1. Introduction
UAS-based remote sensing technology has demonstrated the potential for precision agriculture by providing data at high temporal and spatial resolution [
1]. In precision agriculture, remote sensing has been used to monitor crop growth and health by computing a range of spectral vegetation indices [
2,
3,
4,
5,
6,
7,
8,
9]. However, recently more attention has been given to the use of crop height modelling for yield estimation [
10,
11,
12]. In this context, several studies have shown that crop productivity, in certain crop types (e.g., maize, potato, barley, wheat, corn, rice, sunflower and poppy [
11,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22], can be assessed from biophysical characteristics, such as crop height and biomass. Crop height is an important factor for yield estimation and crop management [
11,
19,
20,
21,
22,
23]. Crop height is a significant indicator of yield estimation in maize [
10], whereas, in barley, crop height has been utilised to estimate biomass [
11]. In the case of poppy crops, only a single study has looked at the use of remote sensing methods, in which crop height and Leaf Area Index (LAI) were found to be two suitable indicators for estimating opium yield [
21].
UAS have become a viable and cost-effective alternative to manned airborne or satellite remote sensing for precision agriculture, given their ability to collect high spatial resolution data at critical times during the growing season [
1,
24,
25,
26,
27]. Moreover, UAS aerial photography allows overlapping imagery to be collected, which is widely used for three-dimensional (3D) image reconstruction and facilitates change analysis [
11,
28,
29,
30,
31]. Three-dimensional measurements of crop structure are possible by generating Crop Surface Models (CSMs), which enable crop height measurement [
11,
26,
27,
32]. Availability of CSM at high spatial resolution and high vertical accuracy is of increasing importance for site-specific precision agriculture. The change in height values between the DTM at the time of sowing and DSM at crop maturity can be used to derive plant height, which can improve understanding of the relation between plant height, biomass, and yield [
11,
32].
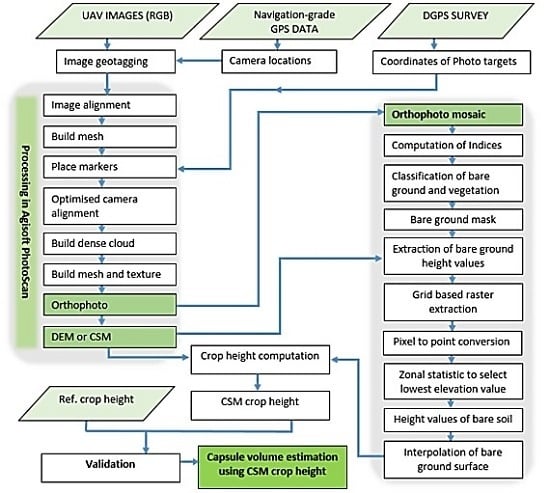
For the reliability and accuracy of estimated crop biomass or crop yield, the availability of timely information on crop height is important. In recent years, automated image orientation technique Structure from Motion (SfM) complemented by dense image-matching through Multi-View Stereo (MVS) has become a popular technique to derive 3D information about terrain and vegetation from UAS images [
11,
26,
27,
29,
32,
33,
34,
35]. The SfM-MVS workflow starts with automated image feature detection and identification followed by feature matching between overlapping images. Next, SfM-MVS uses bundle adjustment algorithms to simultaneously estimate the 3D geometry, the different camera poses (extrinsic calibration) and the camera intrinsic parameters (intrinsic calibration) [
34,
35,
36,
37]. The output of the SfM stage is a sparse, unscaled 3D point cloud in arbitrary units along with camera models and orientation [
29,
30,
34,
37]. The 3D point cloud can be registered to a projected coordinate system by identifying and incorporating ground control points in the bundle adjustment [
26,
27,
29,
38]. A dense image matching algorithm is then employed to extract a dense 3D point cloud from the images [
29,
34,
35,
36,
39,
40,
41,
42].
The SfM-MVS workflow has been successfully applied for estimation of crop height [
11,
18,
26,
32]. DTMs have been produced with imagery acquired at the sowing stage, and subsequent DSMs have been derived from UAS imagery at several critical crop stages [
11]. The vertical difference of both surface models (DSMs) and the DTM was used to estimate the crop height. A challenge with this method is that it requires either a precise DTM or at least two flights to collect the DTM before crop sowing and crop DSM during the growing season. It is not always feasible to collect a DTM at the start of the growing season, and, therefore, our study aims to estimate crop height from a single UAS flight.
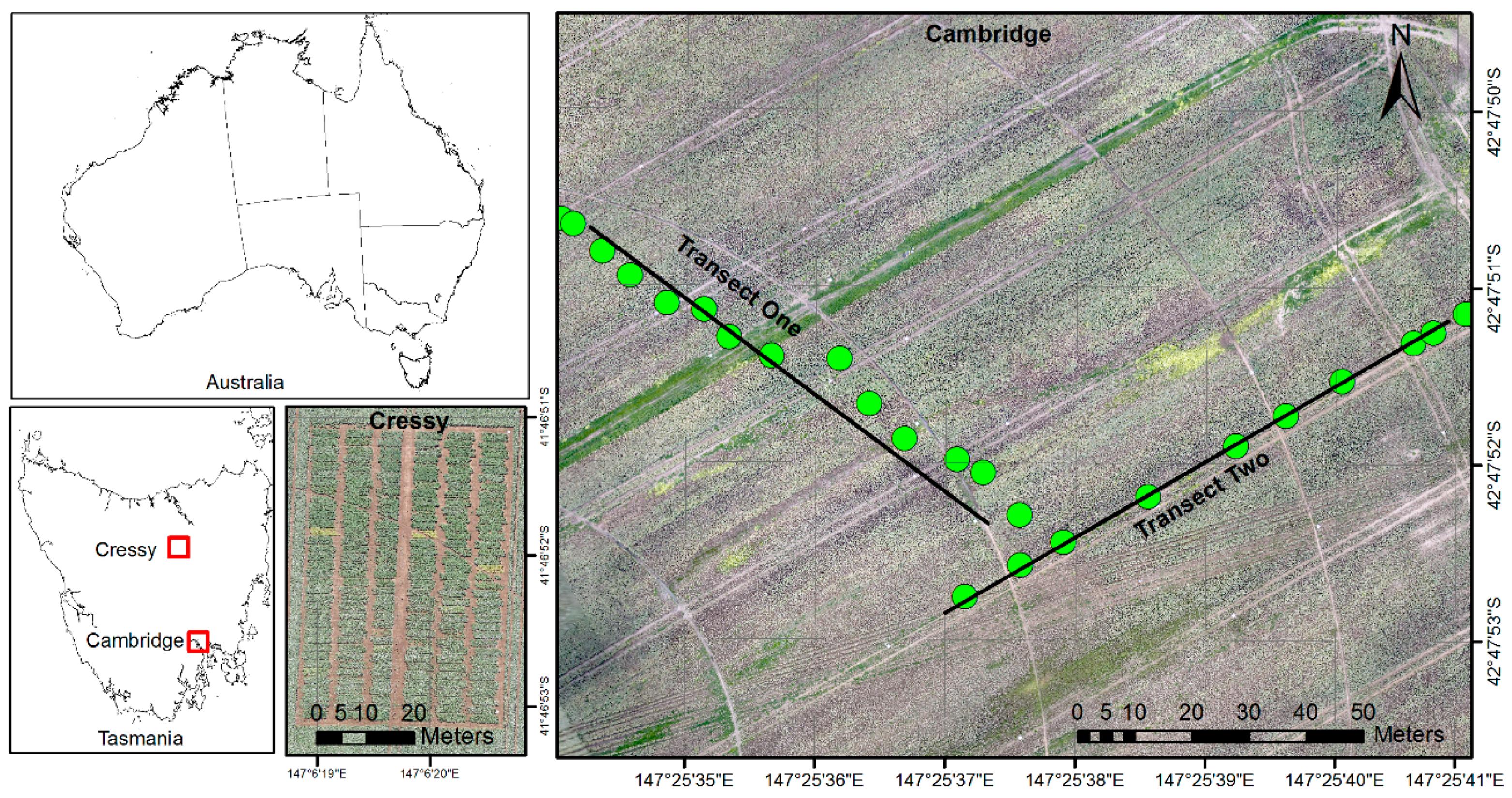
In this study, we focus on a high-value alkaloid poppy crop (
Papaver somniferum L.), grown for pharmaceutical applications, to demonstrate the application of UAS-based crop height assessment. In earlier studies, different physiological indicators, such as plant height, have been assessed to predict opium yield [
20,
21,
43,
44,
45,
46,
47,
48]. Spectral indices have been reported to estimate capsule volume and showed a significant correlation between NDVI and capsule volume from flowering till harvesting stage [
7]. In most fields, poppy crop growth is spatially highly heterogeneous and requires a substantial amount of data collection to develop models for yield estimation [
45]. Several studies have highlighted this phenomenon based on field observation of poppy crops grown under controlled conditions [
43,
45,
48,
49,
50]. Few studies have used remote sensing technologies for poppy yield estimation [
7,
21]. Data collected from field measurements, particularly physical measurements of the crop, provide reference information on crop growth. The spatial coverage of detailed field measurements is often limited, however remote sensing techniques can help to identify the spatial variability of key yield indicators at a much broader scale. This paper aims to investigate the potential use of low-cost image acquisition using a UAS-based platform for the estimation of poppy crop height and capsule volume. More specifically, the first objective of our study is to develop a new approach that facilitates crop height estimation from a single UAS flight. The second objective is to assess the potential of UAS-based CSM to predict poppy capsule volume (CV) using plant height as the predictor variable.
4. Discussion
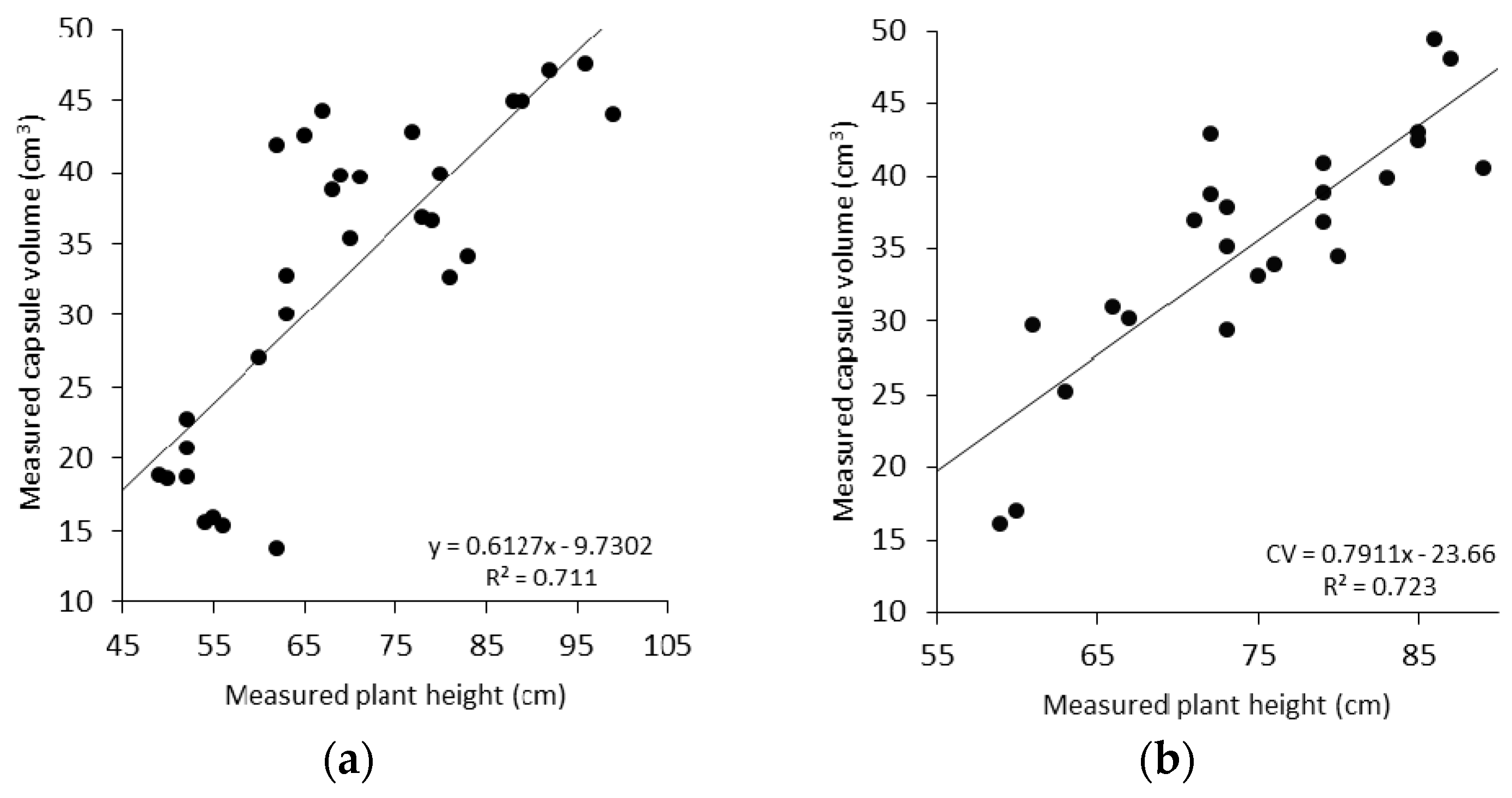
In this research, the analysis was carried out to estimate capsule volume from plant height determined at different developmental stages, and it was concluded that the capsule formation stage gives the best results in predicting capsule volume (R
2 = 0.71), which is in accordance with a previous study [
21]. Capsule volume can also be predicted at the flowering stage, but with lower accuracy (R
2 = 0.67). The coefficient of determination at the flowering stage was found to be low, as plant development was not uniform (i.e., some plants had progressed to the flowering stage while others remained at the stem elongation stage). This shows that at intermediate developmental stages of the crop the accuracy of the relationship between PH and CV is limited, as reported previously [
7].
PH plays an important role in providing information on crop development. Healthier plant development in early stages supports formation of larger capsules [
20,
45]. The current research shows that plant height information can be estimated using UAS-MVS remote sensing. Apart from apparent advantages of UAS remote sensing, there are many challenges associated with routine operations, such as small payload capacity, low spectral resolution, poor geometric and radiometric performance, low software automation, sensitivity to atmospheric conditions, short flight endurance, and possibility of equipment damage. Moreover, UAS users need to consider cost involved in repairs and maintenance, transportation, weather conditions and regulations for flying small UAS. Thus, multiple flight campaigns are not always possible. The weather, especially in Tasmania, is also a restricting factor. Spring and early summer can have high rainfall, so, it can be difficult to conduct UAS campaigns during this period at the relevant crop stages. Therefore, in this study, a simple and reliable method has been developed and tested for robust estimation of PH, based on a single UAS flight campaign, thereby overcoming the restriction of multiple flight campaigns and providing promising results for precision agriculture.
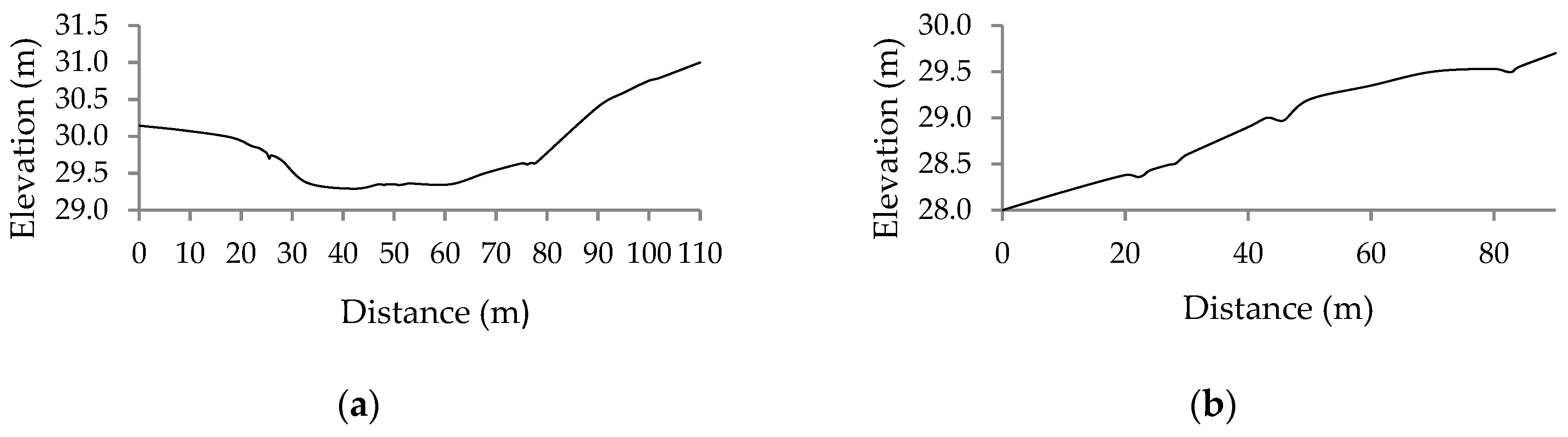
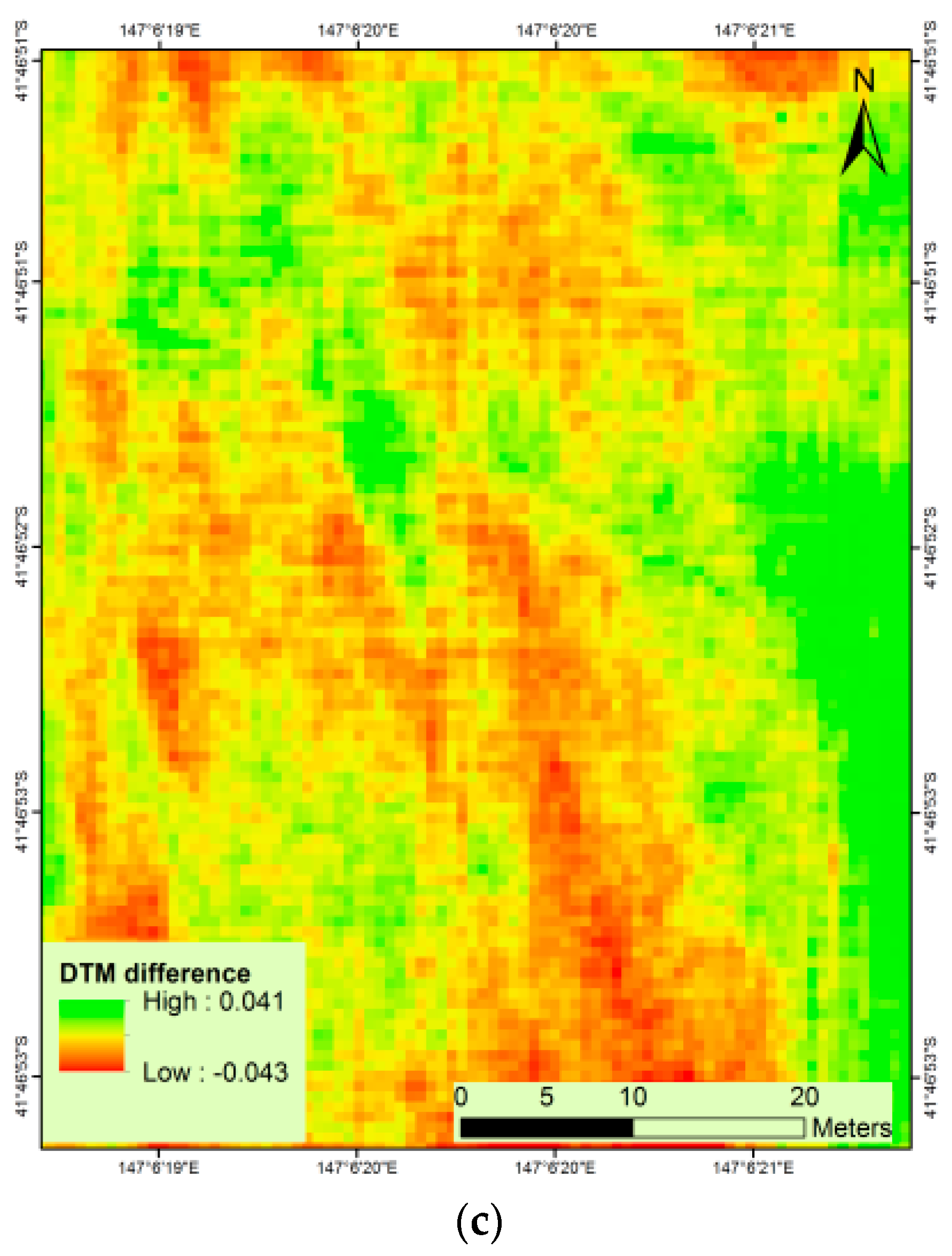
In this study, the visible ground areas within the field were utilised to detect the elevation of bare ground and were used to interpolate the DTM. The height values of soil points could also be extracted from vegetated imagery [
11,
27]. The accuracy of the DTM obtained from the single flight method depends on the distribution and size of bare ground patches visible in the mosaicked image. The error analysis of the Cambridge site DTM showed an underestimation, expressed by an RMSE of 9 cm. This indicates that a smaller number of bare ground patches cannot represent an accurate distribution of height values. Moreover, the accuracy of the DTM depends upon the terrain variation in the field. If the topography throughout the field is highly variable, then a large number of bare ground patches are needed to obtain an accurate DTM. In the case of the Cressy site with flatter topography but fewer bare ground patches, similar results were obtained (
Figure 10). The Cressy site results illustrate that the difference in DTM extracted at capsule formation stages and DTM generated at sowing stage varied approximately ±4 cm, which is much lower than the result of Cambridge site, and therefore more accurate. An accuracy assessment of the CSM resulted in an RMSE of 12.24 cm for the Cambridge site, whereas, Cressy resulted in RMSE of 5.60 cm, in accordance with the result of previous studies [
29,
31,
35,
38].
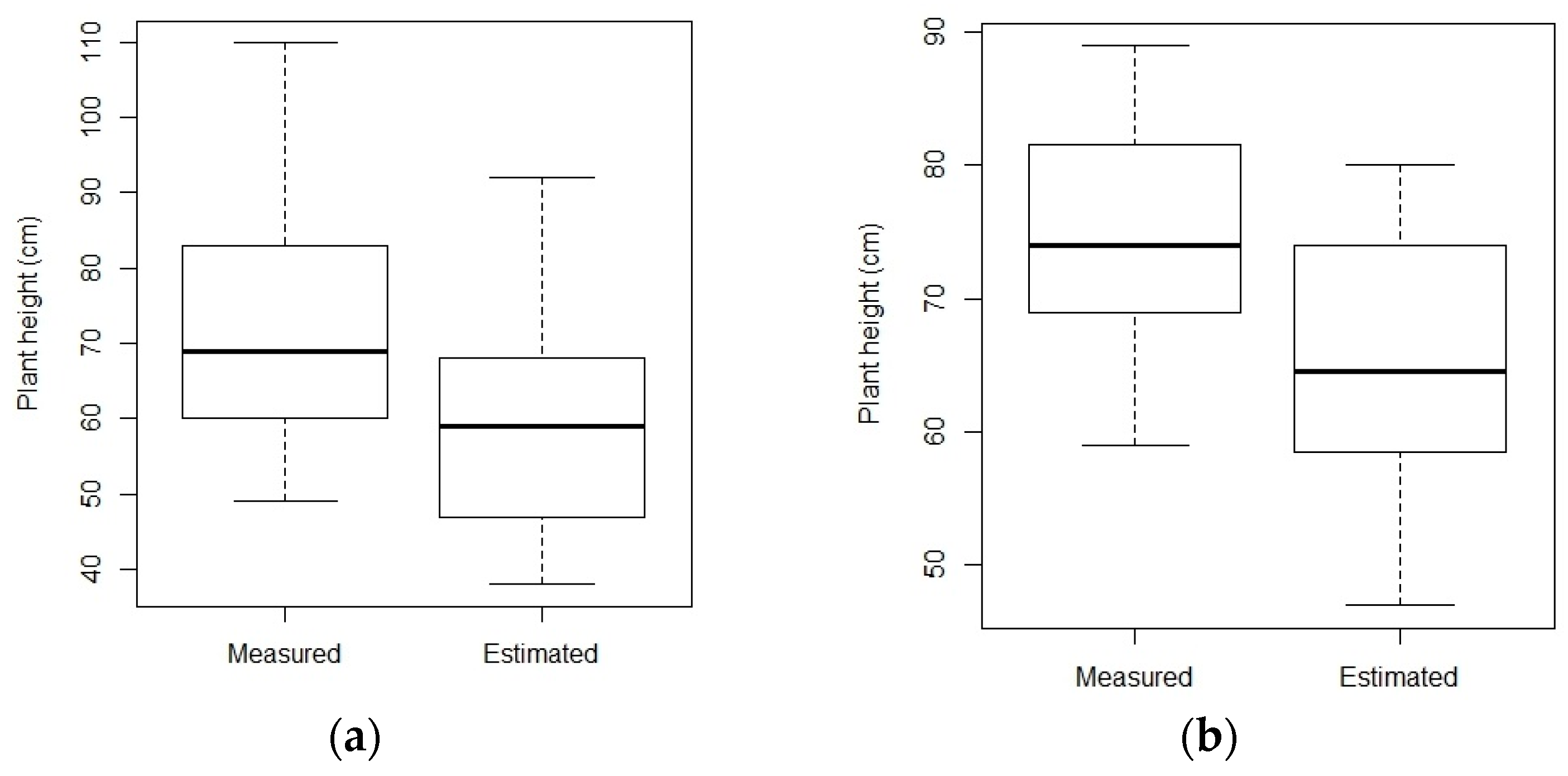
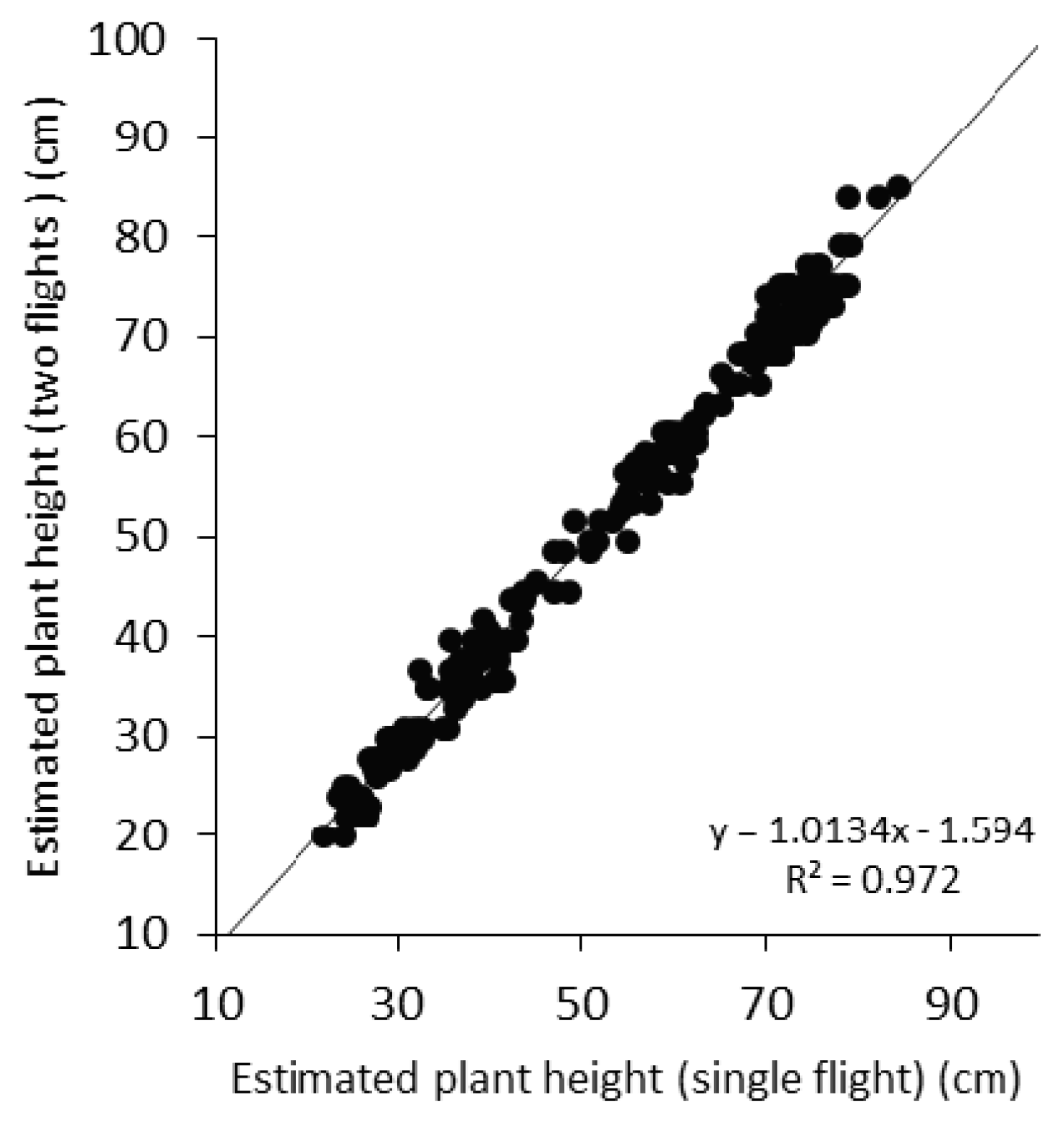
Crop height is generally underestimated, which is a common issue with vegetation height assessment with SfM-MVS [
11]. Crop height estimated from UAS compared with measured crop height resulted in a coefficient of determination of 0.93 in the case of T1, while in T2 it was slightly higher and reached up to 0.97. Cressy site results with a single campaign provide R
2 of 0.972, in accordance with a previous study [
11]. Results with two flights showed higher R
2 of 0.988, in accordance with other findings [
55]. Overall, the RMSE also varied depending on topography. In Cambridge farm, the relative error being 13.60% and 18.72% for transect T1 and T2, respectively. Conversely, in Cressy experiment, results illustrates that the crop height extracted with the single flight can provide similar results as compared to two flight campaigns, with the relative error ranging from 11.01% to 10.36%, respectively. This is because topography of Cressy farm was well levelled as compared to Cambridge site. Moreover, well distributed bare soil patches were available to detect, therefore, resulted in better estimation.
UAS remote sensing derived PH estimates used to simulate capsule volume showed the coefficient of determination of R2 = 0.738 with RMSE of 6.95 cm3 with a relative error of 19.62% at T2. The error is slightly higher for UAS derived CV as compared to the field-based model because of underestimation of UAS-derived plant height. The results showed a higher agreement between the estimated capsule volume and estimated plant height. To improve the model prediction for future research, the error inherited from DTM and DSM can be minimised by using an on-board RTK GNSS receiver as it will enable collection of accurate location and position information associated with each image and improve the georeferencing of acquired imagery. Moreover, an important factor is the size and distribution of bare ground patches. A larger number of bare ground patches with larger GCPs can be used to cover the whole variation of terrain, which would improve the accuracy of the DTM and DSM, resulting in a higher accuracy of the CSM.
The poppy capsule volume estimation model used in this study is an empirical model, and if implemented elsewhere, would require a new regression analysis based on field survey data, as the slope and offset of the regression equation would alter based on environmental circumstances and impacts of treatments, such as growth regulators. In Tasmania, poppy is grown as a legal crop and farmers are paid based on the alkaloid output of crops. Therefore, the poppy industry selects specific varieties for cropping, thus, the applicability of this model would need to be investigated on other varieties as well. Other factors that may change the relationship of PH with CV include different soil types, irrigation applications, and nutrients. Crop height estimation using the proposed methodology provides a cost-effective and time-saving way to acquire crop information. The proposed method reduces the time for field campaigns and reduces the cost of each flight campaign (GPS, UAV pilot, transportation, image processing etc.), which can help farm managers and researchers to manage crop zones according to crop requirement and therefore optimise yield.