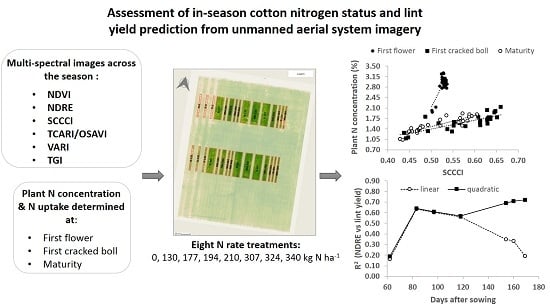
Assessment of In-Season Cotton Nitrogen Status and Lint Yield Prediction from Unmanned Aerial System Imagery
Abstract
:1. Introduction
1.1. Sensitive Wavebands to Crop Nitrogen Deficiency
1.2. Unmanned Aerial Systems for Monitoring Crop Performance
2. Material and Methods
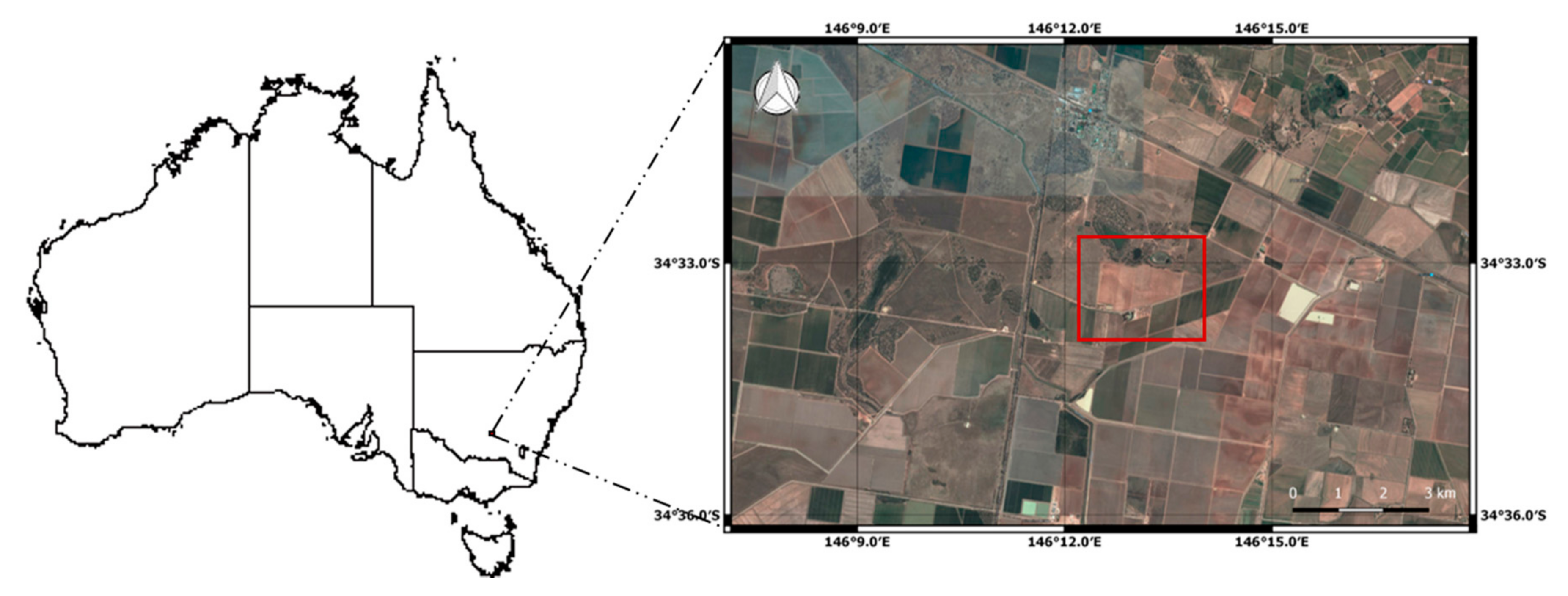
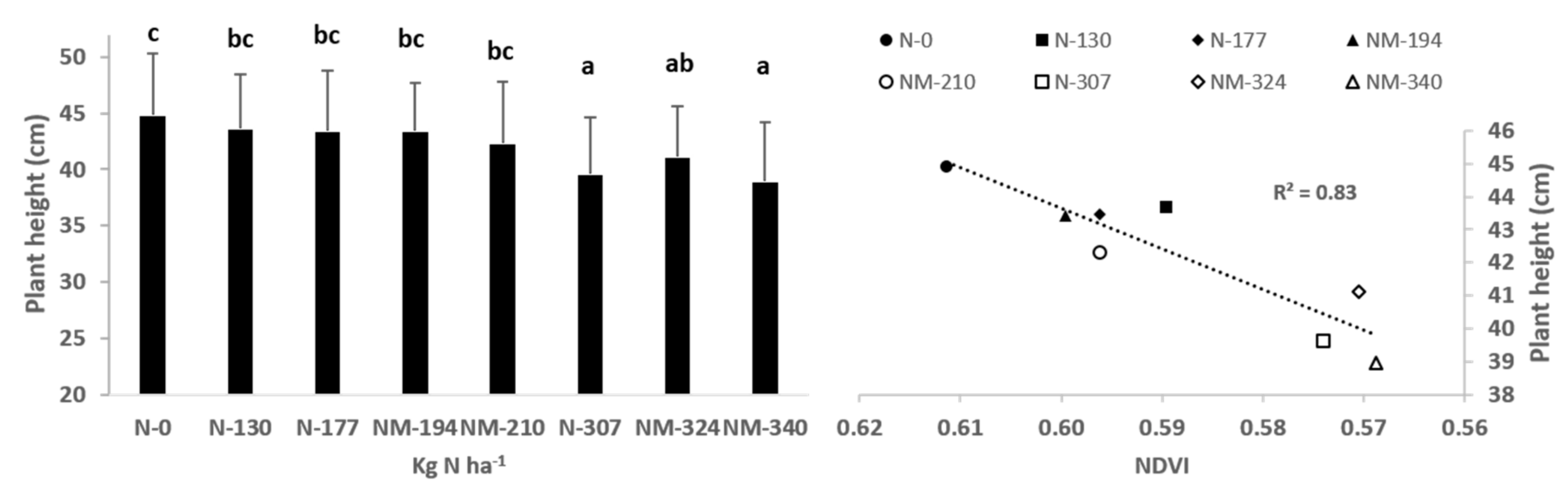
2.1. Experimental Site and Fertilizer Treatments Applied
2.2. In-Field Determinations
2.3. Multispectral Imagery Acquisition and Processing
2.4. Statistical Analysis
3. Results
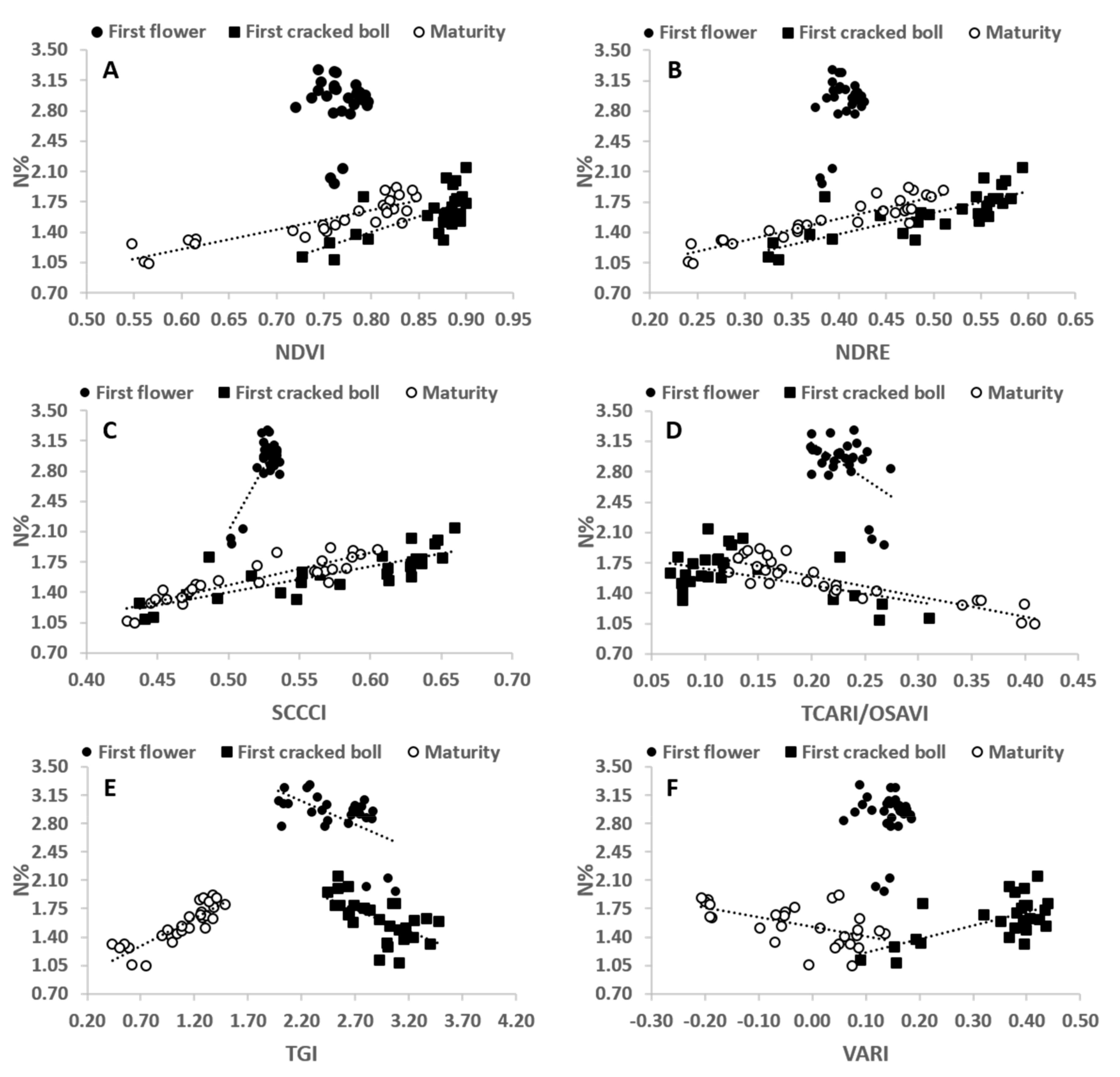
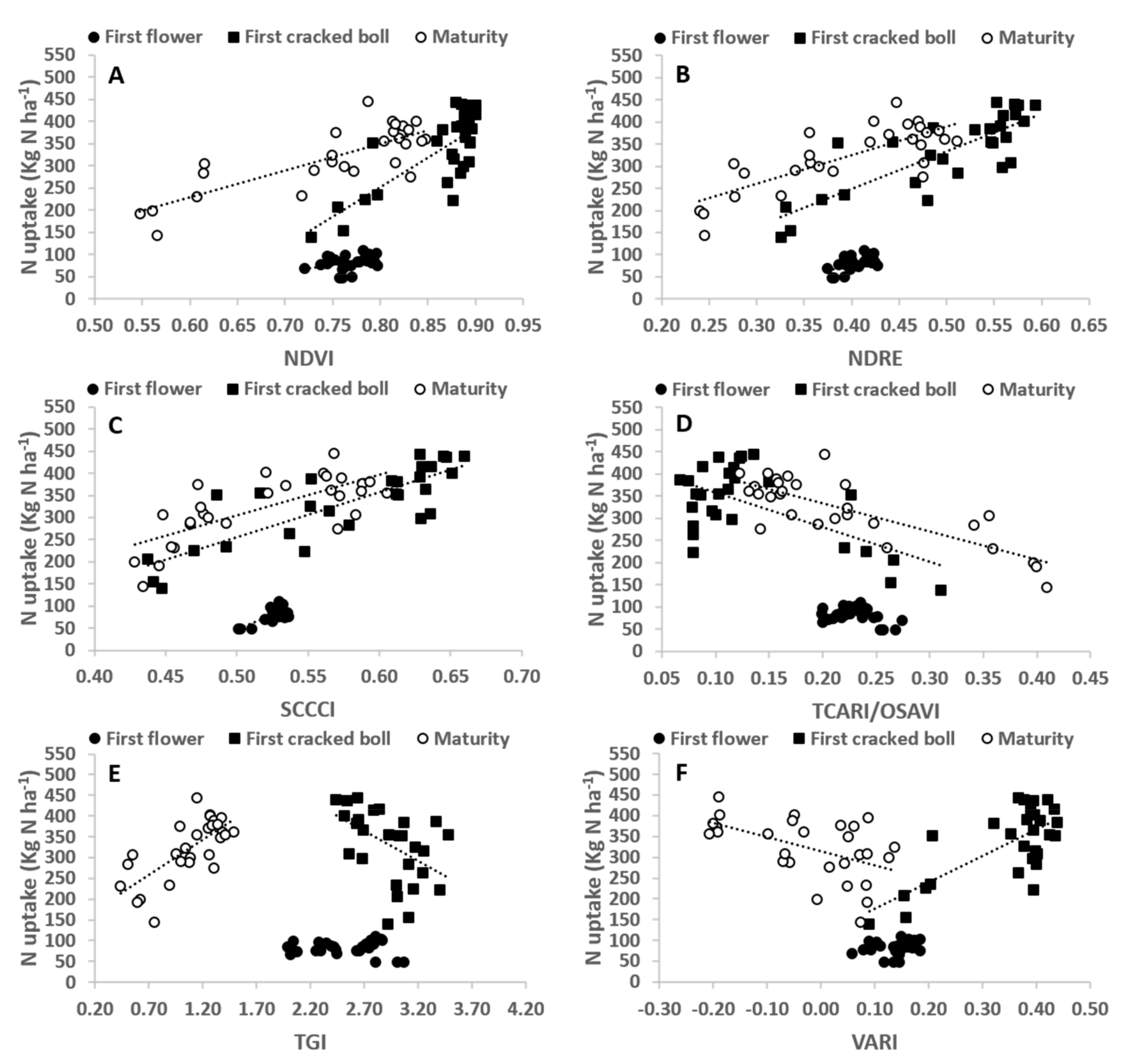
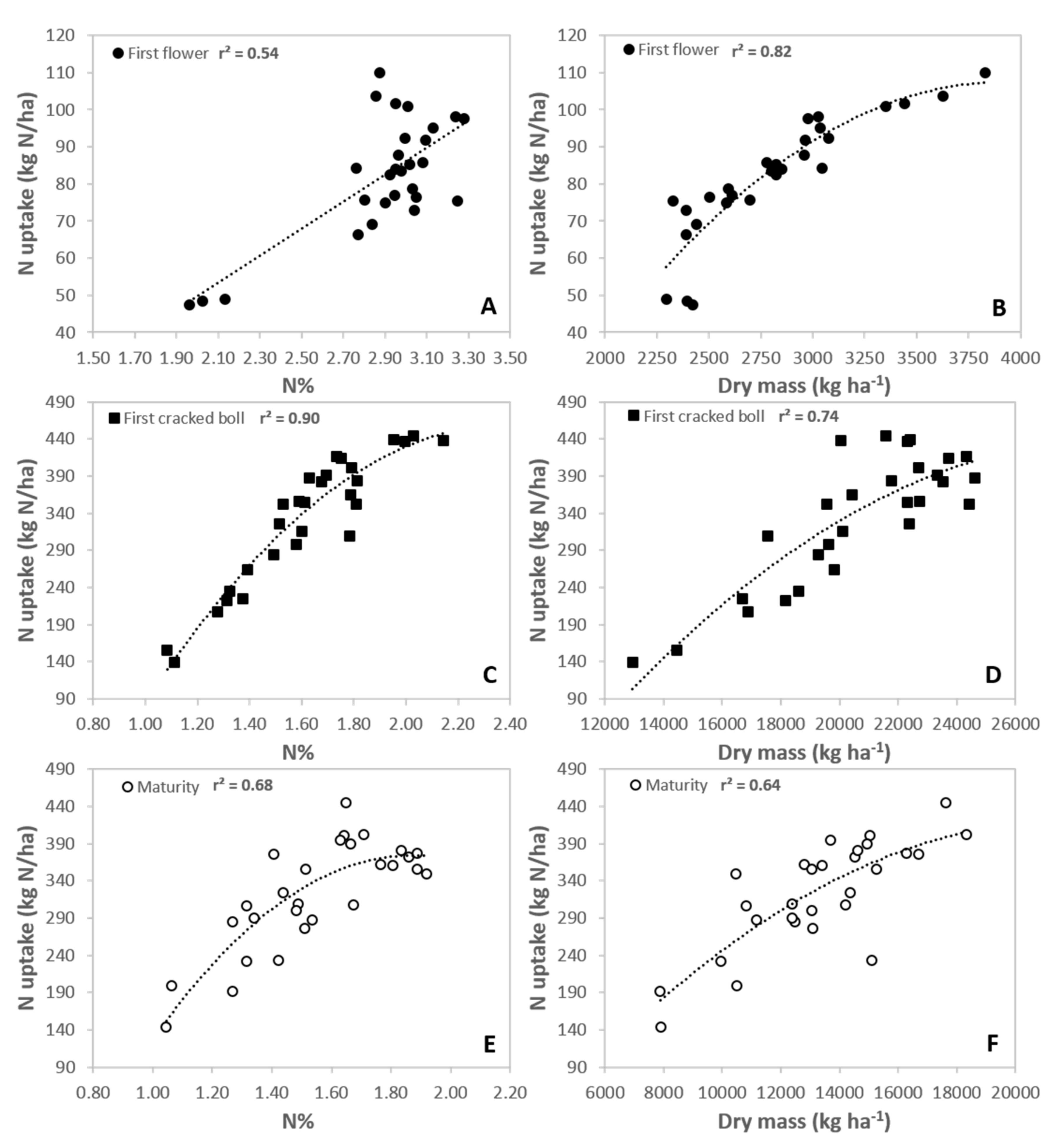
3.1. N% and N Uptake at First Flower, First Cracked Boll and Maturity
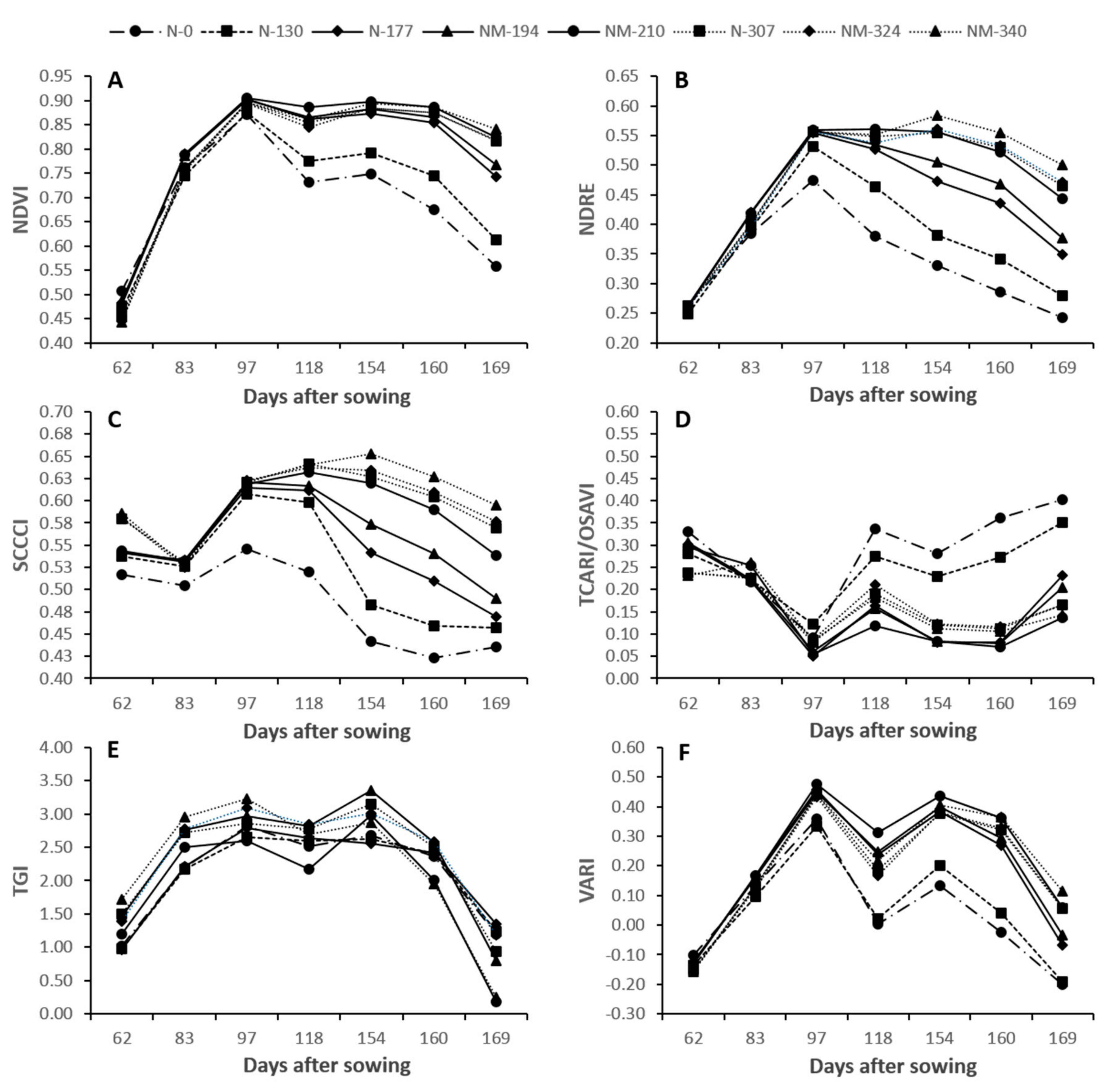
3.2. VIs and Their Relationship with Plant N% and N Uptake at Different Stages of the Crop
3.3. Lint Yield and Its Relationship with the VIs
4. Discussion
4.1. Plant N% and N Uptake Estimations across the Season
4.2. Lint Yield Prediction
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gerik, T.J.; Oosterhuis, D.M.; Torbert, H.A. Managing cotton nitrogen supply. Adv. Agron. 1998, 64, 115–147. [Google Scholar]
- World Fertilizer Trends and Outlook to 2018; FAO: Rome, Italy, 2015; p. 66.
- Ali, N. Review: Nitrogen utilization features in cotton crop. Am. J. Plant Sci. 2015, 6, 987–1002. [Google Scholar] [CrossRef]
- Good, A.G.; Beatty, P.H. Fertilizing nature: A tragedy of excess in the commons. PLoS Biol. 2011, 9, e1001124. [Google Scholar] [CrossRef] [PubMed]
- Snyder, C.S.; Davidson, E.A.; Smith, P.; Venterea, R.T. Agriculture: Sustainable crop and animal production to help mitigate nitrous oxide emissions. Curr. Opin. Environ. Sustain. 2014, 9–10, 46–54. [Google Scholar] [CrossRef]
- Cottoninfo. Cottoninfo on-Farm Nitrogen Trials and N-Use Practices. Available online: https://www.cottoninfo.com.au/publications/cottoninfo-nitrogen-trials-report (accessed on 8 November 2017).
- Zhou, G.; Yin, X. Assessing nitrogen nutritional status, biomass and yield of cotton with ndvi, spad and petiole sap nitrate concentration. Exp. Agric. 2017, 1–18. [Google Scholar] [CrossRef]
- Mulla, D.J. Twenty five years of remote sensing in precision agriculture: Key advances and remaining knowledge gaps. Biosyst. Eng. 2013, 114, 358–371. [Google Scholar] [CrossRef]
- Buscaglia, H.J.; Varco, J.J. Early detection of cotton leaf nitrogen status using leaf reflectance. J. Plant Nutr. 2002, 25, 2067. [Google Scholar] [CrossRef]
- Tarpley, L.; Reddy, K.R.; Sassenrath-Cole, G.F. Reflectance indices with precision and accuracy in predicting cotton leaf nitrogen concentration. Crop Sci. 2000, 40, 1814–1819. [Google Scholar] [CrossRef]
- Read, J.J.; Tarpley, L.; McKinion, J.M.; Reddy, K.R. Narrow-waveband reflectance ratios for remote estimation of nitrogen status in cotton. J. Environ. Qual. 2002, 31, 1442–1452. [Google Scholar] [CrossRef] [PubMed]
- Raper, T.B.; Varco, J.J. Canopy-scale wavelength and vegetative index sensitivities to cotton growth parameters and nitrogen status. Precis. Agric. 2015, 16, 62–76. [Google Scholar] [CrossRef]
- Yang, C.; Bradford, J.M.; Wiegand, C.L. Airborne multispectral imagery for mapping variable growing conditions and yields of cotton, grain sorghum, and corn. Trans. ASAE 2001, 44, 1983–1994. [Google Scholar] [CrossRef]
- Raper, T.B.; Varco, J.J.; Hubbard, K.J. Canopy-based normalized difference vegetation index sensors for monitoring cotton nitrogen status. Agron. J. 2013, 105, 1345–1354. [Google Scholar] [CrossRef]
- Gutierrez, M.; Norton, R.; Thorp, K.R.; Wang, G. Association of spectral reflectance indices with plant growth and lint yield in upland cotton. Crop Sci. 2012, 52, 849–857. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Ustin, S.L.; Whiting, M. Temporal and spatial relationships between within-field yield variability in cotton and high-spatial hyperspectral remote sensing imagery. Agron. J. 2005, 97, 641–653. [Google Scholar] [CrossRef]
- Zhao, D.; Reddy, K.R.; Kakani, V.G.; Read, J.J.; Koti, S. Canopy reflectance in cotton for growth assessment and lint yield prediction. Eur. J. Agron. 2007, 26, 335–344. [Google Scholar] [CrossRef]
- Zhang, C.; Kovacs, J.M. The application of small unmanned aerial systems for precision agriculture: A review. Precis. Agric. 2012, 13, 693–712. [Google Scholar] [CrossRef]
- Salamí, E.; Barrado, C.; Pastor, E. Uav flight experiments applied to the remote sensing of vegetated areas. Remote Sens. 2014, 6, 11051–11081. [Google Scholar] [CrossRef] [Green Version]
- Muharam, F.; Maas, S.; Bronson, K.; Delahunty, T. Estimating cotton nitrogen nutrition status using leaf greenness and ground cover information. Remote Sens. 2015, 7, 7007–7028. [Google Scholar] [CrossRef]
- Maresma, Á.; Ariza, M.; Martínez, E.; Lloveras, J.; Martínez-Casasnovas, J. Analysis of vegetation indices to determine nitrogen application and yield prediction in maize (zea mays l.) from a standard uav service. Remote Sens. 2016, 8, 973. [Google Scholar]
- Caturegli, L.; Corniglia, M.; Gaetani, M.; Grossi, N.; Magni, S.; Migliazzi, M.; Angelini, L.; Mazzoncini, M.; Silvestri, N.; Fontanelli, M.; et al. Unmanned aerial vehicle to estimate nitrogen status of turfgrasses. PLoS ONE 2016, 11, e0158268. [Google Scholar] [CrossRef] [PubMed]
- Hunt, E.R.; Horneck, D.A.; Spinelli, C.B.; Turner, R.W.; Bruce, A.E.; Gadler, D.J.; Brungardt, J.J.; Hamm, P.B. Monitoring nitrogen status of potatoes using small unmanned aerial vehicles. Precis. Agric. 2017, 1–20. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, K.; Deng, J.; Harmon, T. Quantifying nitrogen status of rice using low altitude uav-mounted system and object-oriented segmentation methodology. In Proceedings of the ASME/IEEE International Conference on Mechatronic and Embedded Systems and Applications, San Diego, CA, USA, 30 August–2 September 2009; pp. 603–609. [Google Scholar]
- Isbell, R.F. The Australian Soil Classification; CSIRO: Collingwood, Australia, 2002; Volume 4. [Google Scholar]
- Grabham, M. Bankless Channel Irrigation Systems. In WATERpack—A Guide for Irrigation Management in Cotton and Grain Farming Systems, 3rd ed.; Cotton Research and Development Corporation (CRDC): Narrabri, Australia, 2012; pp. 388–391. [Google Scholar]
- Hunt, E.R.; Doraiswamy, P.C.; McMurtrey, J.E.; Daughtry, C.S.T.; Perry, E.M.; Akhmedov, B. A visible band index for remote sensing leaf chlorophyll content at the canopy scale. Int. J. Appl. Earth Obs. GeoInf. 2013, 21, 103–112. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Kaufman, Y.J.; Stark, R.; Rundquist, D. Novel algorithms for remote estimation of vegetation fraction. Remote Sens. Environ. 2002, 80, 76–87. [Google Scholar] [CrossRef]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring Vegetation Systems in the Great Plains with ERTS. In Proceedings of the Third Earth Resources Technology Satellite-1 Symposium, Washington, DC, USA, 10–14 December 1973; Freden, S.C., Mercanti, E.P., Becker, M.A., Eds.; NASA: Washington, DC, USA, 1974; pp. 309–317. [Google Scholar]
- Gitelson, A.A.; Merzlyak, M.N. Remote estimation of chlorophyll content in higher plant leaves. Int. J. Remote Sens. 1997, 18, 2691–2697. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Tremblay, N.; Zarco-Tejada, P.J.; Dextraze, L. Integration of hyperspectral vegetation indices for prediction of crop chlorophyll content for application to precision agriculture. Remote Sens. Environ. 2002, 81, 416–426. [Google Scholar] [CrossRef]
- Li, F.; Miao, Y.; Hennig, S.D.; Gnyp, M.L.; Chen, X.; Jia, L.; Bareth, G. Evaluating hyperspectral vegetation indices for estimating nitrogen concentration of winter wheat at different growth stages. Precis. Agric. 2010, 11, 335–357. [Google Scholar] [CrossRef]
- Yu, K.; Leufen, G.; Hunsche, M.; Noga, G.; Chen, X.; Bareth, G. Investigation of leaf diseases and estimation of chlorophyll concentration in seven barley varieties using fluorescence and hyperspectral indices. Remote Sens. 2014, 6, 64–86. [Google Scholar] [CrossRef]
- Yu, K.; Li, F.; Gnyp, M.L.; Miao, Y.; Bareth, G.; Chen, X. Remotely detecting canopy nitrogen concentration and uptake of paddy rice in the northeast china plain. ISPRS J. Photogramm. Remote Sens. 2013, 78, 102–115. [Google Scholar] [CrossRef]
- Yang, C.; Greenberg, S.M.; Everitt, J.H.; Fernandez, C.J. Assessing cotton defoliation, regrowth control and root rot infection using remote sensing technology. Int. J. Agric. Biol. Eng. 2011, 4, 1–11. [Google Scholar]
- Rochester, I.J. Nutrient uptake and export from an australian cotton field. Nutr. Cycl. Agroecosyst. 2007, 77, 213–223. [Google Scholar] [CrossRef]
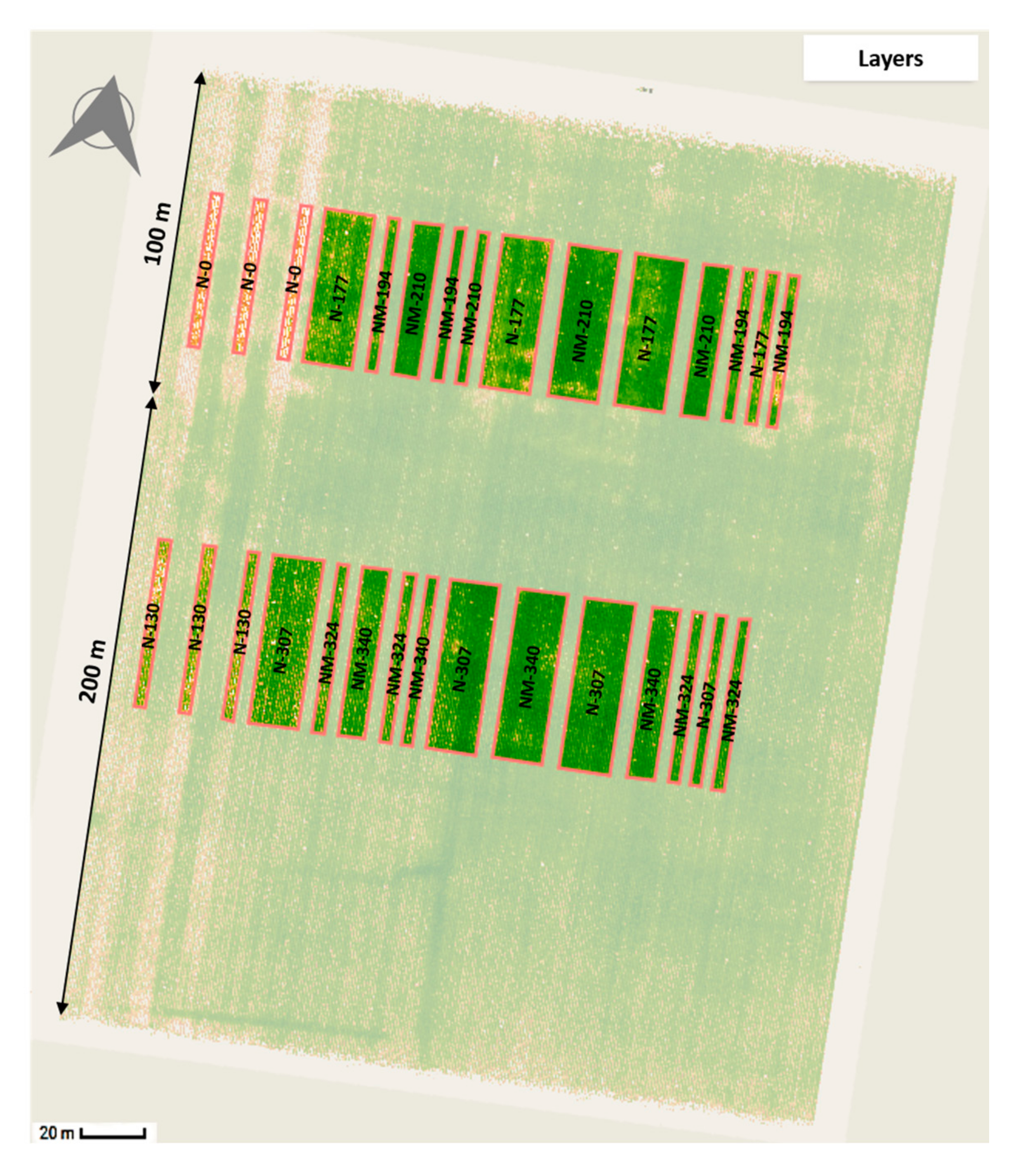


| Treatment | DAP * | NH3-N * | Poultry Manure * | Urea y | Total N Applied (kg ha−1) | Replicates |
|---|---|---|---|---|---|---|
| N-0 | 0 | 0 | 0 | 0 | 0 | 3 |
| N-130 | 0 | 0 | 0 | 130 | 130 | 3 |
| N-177 | 27 | 150 | 0 | 0 | 177 | 4 |
| NM-194 | 27 | 150 | 16.6 | 0 | 194 | 4 |
| NM-210 | 27 | 150 | 33.2 | 0 | 210 | 4 |
| N-307 | 27 | 150 | 0 | 130 | 307 | 4 |
| NM-324 | 27 | 150 | 16.6 | 130 | 324 | 4 |
| NM-340 | 27 | 150 | 33.2 | 130 | 340 | 4 |
| Vegetation Index | Formulation | Reference |
|---|---|---|
| NDVI | (NIR − R)/(NIR + R) | [29] |
| NDRE | (NIR − RE)/(NIR + RE) | [30] |
| SCCCI | NDRE/NDVI | [12] |
| TCARI/OSAVI | [3[(RE − R) − 0.2 (RE/G) (RE/R)]]/[(1 + 0.16) (NIR − R)/(NIR + R + 0.16)] RE/R | [31] |
| TGI | −0.5[(668 − 475)(R − G) − (668 − 560)(R − B)] | [27] |
| VARI | (G − R)/(G + R − B) | [28] |
| Treatment | First Flower (83 DAS *) | First Cracked Boll (154 DAS *) | Maturity (169 DAS *) | Lint Yield | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N% | N Uptake | DM | N% | N Uptake | DM | N% | N Uptake | DM | ||
| N-0 | 2.04 ± 0.09 a | 48.3 ± 0.8 a | 2.4 ± 0.1 a | 1.16 ± 0.10 a | 167.0 ± 35.6 a | 14.7 ± 1.9 a | 1.13 ± 0.12 a | 178.3 ± 30.1 a | 8.7 ± 1.5 a | 2.11 ± 0.13 a |
| N-130 | 2.89 ± 0.12 b | 74.5 ± 4.8 b | 2.6 ± 0.1 ab | 1.50 ± 0.27 ab | 270.5 ± 71.0 ab | 18.3 ± 1.5 ab | 1.30 ± 0.03 ab | 273.9 ± 38.3 ab | 11.1 ± 1.3 ab | 2.34 ± 0.15 ab |
| N-177 | 2.93 ± 0.07 bc | 97.3 ± 11.8 d | 3.3 ± 0.5 c | 1.51 ± 0.14 ab | 322.8 ± 71.4 bc | 21.9 ± 2.8 b | 1.44 ± 0.04 bc | 310.3 ± 59.0 bc | 14.6 ± 1.7 bc | 3.10 ± 0.22 c |
| NM-194 | 2.97 ± 0.09 bc | 83.3 ± 6.9 cd | 2.8 ± 0.2 abc | 1.52 ± 0.10 ab | 304.4 ± 39.8 bc | 20.3 ± 1.3 b | 1.47 ± 0.09 bc | 308.1 ± 32.0 bc | 13.0 ± 1.7 abc | 3.12 ± 0.11 c |
| NM-210 | 2.99 ± 0.04 bc | 95.9 ± 9.2 cd | 3.2 ± 0.3 bc | 1.72 ± 0.19 bc | 384.2 ± 57.8 bc | 23.5 ± 1.7 b | 1.77 ± 0.11 d | 386.6 ± 19.4 c | 15.6 ± 2.0 c | 3.03 ± 0.07 c |
| N-307 | 2.88 ± 0.14 b | 75.1 ± 7.5 bc | 2.6 ± 0.3 abc | 1.70 ± 0.09 bc | 350.8 ± 56.5 bc | 21.1 ± 3.0 b | 1.65 ± 0.10 cd | 367.9 ± 70.6 bc | 14.6 ± 2.2 bc | 2.87 ± 0.06 c |
| NM-324 | 3.17 ± 0.10 c | 91.8 ± 10.4 bcd | 2.9 ± 0.3 abc | 1.87 ± 0.15 bc | 409.9 ± 38.2 c | 21.9 ± 1.2 b | 1.78 ± 0.15 d | 357.0 ± 38.1 bc | 13.7 ± 2.4 bc | 2.87 ± 0.20 c |
| NM-340 | 3.09 ± 0.14 bc | 83.0 ± 6.6 bcd | 2.7 ± 0.3 ab | 2.02 ± 0.17 c | 428.6 ± 19.8 c | 21.7 ± 1.4 b | 1.83 ± 0.04 d | 364.5 ± 12.4 bc | 13.6 ± 0.7 bc | 2.78 ± 0.26 bc |
| Cotton Growth Stage | N% | N Uptake | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NDVI | NDRE | SCCCI | TCARI/OSAVI | TGI | VARI | NDVI | NDRE | SCCCI | TCARI/OSAVI | TGI | VARI | |
| FF | 0.00 | 0.14 | 0.61 *** | 0.26 ** | 0.33 ** | 0.00 | 0.11 | 0.30 ** | 0.47 *** | 0.12 | 0.01 | 0.06 |
| FCB | 0.47 *** | 0.62 *** | 0.65 *** | 0.25 ** | 0.37 *** | 0.40 *** | 0.58 *** | 0.67 *** | 0.68 *** | 0.38 *** | 0.25 ** | 0.52 *** |
| Maturity | 0.77 *** | 0.84 *** | 0.80 *** | 0.76 *** | 0.74 *** | 0.29 ** | 0.65 *** | 0.62 *** | 0.53 *** | 0.65 *** | 0.51 *** | 0.26 ** |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ballester, C.; Hornbuckle, J.; Brinkhoff, J.; Smith, J.; Quayle, W. Assessment of In-Season Cotton Nitrogen Status and Lint Yield Prediction from Unmanned Aerial System Imagery. Remote Sens. 2017, 9, 1149. https://doi.org/10.3390/rs9111149
Ballester C, Hornbuckle J, Brinkhoff J, Smith J, Quayle W. Assessment of In-Season Cotton Nitrogen Status and Lint Yield Prediction from Unmanned Aerial System Imagery. Remote Sensing. 2017; 9(11):1149. https://doi.org/10.3390/rs9111149
Chicago/Turabian StyleBallester, Carlos, John Hornbuckle, James Brinkhoff, John Smith, and Wendy Quayle. 2017. "Assessment of In-Season Cotton Nitrogen Status and Lint Yield Prediction from Unmanned Aerial System Imagery" Remote Sensing 9, no. 11: 1149. https://doi.org/10.3390/rs9111149