Prediction of Soil Physical and Chemical Properties by Visible and Near-Infrared Diffuse Reflectance Spectroscopy in the Central Amazon
Abstract
:1. Introduction
2. Materials and Methods
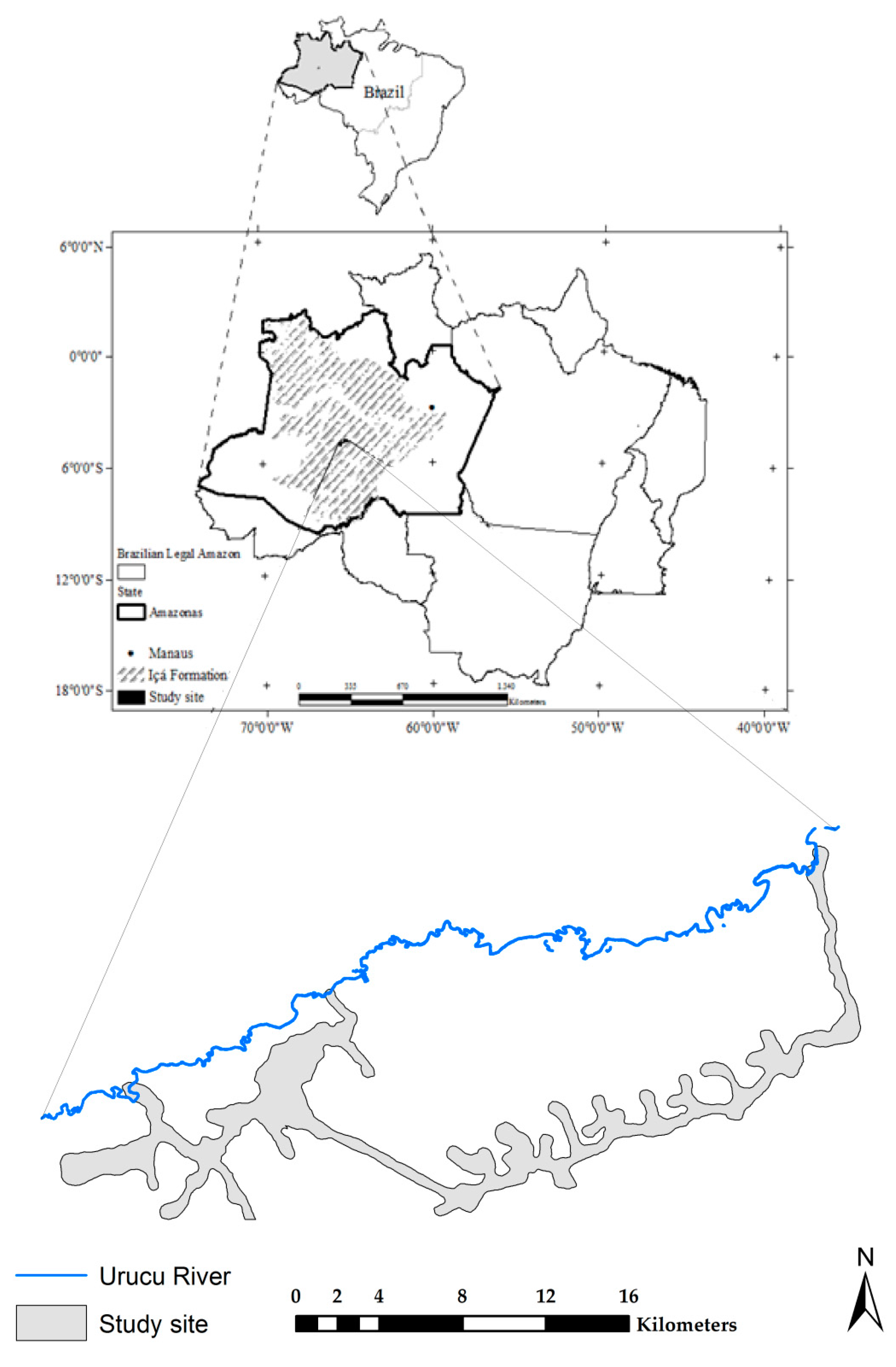
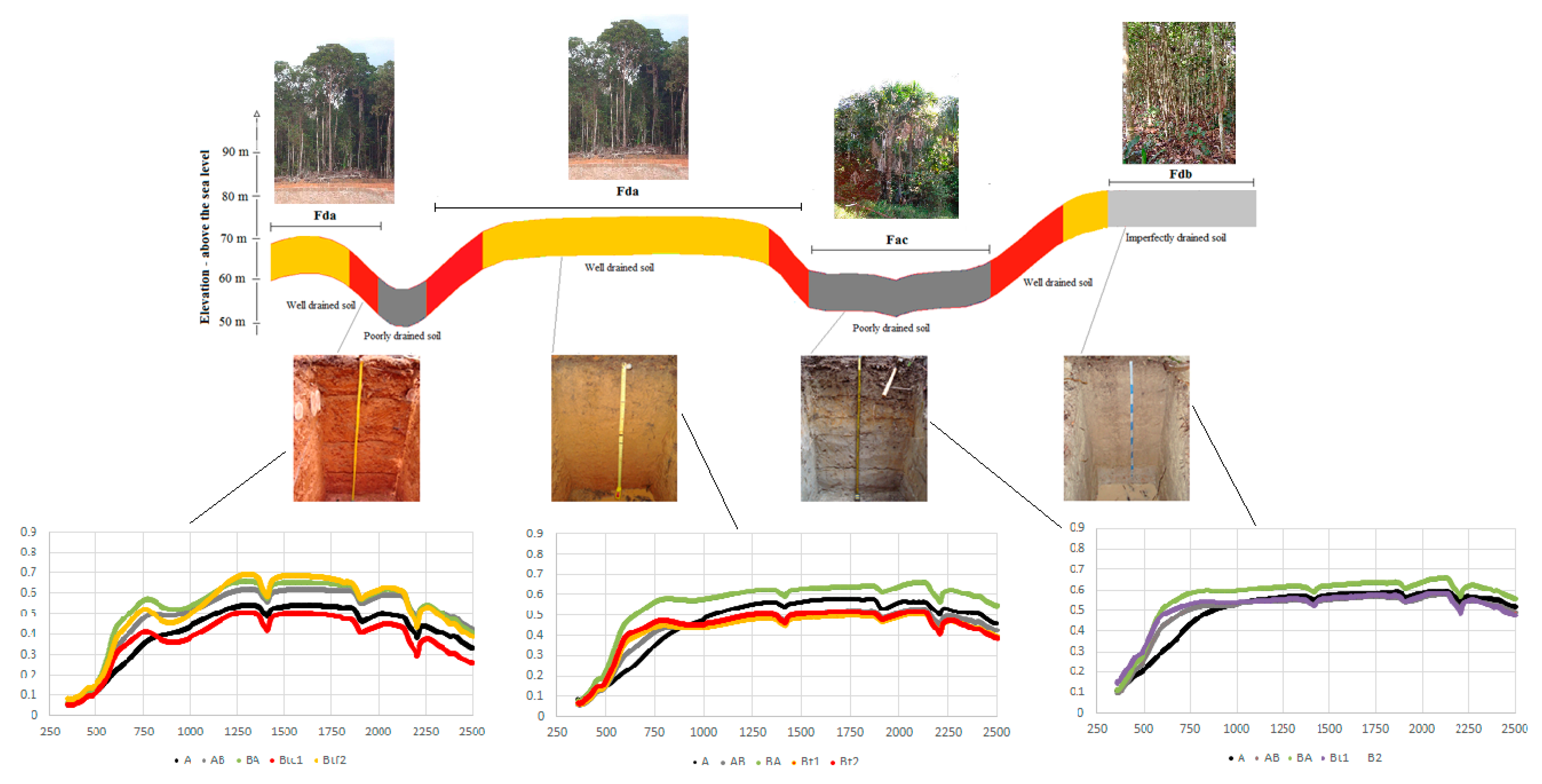
2.1. Study Area
2.2. Soil Sampling and Laboratory Analysis
2.3. Laboratory Spectral Analysis and Pre-Processing
2.4. Statistical Analysis
2.5. PLSR-VIP Method
3. Results
3.1. Descriptive Statistics and Correlations among Soil Properties
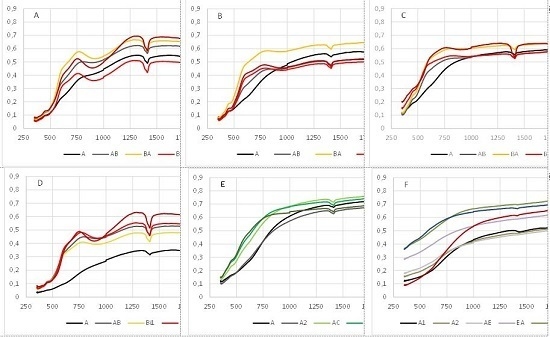
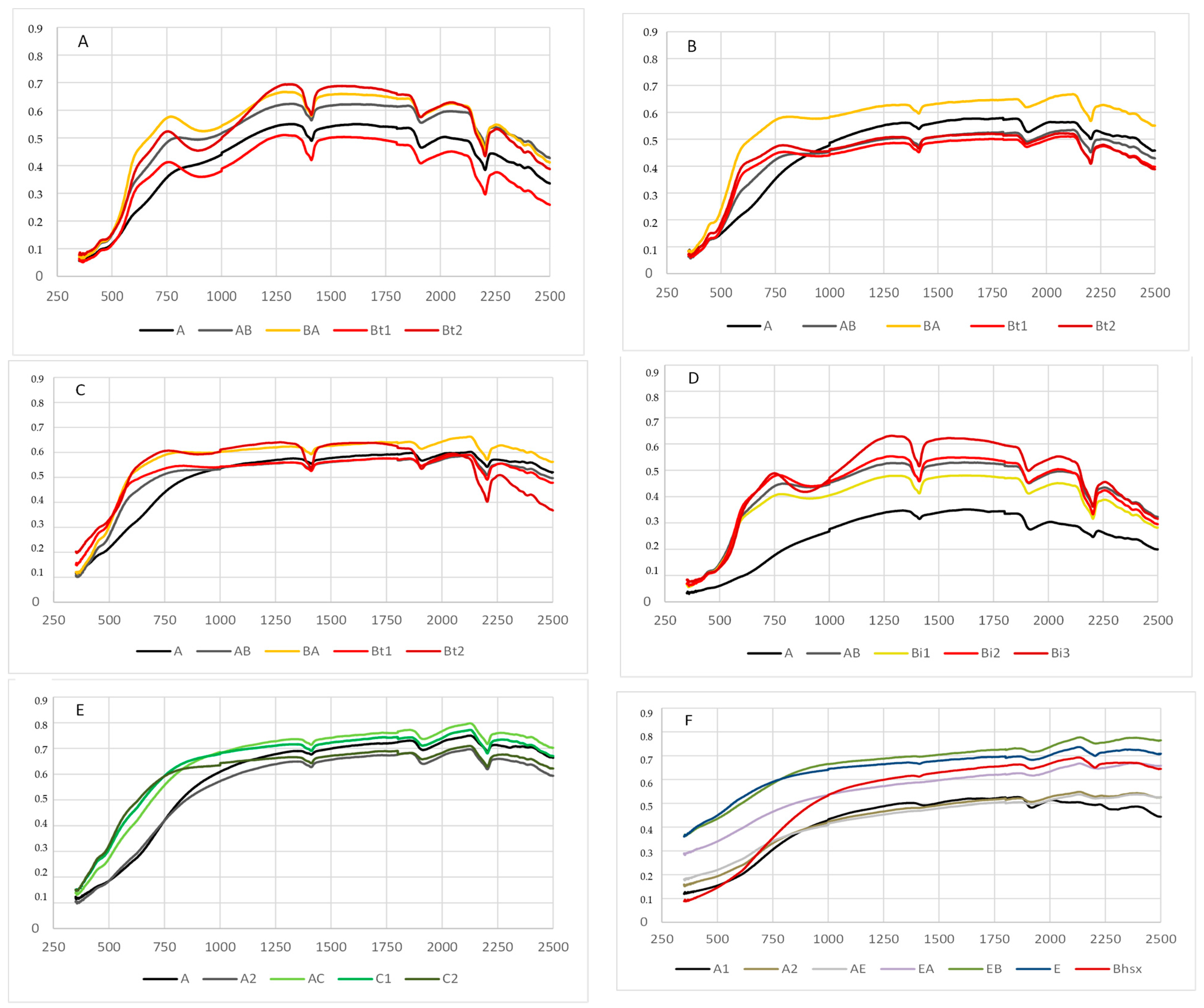
3.2. Qualitative Description of the Soil Spectra
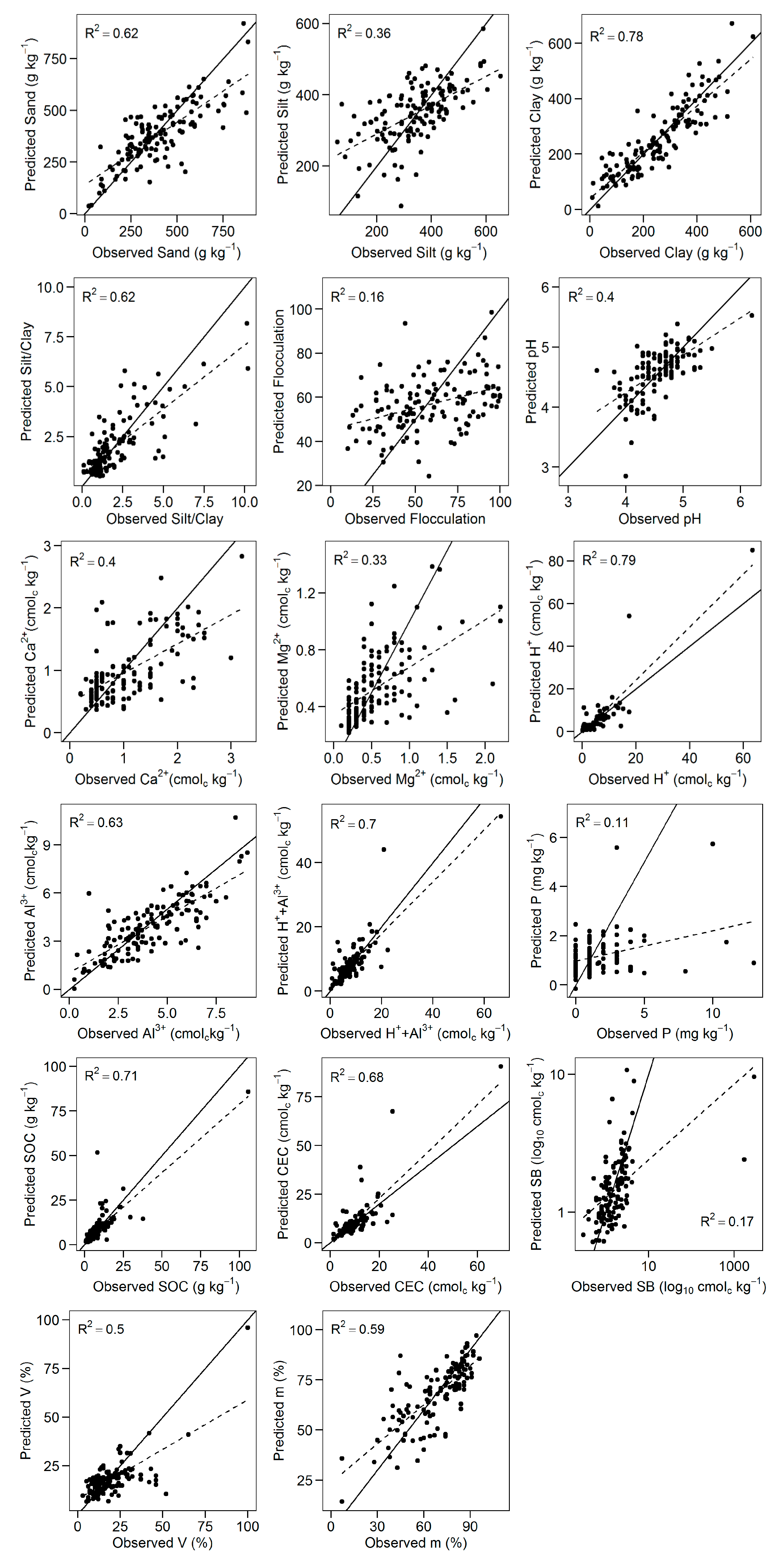
3.3. Predictions of Soil Chemical and Physical Properties
3.4. Explanatory Variable Importance
4. Discussion
4.1. Correlations between Soil Properties
4.2. Predictions of Soil Chemical Properties
4.3. Predictions of Soil Physical Properties
4.4. PLSR-VIP Method
4.5. Relationships between Spectral Reflectance and Soil Type
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of interest
References
- Grunwald, S.; Vasques, G.M. Fusion of soil and remote sensing data to model soil properties. In Advances in Agronomy; Sparks, D.L., Ed.; Advances in Agronomy: Amsterdam, The Netherland, 2015; Volume 131, pp. 1–109. [Google Scholar]
- Vasques, G.M.; Grunwald, S.; Sickman, J.O. Visible/near-infrared spectroscopy modeling of dynamic soil carbon fractions. Soil Sci. Soc. Am. J. 2009, 73, 176–184. [Google Scholar] [CrossRef]
- Stevens, A.; Udelhoven, T.; Denis, A.; Tychon, B.; Lioy, R.; Hoffmann, L.; Wesemael, B.V. Measuring soil organic carbon in croplands at regional scale using airborne imaging spectroscopy. Geoderma 2010, 158, 32–45. [Google Scholar] [CrossRef]
- Gomez, C.; Viscarra Rossel, R.A.; McBrantney, A.B. Comparing predictions of soil organic carbon by field Vis-NIR Spectroscopy and hyper spectral remote sensing. Geophys. Res. Abstr. 2008, 10, 1–2. [Google Scholar]
- Viscarra Rossel, R.A.; McGlynn, R.N.; McBratney, A.B. Determining the composition of mineral-organic mixes using UV-vis-NIR diffuse reflectance spectroscopy. Geoderma 2006, 137, 70–82. [Google Scholar] [CrossRef]
- Reeves, J.; McCarty, G.; Mimmo, T. The potential of diffuse reflectance spectroscopy for the determination of carbon inventories in soils. Environ. Pollut. 2002, 116, 277–284. [Google Scholar] [CrossRef]
- Reeves, J.B. Near- versus mid-infrared diffuse reflectance spectroscopy for soil analysis emphasizing carbon and laboratory versus on-site analysis: Where are we and what needs to be done? Geoderma 2010, 158, 3–14. [Google Scholar] [CrossRef]
- Reeves, J.B.; Follett, R.F.; McCarthy, G.W.; Kimble, J.M. Can near- or mid-infrared diffuse reflectance spectroscopy be used to determine soil carbon pools? Commun. Soil Sci. Plant Anal. 2006, 37, 2307–2325. [Google Scholar] [CrossRef]
- Sorensen, I.K.; Dalsgaard, S. Determination of clay and other soil properties by near infrared spectroscopy. Soil Sci. Soc. Am. J. 2005, 69, 159–167. [Google Scholar] [CrossRef]
- Chong, I.G.; Jun, C.H. Performance of some variable selection methods when multicollinearity is present. Chemom. Intell. Lab. Syst. 2005, 78, 103–112. [Google Scholar] [CrossRef]
- Nanni, M.R.; Demattê, J.A.M. Spectral reflectance methodology in comparison to soil analysis. Soil Sci. Soc. Am. J. 2006, 70, 393–407. [Google Scholar] [CrossRef]
- Viscarra Rossel, R.A.; Behrens, T.; Ben-Dor, E.; Brownd, D.J.; Demattêe, J.A.M.; Shepherdf, K.D.; Shig, Z.; Stenbergh, B.; Stevensi, A.; Adamchuk, V.; et al. Global spectral library to characterize the world’s soil. Earth Sci. Rev. 2016, 155, 198–230. [Google Scholar] [CrossRef]
- Xiong, X.; Grunwald, S.; Myers, D.B.; Kim, J.; Harries, W.G.; Comerford, N.B. Holistic environmental soil landscape modeling of soil organic carbon. Environ. Model. Softw. J. 2014, 57, 202–215. [Google Scholar] [CrossRef]
- Ceddia, M.B.; Villela, A.L.O.; Pinheiro, E.F.M.; Wendroth, O. Spatial variability of soil carbon stock in the Urucu river basin, Central Amazon-Brazil. Sci. Total Environ. 2015, 526, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Reis, N.J.; Almeida, M.E.; Rikes, S.L.; Ferreira, A.L. Geologia e Recursos Minerais do Estado do Amazonas; Serviço Geológico do Brasil: Manaus, Brazil, 2006. [Google Scholar]
- Maia, R.G.N.; Godoy, H.K.; Yamaguti, H.S.; Moura, P.A.; Costa, F.S.F.; Holanda, M.A.; Costa, J.A. Projeto Carvão no Alto Solimões; Serviço Geológico do Brasil: Manaus, Brazil, 1977. [Google Scholar]
- Villela, A.L.O. Mapeamento Digital de Solos da Formação Solimões Sob Floresta Tropical Amazônica. Ph.D. Thesis, Agronomia-Ciência do Solo, Universidade Federal Rural do Rio de Janeiro, Rio de Janeiro, Brazil, 2013. [Google Scholar]
- Embrapa. Sistema Brasileiro de Classificação de Solos, 3rd ed.; Brasília: Distrito Federal, Brazil, 2013. [Google Scholar]
- United States Department of Agriculture. Soil Taxonomy. A Basic System of Soil Classification for Making and Interpreting Soil Surveys; Natural Resources Conservation Service No. 436; United States Department of Agriculture: Washington, DC, USA, 1999.
- Embrapa. Manual de Métodos de Análise de Solo, 2nd ed.; Centro Nacional de Pesquisa de Solos: Rio de Janeiro, Brazil, 1997. [Google Scholar]
- Mehlich, A. Determination of P, Ca, Mg, K, Na, and NH4; North Carolina Soil Test Division: Raleigh, NC, USA, 1953. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Rinnan, A.; Berg, F.V.; Engelsen, S.B. Review of the most common pre-processing techniques for near-infrared spectra. Trends Anal. Chem. 2009, 28, 1201–1222. [Google Scholar] [CrossRef]
- Box, G.E.P.; Cox, D.R. An Analysis of Transformations. J. R. Stat. Soc. 1964, 26, 211–252. [Google Scholar]
- Estatcamp®. Portal Action: Software Action. Available online: http://www.portalaction.com.br/content/sobre-o-action (accessed on 13 December 2013).
- Johnson, R.A.; Wichern, D.W. Applied Multivariate Statistical Analysis, 6th ed.; Pearson Education Inc.: New Jersey, NJ, USA, 2007; p. 700. [Google Scholar]
- Viscarra Rossel, R.A.; Walvoort, D.J.J.; McBratney, A.B.; Janik, L.J.; Skjemstad, J.O. Visible, near infrared, mid infrared or combined diffuse reflectance spectroscopy for simultaneous assessment of various soil properties. Geoderma 2006, 131, 59–75. [Google Scholar] [CrossRef]
- Vasques, G.M.; Grunwald, S.; Sickman, J.O. Comparison of multivariate methods for inferential modeling of soil carbon using visible/near-infrared spectra. Geoderma 2008, 146, 14–25. [Google Scholar] [CrossRef]
- Mevik, B.-H. VIP.R: Implementation of VIP (Variable Importance in Projection) for the ‘pls’ Package. Available online: http://mevik.net/work/software/VIP.R (accessed on 26 October 2016).
- Van Wambeke, A. Soils of the Tropics—Properties and Appraisal; McGraw-Hill: New York, NY, USA, 1992. [Google Scholar]
- Stevenson, F.J. Humus Chemistry: Genesis, Composition, Reaction; Wiley: New York, NY, USA, 1994; p. 496. [Google Scholar]
- Terra, F.S.; Demattê, J.A.M.; Viscarra-Rossel, R.A. Spectral libraries for quantitative analysis of tropical Brazillian soils: Comparing VIS-NIR and MIR reflecytance data. Geoderma 2015, 255–256, 81–93. [Google Scholar] [CrossRef]
- Lee, K.S.; Lee, D.H.; Sudduth, K.A.; Chung, S.O.; Kitchen, N.R.; Drummond, S.T. Wavelength identification and diffuse reflectance estimation for surface and profile soil properties. Trans. ASABE 2009, 52, 683–695. [Google Scholar] [CrossRef]
- Bellon-Maurel, V.; McBratney, A. Near-infrared (NIR) and mid-infrared (MIR) spectroscopic techniques for assessing the amount of carbon stocks in soils—Critical review and research perspectives. Soil Biol. Biochem. 2011, 43, 1398–1410. [Google Scholar] [CrossRef]
- Morgan, C.L.S.; Waiser, T.H.; Brown, D.J.; Hallmark, C.T. Simulated in situ characterization of soil organic and inorganic carbon with visible near-infrared diffuse reflectance spectroscopy. Geoderma 2009, 151, 249–256. [Google Scholar] [CrossRef]
- Janik, L.J.; Merry, R.H.; Skjemstad, J.O. Can mid infra-red diffuse reflectance analysis replace soil extractions? Aust. J. Exp. Agric. 1998, 38, 681–696. [Google Scholar] [CrossRef]
- Araújo, S.R.; Söderström, M.; Eriksson, J.; Isendahl, C.; Stenborg, P.; Demattê, J.M. Determining soil properties in Amazonian Dark Earths by reflectance spectroscopy. Geoderma 2015, 237–238. [Google Scholar] [CrossRef]
- Daniel, K.W.; Tripathi, N.K.; Honda, K. Artificial neural network analysis of laboratory and in situ spectra for the estimation of macronutrients in soils of Lop Buri (Thailand). Aust. J. Soil Res. 2003, 41, 47–59. [Google Scholar] [CrossRef]
- Pirie, A.; Singh, B.; Islam, K. Ultra-violet, visible, near-infra-red, and mid-infra-red diffuse reflectance spectroscopic techniques to predict several soil properties. Aust. J. Soil Res. 2005, 43, 713–721. [Google Scholar] [CrossRef]
- Viscarra Rossel, R.A.; Lark, R.M. Improved analysis and modeling of soil diffuse reflectance spectra using wavelets. Eur. J. Soil Sci. 2009, 60, 453–464. [Google Scholar] [CrossRef]
- Viscarra Rossel, R.A.; Behrens, T. Using data mining to model and interpret soil diffuse reflectance spectra. Geoderma 2010, 158, 46–54. [Google Scholar] [CrossRef]
- Chang, C.-W.; Laird, D.A.; Mausbach, M.J.; Hurburgh, C.R., Jr. Near-infrared reflectance spectroscopy-principal components regression analysis of soil properties. Soil Sci. Soc. Am. J. 2001, 65, 480–490. [Google Scholar] [CrossRef]
- Shepherd, K.D.; Walsh, M.G. Development of reflectance spectral libraries for characterization of soil properties. Soil Sci. Soc. Am. J. 2002, 66, 988–998. [Google Scholar] [CrossRef]
- Cozzolino, D.; Moron, A. The potential of near-infrared reflectance spectroscopy to analyse soil chemical and physical characteristics. J. Agric. Sci. 2003, 140, 65–71. [Google Scholar] [CrossRef]
- Clark, R.N.; King, T.V.V.; Klejwa, M.; Swayze, G.; Vergo, N. High spectral resolution reflectance spectroscopy of minerals. J. Geophys. Res. 1990, 95, 12653–12680. [Google Scholar] [CrossRef]
- Hunt, G.R. Spectral signatures of particulate minerals in the visible and near infrared. Geophysics 1977, 42, 501–513. [Google Scholar] [CrossRef] [Green Version]
- Henderson, T.L.; Baumgardner, M.F.; Franzmeier, D.P.; Stott, D.E.; Coster, D.C. High dimensional reflectance analysis of soil organic matter. Soil Sci. Soc. Am. J. 1992, 56, 865–872. [Google Scholar] [CrossRef]
- Stevens, A.; Wesemael, B.V.; Bartholomeus, H.; Rosillon, D.; Tychon, B.; Bem-Dor, E. Laboratory, field, and airbone spectroscopy for monitoring organic carbon content in agricultural soils. Geoderma 2008, 144, 395–404. [Google Scholar] [CrossRef]
- Vasques, G.M.; Sabine Grunwald, S.; Harris, W. Spectroscopic models of soil organic carbon in Florida, USA. J. Environ. Qual. 2010, 39, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Demattê, J.A.M.; Garcia, G.J. Alteration of soil properties through a weathering sequence as evaluated by spectral reflectance. Soil Sci. Soc. Am. J. 1999, 63, 327–342. [Google Scholar] [CrossRef]
- Demattê, J.A.M.; Campos, R.C.; Alves, M.C.; Fiorio, P.R.; Nanni, M.R. Visible-NIR reflectance: A new approach on soil evaluation. Geoderma 2004, 121, 95–112. [Google Scholar] [CrossRef]
- Hunt, G.R.; Salisbury, J.W. Visible and infrared spectra of minerals and rocks: II. Carbonate. Mod. Geol. 1970, 2, 23–30. [Google Scholar]
- Nguyen, T.T.; Janik, L.J.; Raupach, M. Diffuse Reflectance Infrared Fourier Transform (DRIFT) Spectroscopy in soil studies. Aust. J. Soil Res. 1991, 29, 49–67. [Google Scholar] [CrossRef]
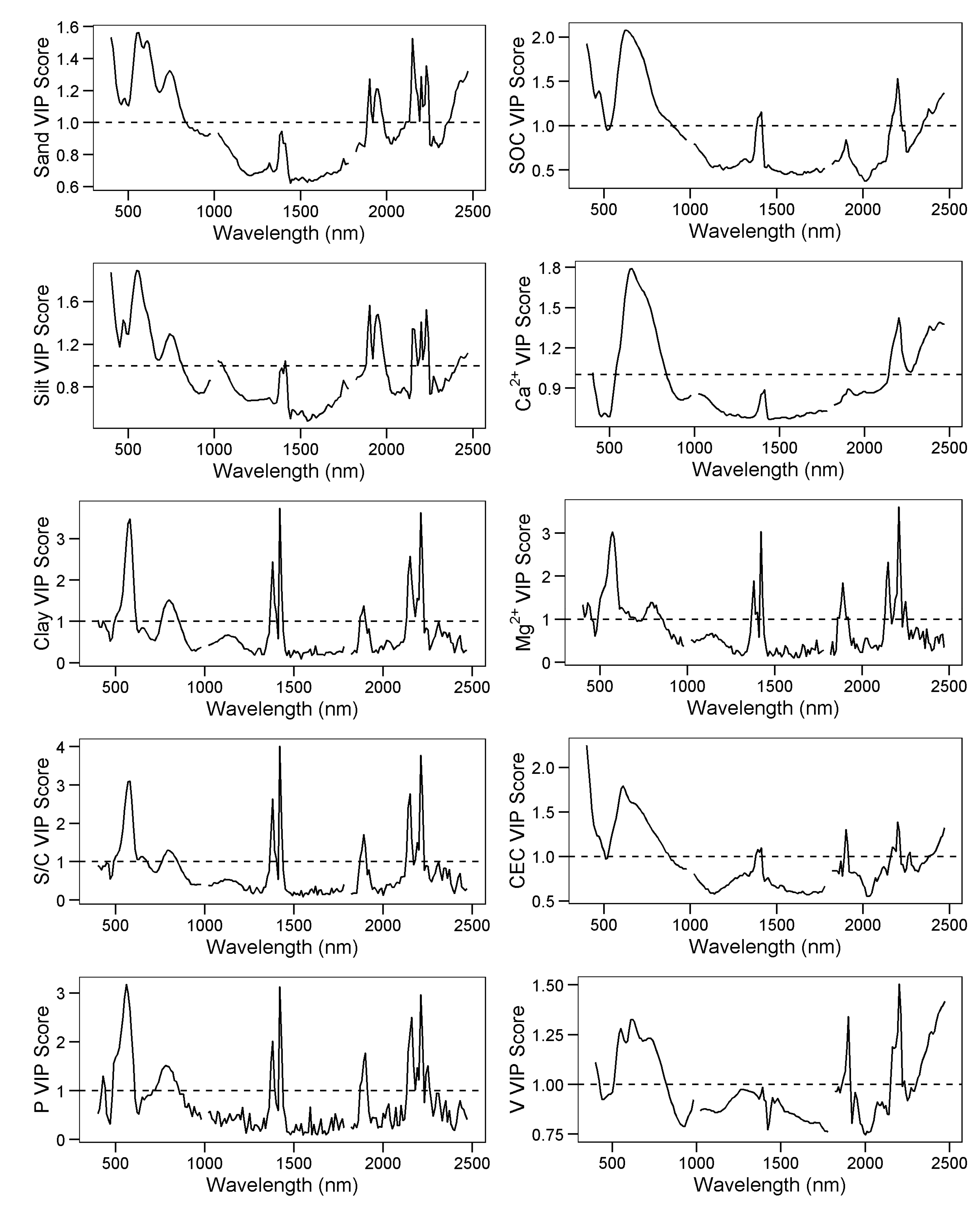
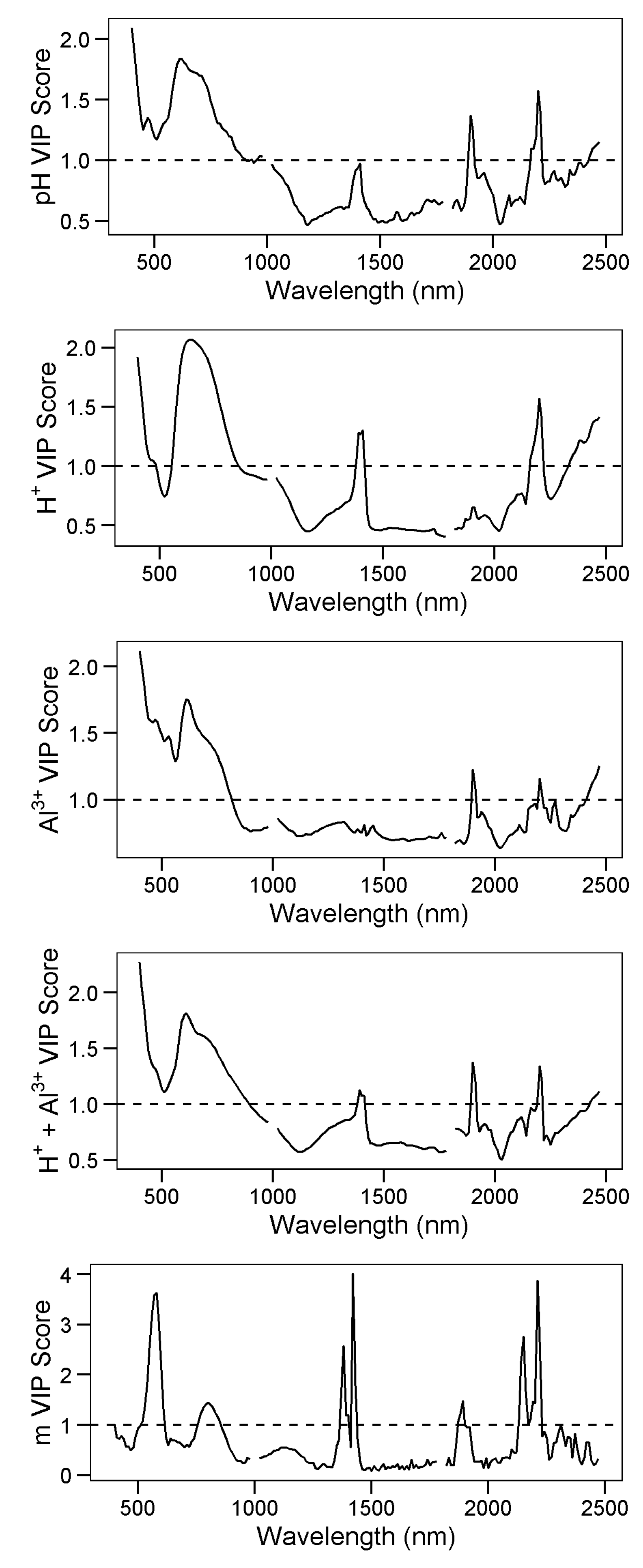
| SiBCS | Soil Taxonomy | n | Frequency (%) |
|---|---|---|---|
| Yellow Argisols | Oxyaquic Hapludults | 27 | 28 |
| Red Argisols | Typic Hapludults | 2 | 2 |
| Red-Yellow Argisols | Typic Hapludults | 21 | 22 |
| Greyish Argisols | Typic Endoaquults | 3 | 3 |
| Haplic Cambisols | Typic Dystrudepts | 39 | 41 |
| Spodosols | Humods | 1 | 1 |
| Quartzarenic Neosols | Quartzipsamments | 2 | 2 |
| Haplic Planosols | Aquults | 1 | 1 |
| Total | 96 | 100 |
| Soil Property | Min. | Mean | Max. | Median | SD | Variance | Skewness | Kurtosis | β |
|---|---|---|---|---|---|---|---|---|---|
| Sand (g·kg−1) | 22 | 397 | 884 | 385 | 177.5 | 31,512.3 | 0.42 | −0.27 | 0.58 |
| Silt (g·kg−1) | 50 | 344 | 660 | 341.5 | 118.8 | 14,122.8 | 0.04 | −0.02 | 0.93 |
| Clay (g·kg−1) | 10 | 257 | 640 | 251.5 | 133.1 | 17,720.2 | 0.24 | −0.68 | 0.68 |
| F (%) | 2 | 58 | 100 | 56 | 25.8 | 666.9 | −0.02 | −1.09 | 0.83 |
| S/C (unitless) | 0.1 | 2.0 | 13.2 | 1.4 | 1.9 | 3.65 | 2.58 | 8.27 | - |
| pH (unitless) | 3.2 | 4.5 | 6.5 | 4.7 | 0.4 | 0.20 | 0.04 | 0.63 | 0.93 |
| Ca (cmolc·kg−1) | 0.2 | 1.0 | 3.2 | 0.8 | 0.6 | 0.39 | 0.77 | −0.44 | - |
| Mg (cmolc·kg−1) | 0.1 | 0.56 | 2.2 | 0.5 | 0.4 | 0.15 | 1.59 | 2.85 | - |
| H (cmolc·kg−1) | 0.1 | 4.0 | 63.5 | 2.5 | 5.0 | 25.26 | 5.39 | 48.93 | - |
| Al (cmolc·kg−1) | 0.1 | 4.0 | 12 | 3.8 | 1.9 | 3.80 | 0.38 | 0.16 | 0.73 |
| H+Al (cmolc·kg−1) | 0.35 | 8.0 | 66.5 | 7.4 | 5.2 | 27.32 | 4.45 | 39.05 | 0.33 |
| P (mg·kg−1) | 0 | 1.37 | 13 | 1 | 1.7 | 3.00 | 2.48 | 9.13 | 2.50 |
| SOC (g·kg−1) | 0.1 | 7.99 | 105.6 | 6.2 | 8.1 | 65.9 | 5.45 | 51.74 | - |
| CEC (cmolc·kg−1) | 1 | 9.6 | 69.6 | 8.8 | 5.6 | 31.56 | 4.04 | 32.76 | - |
| SB (cmolc·kg−1) | 0.3 | 30.6 | 2924 | 1.4 | 251.2 | 63,126.4 | 8.96 | 82.54 | −0.48 |
| V (%) | 3 | 19 | 100 | 17 | 11.7 | 138.1 | 2.66 | 12.18 | - |
| m (%) | 7 | 68 | 96 | 72 | 18.2 | 332.2 | −0.99 | 0.70 | 2.04 |
| Properties | Sand | Silt | Clay | F | S/C | pH | Ca | Mg | SB | Al | H | H+Al | CEC | V | m | P | SOC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sand (g·kg−1) | 1.00 | ||||||||||||||||
| Silt (g·kg−1) | −0.64 | 1.00 | |||||||||||||||
| Clay (g·kg−1) | −0.74 | 0.02 | 1.00 | ||||||||||||||
| F (%) | −0.07 | 0.09 | 0.02 | 1.00 | |||||||||||||
| S/C (unitless) | 0.28 | 0.43 | −0.77 | 0.03 | 1.00 | ||||||||||||
| pH (unitless) | −0.14 | −0.08 | 0.19 | −0.23 | −0.11 | 1.00 | |||||||||||
| Ca (cmolc·kg−1) | 0.24 | 0.01 | −0.30 | −0.03 | 0.24 | −0.37 | 1.00 | ||||||||||
| Mg (cmolc·kg−1) | 0.20 | 0.11 | −0.35 | 0.09 | 0.32 | −0.32 | 0.52 | 1.00 | |||||||||
| SB (cmolc·kg−1) | 0.19 | 0.07 | −0.29 | 0.00 | 0.26 | −0.38 | 0.86 | 0.70 | 1.00 | ||||||||
| Al (cmolc·kg−1) | −0.64 | 0.16 | 0.70 | 0.11 | −0.50 | −0.09 | −0.13 | −0.21 | −0.17 | 1.00 | |||||||
| H (cmolc·kg−1) | 0.16 | 0.10 | −0.26 | 0.13 | 0.19 | −0.50 | 0.43 | 0.42 | 0.43 | 0.00 | 1.00 | ||||||
| H+Al (cmolc·kg−1) | −0.17 | 0.16 | 0.10 | 0.21 | −0.07 | −0.50 | 0.31 | 0.27 | 0.29 | 0.51 | 0.79 | 1.00 | |||||
| CEC (cmolc·kg−1) | −0.15 | 0.17 | 0.06 | 0.19 | −0.02 | −0.51 | 0.47 | 0.41 | 0.45 | 0.47 | 0.80 | 0.96 | 1.00 | ||||
| V (%) | 0.39 | −0.08 | −0.44 | −0.16 | 0.35 | 0.06 | 0.49 | 0.43 | 0.54 | −0.65 | −0.26 | −0.58 | −0.44 | 1.00 | |||
| m (%) | −0.56 | 0.06 | 0.70 | 0.07 | −0.55 | 0.17 | −0.61 | −0.62 | −0.65 | 0.79 | −0.26 | 0.17 | 0.06 | −0.79 | 1.00 | ||
| P (mg·kg−1) | 0.31 | −0.13 | −0.27 | −0.03 | 0.14 | −0.18 | 0.09 | 0.06 | 0.07 | −0.20 | 0.26 | 0.15 | 0.12 | −0.01 | −0.20 | 1.00 | |
| SOC (g·kg−1) | 0.26 | 0.10 | −0.39 | 0.14 | 0.32 | −0.53 | 0.50 | 0.47 | 0.51 | −0.13 | 0.67 | 0.51 | 0.52 | 0.09 | −0.42 | 0.33 | 1.00 |
| Soil Property | Pre-Processing | R2 | RMSE | RPD | RPIQ | Bias |
|---|---|---|---|---|---|---|
| Sand (g·kg−1) | SG.Q.3.0 | 0.62 | 114.74 | 1.61 | 2.05 | −17.37 |
| Silt (g·kg−1) | SNV | 0.36 | 95.15 | 1.25 | 1.68 | 3.20 |
| Clay (g·kg−1) | SG.L.3.1 | 0.78 | 61.70 | 2.14 | 3.35 | −1.75 |
| S/C (unitless) | SG.Q.9.1 | 0.62 | 1.09 | 1.63 | 1.42 | −0.03 |
| F (%) | SG.C.5.1 | 0.16 | 22.83 | 1.08 | 1.73 | −1.61 |
| pH (unitless) | SNV | 0.40 | 0.34 | 1.18 | 1.49 | 0.03 |
| Ca (cmolc·kg−1) | SG.C.5.0 | 0.40 | 0.52 | 1.26 | 1.92 | −0.08 |
| Mg (cmolc·kg−1) | SG.L.3.1 | 0.33 | 0.34 | 1.21 | 1.46 | −0.05 |
| H (cmolc·kg−1) | MSC | 0.79 | 4.40 | 1.45 | 0.70 | 0.32 |
| Al (cmolc·kg−1) | LOG | 0.63 | 1.22 | 1.62 | 2.36 | −0.18 |
| H+Al (cmolc·kg−1) | SNV | 0.70 | 3.61 | 1.80 | 1.08 | 0.15 |
| P (mg·kg−1) | SG.L.9.1 | 0.11 | 1.98 | 1.05 | 1.01 | −0.32 |
| SOC (g·kg−1) | SNV | 0.71 | 5.69 | 1.84 | 1.05 | 0.07 |
| CEC (cmolc·kg−1) | SNV | 0.68 | 5.86 | 1.17 | 0.82 | 0.75 |
| SB (cmolc·kg−1) | SG.L.11.2 | 0.17 | 296.39 | 1.00 | 0.00 | −35.55 |
| V (%) | SG.L.3.0 | 0.50 | 9.13 | 1.40 | 1.31 | −1.51 |
| m (%) | SG.C.7.1 | 0.59 | 11.65 | 1.57 | 2.10 | −0.38 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinheiro, É.F.M.; Ceddia, M.B.; Clingensmith, C.M.; Grunwald, S.; Vasques, G.M. Prediction of Soil Physical and Chemical Properties by Visible and Near-Infrared Diffuse Reflectance Spectroscopy in the Central Amazon. Remote Sens. 2017, 9, 293. https://doi.org/10.3390/rs9040293
Pinheiro ÉFM, Ceddia MB, Clingensmith CM, Grunwald S, Vasques GM. Prediction of Soil Physical and Chemical Properties by Visible and Near-Infrared Diffuse Reflectance Spectroscopy in the Central Amazon. Remote Sensing. 2017; 9(4):293. https://doi.org/10.3390/rs9040293
Chicago/Turabian StylePinheiro, Érika F. M., Marcos B. Ceddia, Christopher M. Clingensmith, Sabine Grunwald, and Gustavo M. Vasques. 2017. "Prediction of Soil Physical and Chemical Properties by Visible and Near-Infrared Diffuse Reflectance Spectroscopy in the Central Amazon" Remote Sensing 9, no. 4: 293. https://doi.org/10.3390/rs9040293