Stay-Green and Associated Vegetative Indices to Breed Maize Adapted to Heat and Combined Heat-Drought Stresses
Abstract
:1. Introduction
2. Materials and Methods
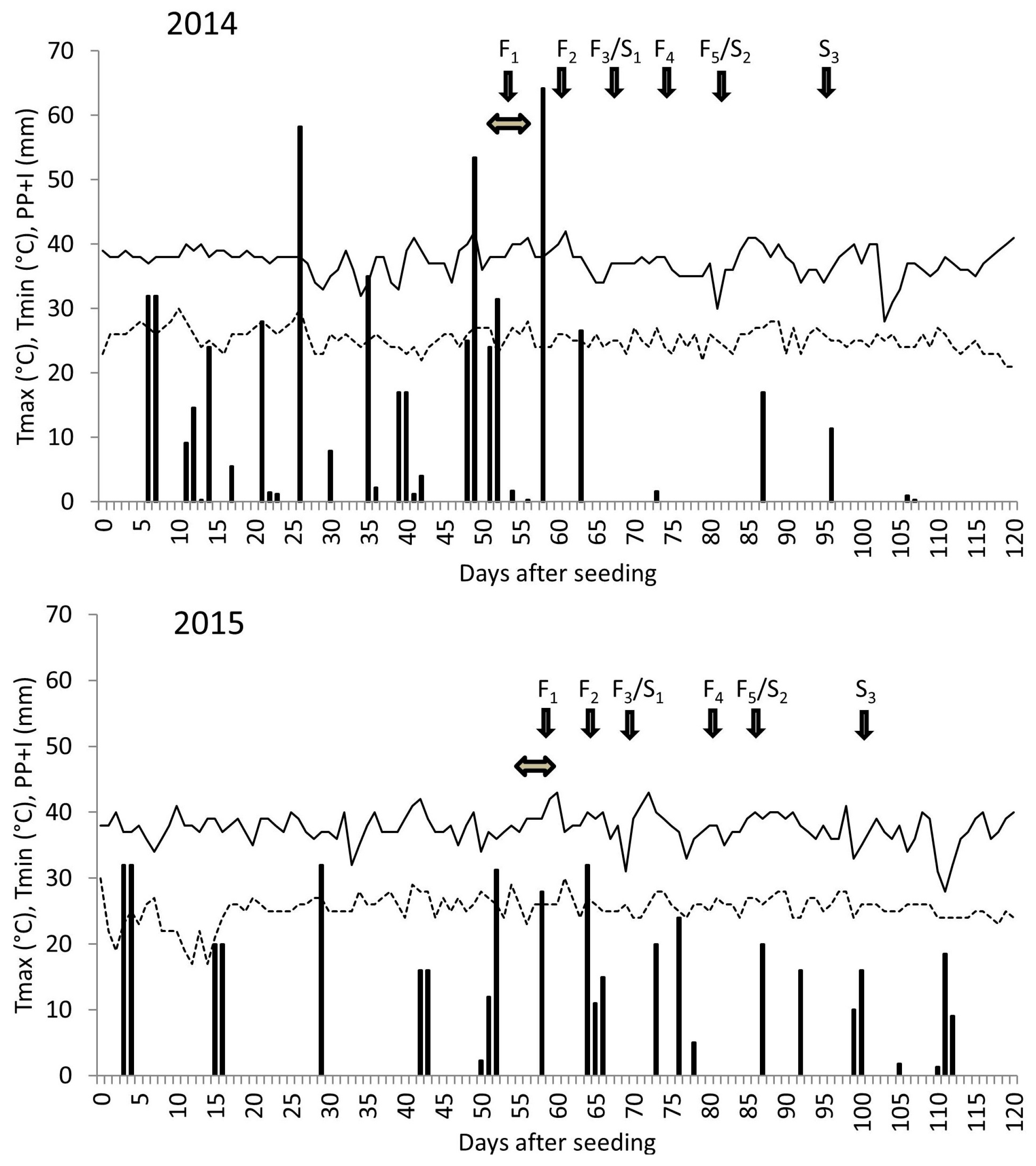
2.1. Field Experiments
2.2. Measurements and Calculations
2.3. Statistical Analysis
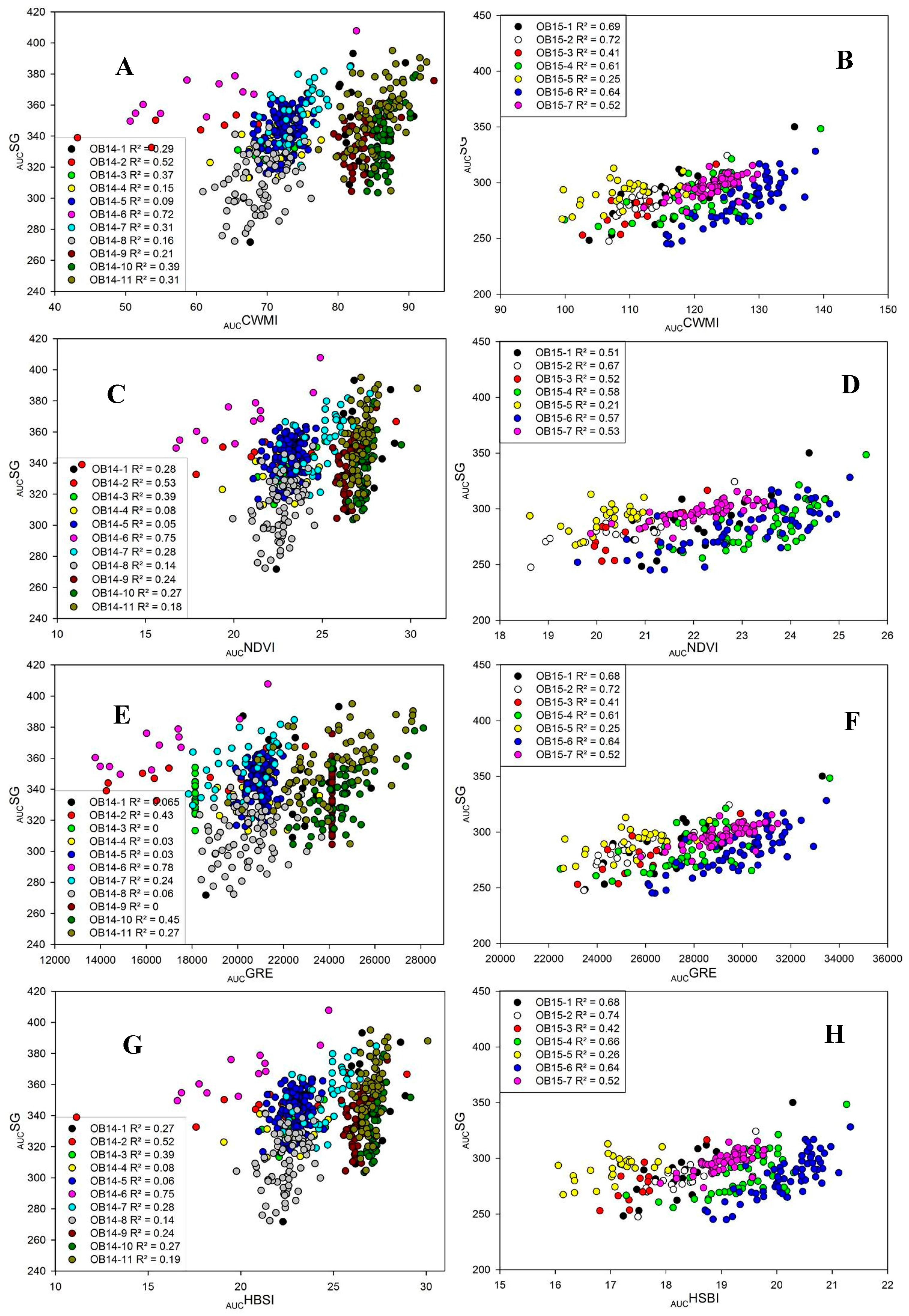
3. Results
3.1. Grain Yield and Stay-Green
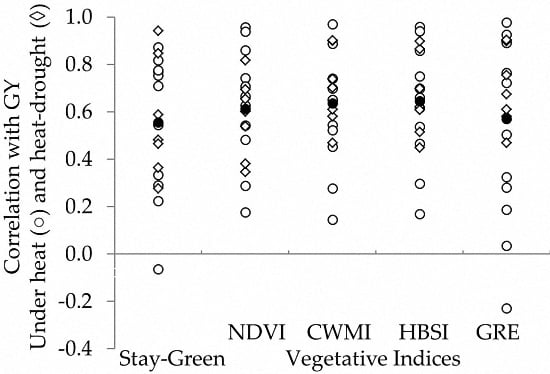
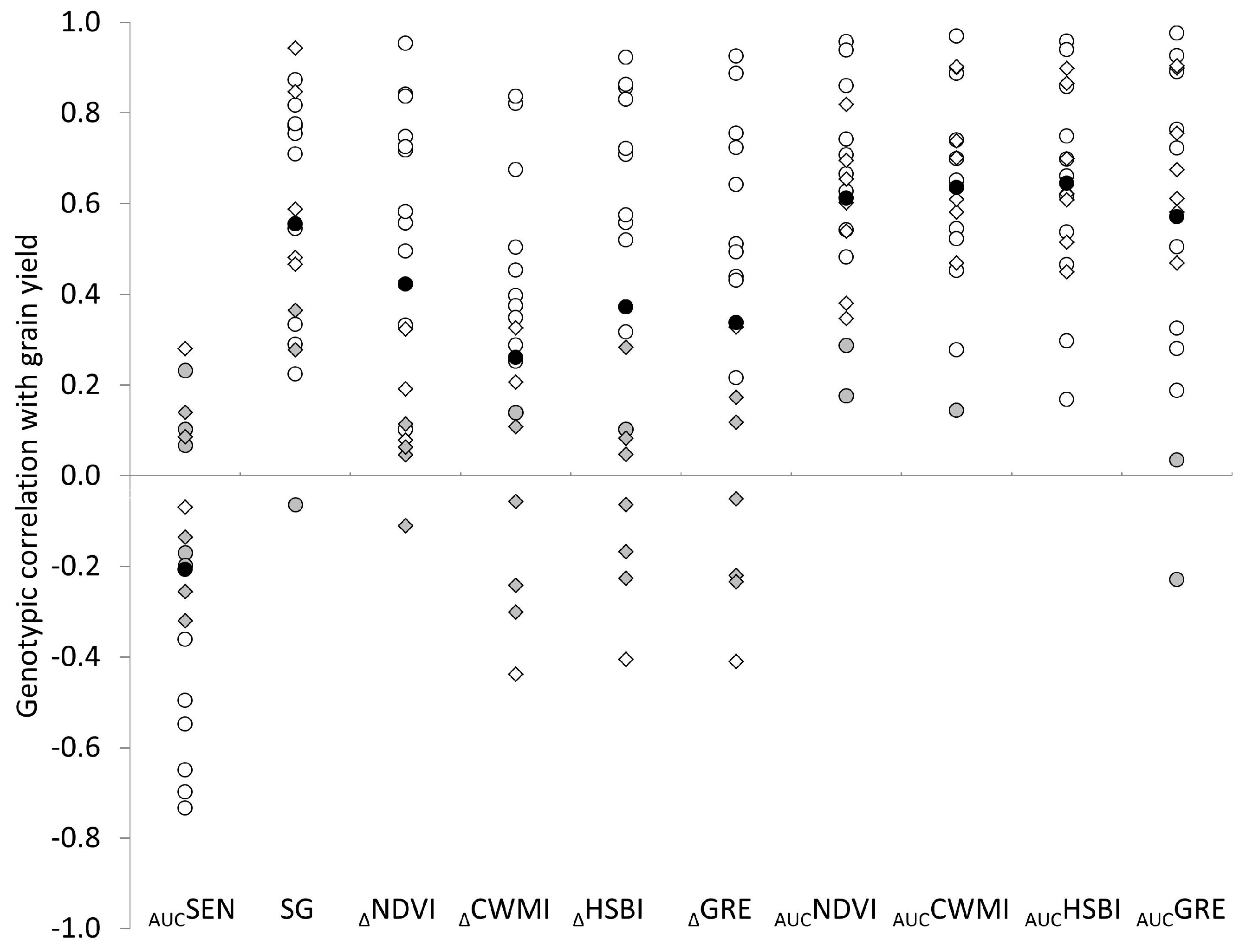
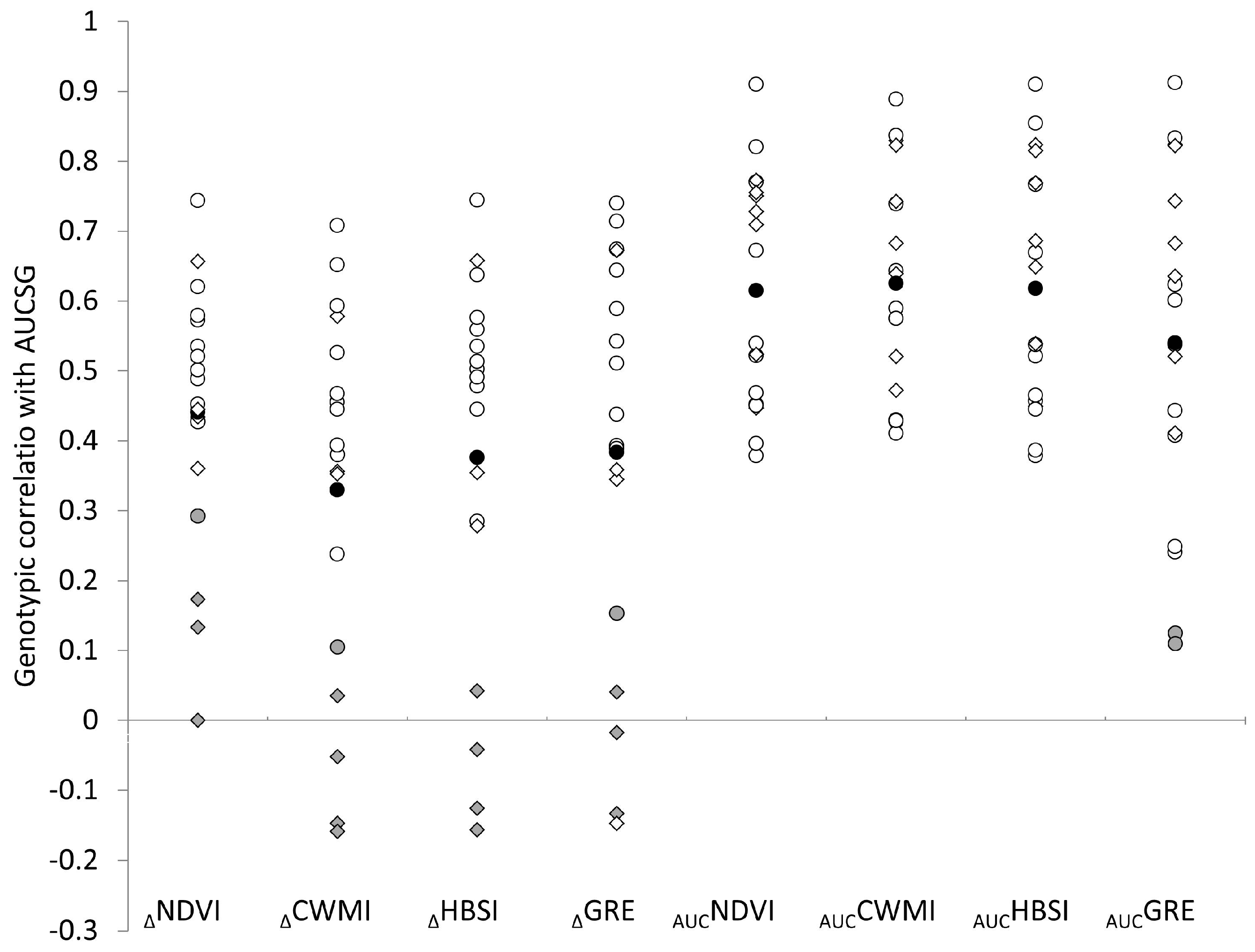
3.2. Genotypic Correlations of Traits with Grain Yield
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chowdhury, S.I.; Wardlaw, I.F. The effect of temperature on kernel development in cereals. Aust. J. Agric. Res. 1978, 29, 205–223. [Google Scholar] [CrossRef]
- Ellis, R.H.; Summerfield, R.J.; Edmeades, G.O.; Roberts, E.H. Photoperiod, temperature, and the interval from sowing to tassel initiation in diverse cultivars of maize. Crop Sci. 1992, 32, 12–25. [Google Scholar] [CrossRef]
- Shaw, R.H. Estimates of yield reductions in corn caused by water and temperature stress. In Crop Reactions to Water and Temperature Stresses in Humid and Temperate Climates; Raper, C.D., Kramer, P.J., Eds.; Westview Press: Boulder, CO, USA, 1983; pp. 49–65. [Google Scholar]
- Rattalino Edreira, J.I.; Otegui, M.E. Heat stress in temperate and tropical maize hybrids: Differences in crop growth, biomass partitioning and reserves use. Field Crop. Res. 2012, 130, 87–98. [Google Scholar] [CrossRef]
- Neiff, N.; Trachsel, S.; Valentinuz, O.R.; Balbi, C.N.; Andrade, F.H. High temperatures around flowering in maize: Effects on photosynthesis and grain yield in three genotypes. Crop Sci. 2016, 56, 2702–2712. [Google Scholar]
- Rosenzweig, C.; Elliott, J.; Deryng, D.; Ruane, A.C.; Müller, C.; Arneth, A.; Boote, K.J.; Folberth, C.; Glotter, M.; Khabarov, N.; et al. Assessing agricultural risks of climate change in the 21st century in a global gridded crop model intercomparison. Proc. Natl. Acad. Sci. USA 2014, 111, 3268–3273. [Google Scholar] [CrossRef] [PubMed]
- Porter, J.R.; Xie, L.; Challinor, A.J.; Cochrane, K.; Howden, S.M.; Iqbal, M.M.; Travasso, M.I. Food security and food production systems. In Climate Change 2014–Impacts, Adaptation and Vulnerability. Part A Global and Sectoral Aspects; Contribution Working Group II to Fifth Assessment Report; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2014; pp. 485–533. [Google Scholar]
- Lobell, D.B.; Bänziger, M.; Magorokosho, C.; Vivek, B. Nonlinear heat effects on African maize as evidenced by historical yield trials. Nat. Clim. Chang. 2011, 1, 42–45. [Google Scholar] [CrossRef]
- Mugo, S.N.; Njoroge, K. Alleviating the effects of drought on maize production in the moisture stress areas of Kenya through escape and tolerance. In Proceedings of the Developing Drought- and Low N-Tolerant Maize, Proceedings of a Symposium, El Batan, Mexico, 25–29 March 1997.
- Monneveux, P.; Sanchez, C.; Tiessen, A. Future progress in drought tolerance in maize needs new secondary traits and cross combinations. J. Agric. Sci. 2008, 146, 287–300. [Google Scholar] [CrossRef]
- Blum, A. Plant Breeding for Stress Environments; CRC Press: Boca Raton, FL, USA, 1988; p. 223. [Google Scholar]
- Mugo, S.N.; Smith, M.E.; Banziger, M.; Setter, T.L.; Edmeades, G.O.; Elings, A. Performance of early maturing Katumani and Kito maize composites under drought at the seedling and flowering stages. Afr. Crop Sci. J. 1998, 6, 329–344. [Google Scholar] [CrossRef]
- Bruns, H.A.; Abbas, H.K. Planting date effects on Bt and Non-Bt corn in the Mid-South USA. Agron. J. 2006, 98, 100–106. [Google Scholar] [CrossRef]
- Trachsel, S.; Burgueno, J.; Suarez, E.A.; San Vicente, F.M.; Rodriguez, C.S.; Dhliwayo, T. Interrelations among early vigor, flowering time, physiological maturity, and grain yield in tropical maize (Zea mays L.) under multiple abiotic stresses. Crop Sci. 2017, 57, 1–14. [Google Scholar] [CrossRef]
- Thomas, H.; Howarth, C.J. Five ways to stay-green. J. Exp. Bot. 2000, 51, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Tollenaar, M.; Wu, J. Yield improvement in temperate maize is attributable to greater stress tolerance. Crop Sci. 1999, 39, 1597–1604. [Google Scholar] [CrossRef]
- Chapman, S.C.; Edmeades, G.O. Genotype by environment effects and selection for drought tolerance in tropical maize I. Two mode pattern analysis of yield. Euphytica 1997, 95, 1–9. [Google Scholar] [CrossRef]
- Bänziger, M.; Lafitte, H.R. Efficiency of secondary traits for improving maize for low-nitrogen target environments. Crop Sci. 1997, 37, 1110–1117. [Google Scholar] [CrossRef]
- Cairns, J.E.; Sanchez, C.; Vargas, M.; Ordoñez, R.; Araus, J.L. Dissecting maize productivity: Ideotypes associated with grain yield under drought stress and well-watered conditions. J. Integr. Plant Biol. 2012, 54, 1007–1020. [Google Scholar] [CrossRef] [PubMed]
- Pinto, R.S.; Lopes, M.S.; Collins, N.C.; Reynolds, M.P. Modelling and genetic dissection of staygreen under heat stress. Theor. Appl. Genet. 2016, 129, 2055–2074. [Google Scholar] [CrossRef] [PubMed]
- Bänziger, M.; Edmeades, G.O.; Beck, D.; Bellon, M. Breeding for Drought and Nitrogen Stress Tolerance in Maize: From Theory to Practice; Cimmyt: Mexico Distrito Federal, Mexico, 2000. [Google Scholar]
- Vina, A.; Gitelson, A.A.; Rundquist, D.C.; Keydan, G.; Leavitt, B.; Schepers, J. Remote sensing—Monitoring maize (Zea mays L.) phenology with remote sensing. Agron. J. 2004, 96, 1139–1147. [Google Scholar] [CrossRef]
- Liebisch, F.; Kirchgessner, N.; Schneider, D.; Walter, A.; Hund, A. Remote, aerial phenotyping of maize traits with a mobile multi-sensor approach. Plant Methods 2015, 11, 1746–4811. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, Q.; Huang, D.A. Review of imaging techniques for plant phenotyping. Sensors 2014, 14, 20078–20111. [Google Scholar] [CrossRef] [PubMed]
- Tucker, C.J. Red and photographic infrared linear combinations for monitoring vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Viña, A.; Arkebauer, T.J.; Rundquist, D.C.; Keydan, G.; Leavitt, B. Remote estimation of leaf area index and green leaf biomass in maize canopies. Geophys. Res. Lett. 2003, 30. [Google Scholar] [CrossRef]
- Thenkabail, P.S.; Murali Krishna Gumma, P.T.; Mohammed, I. Hyperspectral remote sensing of vegetation and agricultural crops. Photogramm. Eng. Remote Sens. 2014, 80, 679–710. [Google Scholar]
- Winterhalter, L.; Mistele, B.; Jampatong, S.; Schmidhalter, U. High-throughput sensing of aerial biomass and above-ground nitrogen uptake in the vegetative stage of well-watered and drought stressed tropical maize hybrids. Crop Sci. 2011, 51, 479–489. [Google Scholar] [CrossRef]
- Weber, V.S.; Araus, J.L.; Cairns, J.E.; Sanchez, C.; Melchinger, A.E.; Orsini, E. Prediction of grain yield using reflectance spectra of canopy and leaves in maize plants grown under different water regimes. Field Crop. Res. 2012, 128, 82–90. [Google Scholar] [CrossRef]
- Romano, G.; Zia, S.; Spreer, W.; Sanchez, C.; Cairns, J.; Araus, J.L.; Müller, J. Use of thermography for high throughput phenotyping of tropical maize adaptation in water stress. Comput. Electron. Agric. 2011, 79, 67–74. [Google Scholar] [CrossRef]
- Neiff, N.; Dhliwayo, T.; Suarez, E.A.; Burgueno, J.; Trachsel, S. Using an airborne platform to measure canopy temperature and NDVI under heat stress in maize using an airborne platform to measure canopy temperature and NDVI under heat stress. J. Crop Improv. 2015, 29, 669–690. [Google Scholar] [CrossRef]
- Cooper, M.; Delacy, I.H. Relationships among analytical methods used to study genotypic variation and genotype-by-environment interaction in plant breeding multi-environment experiments. Theor. Appl. Genet. 1994, 88, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, G.M.; López, M.; Vargas, A.; Pacheco, F.; Rodríguez, J.; Burgueño, J.; Crossa, J. META-R (Multi Environment Trial Analysis with R for Windows) Version 5.0; International Maize and Wheat Improvement Center: Texcoco, Mexico, 2015. [Google Scholar]
- Almeida, G.D.; Makumbi, D.; Magorokosho, C.; Nair, S.; Borm, A.; Ribaut, J.M.; Banziger, M.; Prasanna, B.M.; Crossa, J.; Babu, R. QTL mapping in three tropical maize populations reveals a set of constitutive and adaptive genomic regions for drought tolerance. Theor. Appl. Genet. 2013, 126, 583–600. [Google Scholar] [CrossRef] [PubMed]
- Trachsel, S.; Sun, D.; San Vicente, F.M.; Zheng, H.; Atlin, G.N.; Suarez, E.A.; Babu, R.; Zhang, X. Identification of QTL for early vigor and stay-green conferring tolerance to drought in two connected advanced backcross populations in tropical maize (Zea mays L.). PLoS ONE 2016, 11, e0149636. [Google Scholar]
- Gasura, E.; Setimela, P.; Edema, R.; Gibson, P.T.; Okori, P.; Tarekegne, A. Exploiting grain-filling rate and effective grain-filling duration to improve grain yield of early-maturing maize. Crop Sci. 2013, 53, 2295–2303. [Google Scholar] [CrossRef]

| Index | Equation | Traits Ralated | Reference |
|---|---|---|---|
| NDVI | (R800 − R670)/(R800 + R670) | Productivity | [25,26] |
| Greenness | |||
| Canopy cover | |||
| CWMI | R800/R710 | Crop weight mass | [28] |
| HBSI | (R800 − R670)/(R800 + R670) | Biomass | [27] |
| GRE | 500*((R800/R710) − 1) + 27 | Chlorophyll content | [26] |
| Trial ID | Reps | Genotypes | Year | Trial Mean | Heritability | IQ Range | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Anthesis | GY | SG | GY | SG | GY | SG | ||||
| OB14-1 | 3 | 16 | 2014 | 52 | 2.92 | 345 | 0.91 | 0.73 | 0.64 | 48.6 |
| OB14-2 | 3 | 12 | 2014 | 58 | 3.04 | 348 | 0.90 | 0.76 | 1.55 | 35.8 |
| OB14-3 | 3 | 21 | 2014 | 56 | 3.09 | 334 | 0.88 | 0.81 | 0.85 | 49.1 |
| OB14-4 | 3 | 15 | 2014 | 54 | 3.89 | 335 | 0.88 | 0.89 | 0.85 | 26.7 |
| OB14-5 | 2 | 116 | 2014 | 54 | 3.66 | 343 | 0.98 | 0.86 | 0.90 | 37.2 |
| OB14-6 | 3 | 12 | 2014 | 57 | 2.68 | 369 | 0.64 | 0.47 | 1.13 | 26.4 |
| OB14-7 | 2 | 39 | 2014 | 55 | 3.80 | 354 | 0.87 | 0.86 | 1.13 | 36.6 |
| OB14-8 | 3 | 76 | 2014 | 55 | 3.49 | 311 | 0.48 | 0.63 | 1.10 | 36.3 |
| OB14-9 | 2 | 45 | 2014 | 53 | 4.09 | 336 | 0.98 | 0.87 | 0.90 | 40.2 |
| OB14-10 | 2 | 60 | 2014 | 52 | 3.71 | 336 | 0.79 | 0.81 | 0.80 | 37.0 |
| OB14-11 | 2 | 75 | 2014 | 52 | 4.00 | 354 | 0.85 | 0.81 | 1.15 | 38.3 |
| OB15-1 | 3 | 20 | 2015 | 59 | 1.85 | 288 | 0.92 | 0.93 | 0.90 | 25.5 |
| OB15-2 | 3 | 20 | 2015 | 58 | 0.95 | 282 | 0.90 | 0.77 | 0.68 | 35.1 |
| OB15-3 | 2 | 12 | 2015 | 59 | 1.55 | 277 | 0.97 | 0.74 | 0.50 | 23.7 |
| OB15-4 | 2 | 44 | 2015 | 59 | 1.64 | 285 | 0.88 | 0.84 | 0.80 | 37.2 |
| OB15-5 | 2 | 28 | 2015 | 59 | 1.27 | 291 | 0.80 | 0.71 | 0.65 | 29.5 |
| OB15-6 | 2 | 60 | 2015 | 55 | 1.08 | 284 | 0.58 | 0.75 | 0.70 | 42.2 |
| OB15-7 | 2 | 60 | 2015 | 55 | 0.96 | 297 | 0.37 | 0.48 | 1.00 | 40.6 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerrudo, D.; González Pérez, L.; Mendoza Lugo, J.A.; Trachsel, S. Stay-Green and Associated Vegetative Indices to Breed Maize Adapted to Heat and Combined Heat-Drought Stresses. Remote Sens. 2017, 9, 235. https://doi.org/10.3390/rs9030235
Cerrudo D, González Pérez L, Mendoza Lugo JA, Trachsel S. Stay-Green and Associated Vegetative Indices to Breed Maize Adapted to Heat and Combined Heat-Drought Stresses. Remote Sensing. 2017; 9(3):235. https://doi.org/10.3390/rs9030235
Chicago/Turabian StyleCerrudo, Diego, Lorena González Pérez, José Alberto Mendoza Lugo, and Samuel Trachsel. 2017. "Stay-Green and Associated Vegetative Indices to Breed Maize Adapted to Heat and Combined Heat-Drought Stresses" Remote Sensing 9, no. 3: 235. https://doi.org/10.3390/rs9030235