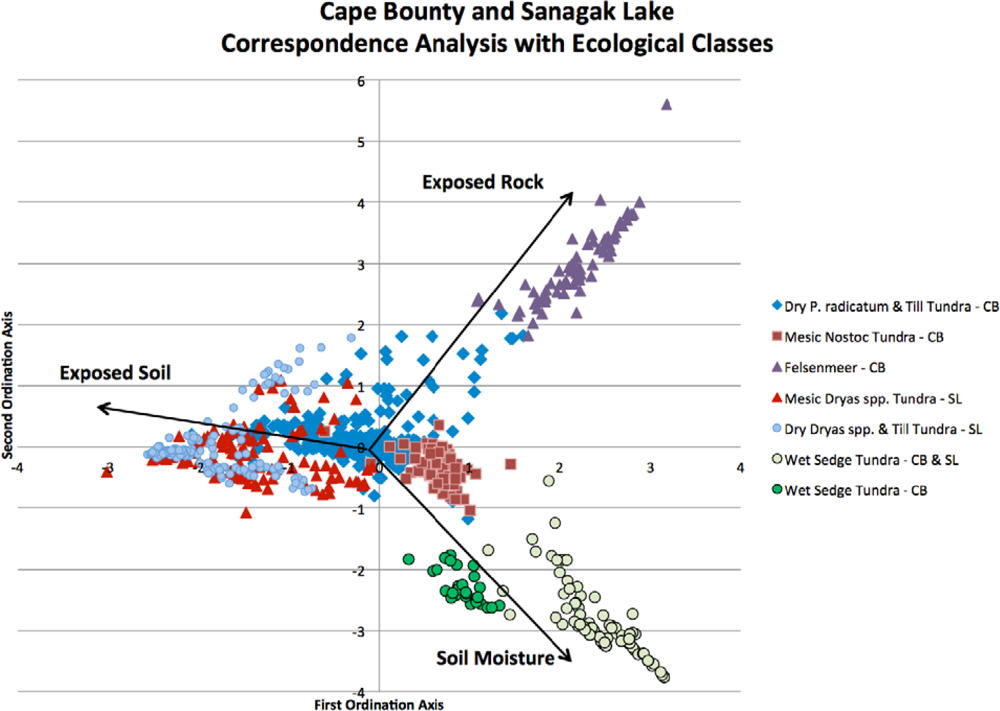
| CA Axis | 1 | 2 | 3 | 4 | Sum | Total Eigenvalue |
|---|---|---|---|---|---|---|
| Eigenvalue | 0.5 | 0.45 | 0.34 | 0.22 | 1.51 | 3.14 |
| % Variance | 16 | 14.4 | 10.9 | 7 | 48.3 |
| CB and SL CA | 1 | 2 | 3 | 4 | 5 | 6* | 8* | 10 |
| Cluster | ||||||||
| % Soil Moisture | 13.4 | 20.6 | 37.3 | 15.9 | 41.2 | 17.2 | 123.9 | 83.3 |
| PVC | 25.4 | 73.4 | 101.7 | 48.0 | 80.4 | 30.4 | 154.0 | 139.6 |
| Primary Cover | Till (75.4%) | Till (40.6%) | Nostoc commune (48.6%) | Rock (68.8%) | Dryas spp. (46.0%) | Till (60.5%) | Sphagnum spp (82.7%) | Sphagnum spp (46.8%) |
| Secondary Cover | Mosses (8.8%) | Mosses (27.8%) | Mosses (32.6%) | Mosses (22.1%) | Carex spp. (10.6%) | Dryas spp. (18.4%) | Eriophorum spp. (60.1%) | Dryas spp. (42.7%) |
| Primary IV | P. radicatu m (42.2) | Thamnolia subliformis (31.8) | Nostoc commune (49.0) | Umbilicari a spp. (91.3) | Dryas spp. (38.6) | n/a | Eriophorum spp. (56.4) | n/a |
| Secondary IV | Till (38.1) | Cetraria nivalis (20.7 ) | Salix arctica (29.8) | Rock (88.3) | Saxifraga oppositifola (17.8) | n/a | Sphagnum spp (31.1) | n/a |
| Majority Sites | Cape Bounty | Cape Bounty | Cape Bounty | Cape Bounty | Sanagak Lake | Sanagak Lake | Mixed | Sanagak Lake |
| CB and SL CA | 1 (Combined Cluster 1 & 2) | 3 | 4 | 5 | 6 | 8 | 10 Combined 8 & 10 (for Sanagak Lake Only) | |
| Final Cluster | ||||||||
| % Soil Moisture | 17.1 | 37.3 | 15.9 | 41.2 | 17.2 | 123.9 | 123.3 | |
| PVC | 50.2 | 101.7 | 48.0 | 80.4 | 30.4 | 154.0 | 144.8 | |
| Primary Cover | Till (57.6%) | Nostoc commune (48.6%) | Rock (68.8%) | Dryas spp. (46.0%) | Till (60.5%) | Sphagnum spp (82.7%) | Sphagnum spp (69.3%) | |
| Secondary Cover | Mosses (18.5%) | Mosses (32.6%) | Mosses (22.1%) | Carex spp. (10.6%) | Dryas spp. (18.4%) | Eriophorum spp. (60.1%) | Eriophorum spp. (47.5%) | |
| Primary IV | P. radicatum (38.4) | Nostoc commune (49.0) | Umbilicari a spp. (91.3) | Dryas spp. (38.6%) | n/a | Eriophorum spp. (56.4) | Eriophorum spp. (43) | |
| Secondary IV | Till (38.0) | Salix arctica (29.8) | Rock (88.3) | Saxifraga oppositifolia (17.8) | n/a | Sphagnum spp (31.1) | Sphagnum spp (27) | |
| Classification | Cape Bounty | Cape Bounty | Cape Bounty | Sanagak Lake | Sanagak Lake | Cape Bounty | Sanagak Lake | |
| Bioclimactic Class | P1 | G1 | n/a | G2 | P1 | W1 | W1 | |
| Ecological Class Name | Dry P. radicatum & Till Tundra | Mesic Nostoc Tundra | Felsenmeer | Mesic Dryas spp. Tundra | Dry Dryas spp. & Till Tundra | Wet Sedge Tundra | Wet Sedge Tundra | |
| Reference Class | # of Pixels | Percent Classified into Class | |||
|---|---|---|---|---|---|
| 1 (Dry P. radicatum & Till Tundra) | 3 (Mesic Nostoc Tundra) | 4 (Felsenmeer) | 8 (Wet Sedge Tundra) | ||
| Unclassified | 2 | 0.22 | 0 | 0 | 0 |
| 1 | 598 | 63.0 | 8.6 | 0 | 1.8 |
| 3 | 406 | 15.7 | 85.9 | 0 | 6.1 |
| 4 | 179 | 6.4 | 0 | 100 | 0 |
| 8 | 858 | 14.7 | 5.5 | 0 | 92.1 |
| Overall Accuracy | 79.1% | ||||
| Kappa Coefficient | 0.69 | ||||
| 95% Confidence Interval | 0.669–0.720 | ||||
| Reference Class | # of Pixels | Percent Classified into Class | |||
|---|---|---|---|---|---|
| 6 (Dry Dryas spp. & Till Tundra) | 5 (Mesic Dryas spp. & Till Tundra) | 10 (Wet Sedge Tundra) | |||
| Unclassified | 18 | 0 | 2.6 | 1.0 | |
| 6 | 523 | 68.9 | 25.5 | 2.6 | |
| 5 | 487 | 28.7 | 55.3 | 0 | |
| 10 | 405 | 2.4 | 16.6 | 96.4 | |
| Overall Accuracy | 69.2% | ||||
| Kappa Coefficient | 0.54 | ||||
| 95% Confidence Interval | 0.509–0.583 | ||||
Share and Cite
Atkinson, D.M.; Treitz, P. Arctic Ecological Classifications Derived from Vegetation Community and Satellite Spectral Data. Remote Sens. 2012, 4, 3948-3971. https://doi.org/10.3390/rs4123948
Atkinson DM, Treitz P. Arctic Ecological Classifications Derived from Vegetation Community and Satellite Spectral Data. Remote Sensing. 2012; 4(12):3948-3971. https://doi.org/10.3390/rs4123948
Chicago/Turabian StyleAtkinson, David M., and Paul Treitz. 2012. "Arctic Ecological Classifications Derived from Vegetation Community and Satellite Spectral Data" Remote Sensing 4, no. 12: 3948-3971. https://doi.org/10.3390/rs4123948




