Effects of Sleeve Gastrectomy vs. Roux-en-Y Gastric Bypass on Eating Behavior and Sweet Taste Perception in Subjects with Obesity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Experimental Procedures
2.2.1. Sensory-Discriminative Component of Taste Perception
- Detection Thresholds: Sucrose, glucose, NaCl, and MSG (monosodium glutamate, for savory/umami quality) detection thresholds were determined by utilizing a two-alternative, forced-choice staircase procedure [27].
- Suprathreshold: Subjects were trained on the use of the general labelled magnitude scale (gLMS) before we measured perceived intensities [28]. Two trials consisting of 4 ascending concentrations of each stimulus (sucrose, glucose, NaCl, MSG) with the first “concentration” being water were presented to the subjects. All four concentrations were presented in random order without repeat. Subjects rated the perceived intensity of the stimulus using the gLMS and we used the mean intensities of the two trials at each concentration for each stimulus to evaluate subjects’ taste intensity perception.
2.2.2. Hedonic Component of Taste Perception
- Preference Tests. We measured preferred concentrations of sucrose and MSG by using the Monell forced-choice, paired comparison tracking procedure [29]. Subjects were initially presented with two mid-range concentrations (180 vs. 700 mmol/L for sucrose or 11 vs. 37 mmol/L for MSG). They tasted the concentrations without swallowing and chose which solution they preferred. This procedure continued until they chose one concentration over concentrations that were both higher and lower, or the highest or lowest concentrations were chosen twice in a row. We presented the pairs in reverse order for the second trial. Subjects were allowed a 1 min interval between each pair, during which they rinsed their palette with water [27,29].
- Sweet Taste Palatability Test. Subjects tasted, without swallowing, a series of 10 samples containing 10 mL of 700 mmol/L (24% w/v) sucrose solution. They were asked to taste each sample for 10 s and received a new sample every two minutes with no interstimulus rinse. Immediately after tasting each sample, subjects rated the following two questions: “How pleasant was the taste?”, and “How strong is your desire for a different taste?” using a hedonic gLMS [28].
2.3. Taste Stimuli
2.4. Eating Behavior
2.5. Surgical Procedures
2.6. Diet Management after Surgery
2.7. Statistical Analyses
2.8. Power Analysis
3. Results
3.1. Demographics of Subjects
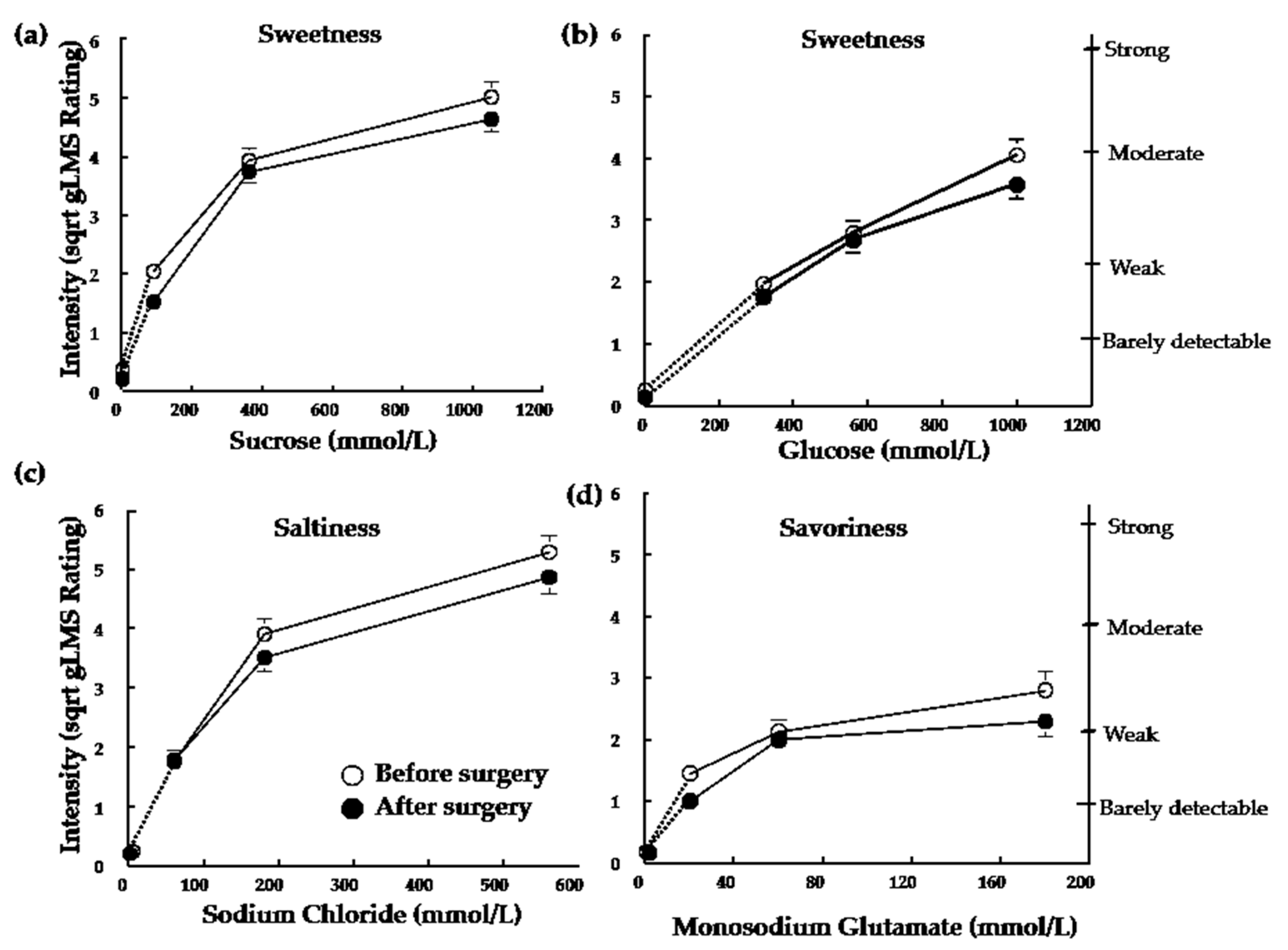
3.2. Sensory-Discriminative Component of Taste Perception
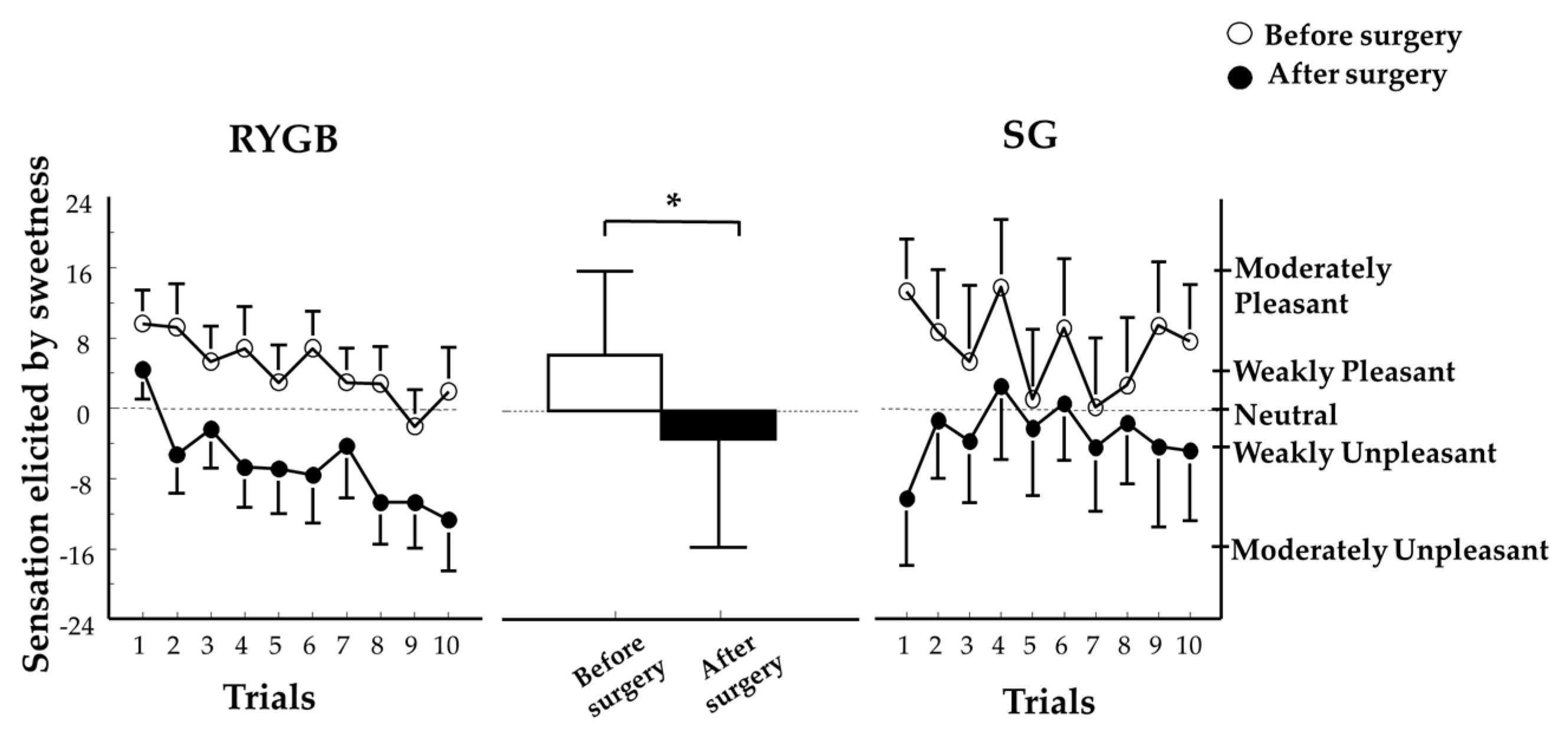
3.3. Hedonic Component of Taste Perception
3.3.1. Preferences
3.3.2. Sweet Taste Palatability
3.4. Eating Behavior
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brolin, R.E. Bariatric surgery and long-term control of morbid obesity. JAMA 2002, 288, 2793–2796. [Google Scholar] [CrossRef] [PubMed]
- Angrisani, L.; Santonicola, A.; Iovino, P.; Formisano, G.; Buchwald, H.; Scopinaro, H. Bariatric Surgery Worldwide 2013. Obes. Surg. 2017, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Rondelli, F.; Bugiantella, W.; Vedovati, M.C.; Mariani, E.; Canger, R.C.B.; Federici, S.; Guerra, A.; Boni, M. Laparoscopic gastric bypass versus laparoscopic sleeve gastrectomy: A retrospective multicenter comparison between early and long-term post-operative outcomes. Int. J. Surg. 2017, 37, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Albeladi, B.; Bourbao-Tournois, C.; Huten, N. Short-and midterm results between laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy for the treatment of morbid obesity. J. Obes. 2013. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Chong, K.; Ser, K.H.; Lee, Y.C.; Chen, S.C.; Chen, J.C.; Tsai, M.H.; Chuang, L.M. Gastric bypass vs. sleeve gastrectomy for type 2 diabetes mellitus: A randomized controlled trial. Arch. Surg. 2011, 146, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.; Clarke, M.G.; Evennett, N.J.; Robinson, S.J.; Humphreys, M.L.; Hammodat, H.; Jones, B.; Kim, D.D.; Cutfield, R.; Johnson, M.H.; et al. Laparoscopic sleeve gastrectomy versus banded Roux-en-Y gastric bypass for diabetes and obesity: A prospective randomised double-blind trial. Obes. Surg. 2017. [Google Scholar] [CrossRef] [PubMed]
- Schauer, P.R.; Bhatt, D.L.; Kirwan, J.P.; Wolski, K.; Aminian, A.; Brethauer, S.A.; Navaneethan, S.D.; Singh, R.P.; Pothier, C.E.; Nissen, S.R.; et al. Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N. Engl. J. Med. 2017, 376, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Breslin, P.A.S.; Spector, A.C. Mammalian taste perception. Curr. Biol. 2008, 18, R148–R155. [Google Scholar] [CrossRef] [PubMed]
- Sugerman, H.J.; Starkey, J.V.; Birkenhauer, R. A randomized prospective trial of gastric bypass versus vertical banded gastroplasty for morbid obesity and their effects on sweets versus non-sweets eaters. Ann. Surg. 1987, 205, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Brolin, R.E.; Robertson, L.B.; Kenler, H.A.; Cody, R.P. Weight loss and dietary intake after vertical banded gastroplasty and Roux-en-Y gastric bypass. Ann. Surg. 1994, 220, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Olbers, T.; Björkman, S.; Lindroos, A.; Maleckas, A.; Lönn, L.; Sjöström, L.; Lönroth, H. Body composition, dietary intake, and energy expenditure after laparoscopic Roux-en-Y gastric bypass and laparoscopic vertical banded gastroplasty: A randomized clinical trial. Ann. Surg. 2006, 244, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Ochner, C.N.; Kwok, Y.; Conceição, E.; Pantazatos, S.P.; Puma, L.M.; Carnell, S.; Teixeira, J.; Hirsch, J.; Geliebter, A. Selective reduction in neural responses to high calorie foods following gastric bypass surgery. Ann. Surg. 2011, 253, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Scholtz, S.; Miras, A.D.; Chhina, N.; Prechtl, C.G.; Sleeth, M.L.; Daud, N.M.; Ismail, N.A.; Durighel, G.; Ahmed, A.R.; Olbers, T.; et al. Obese patients after gastric bypass surgery have lower brain-hedonic responses to food than after gastric banding. Gut 2014, 63, 891–902. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Yang, Q.; Hajnal, A.; Rogers, A.M. A pilot functional MRI study in Roux-en-Y gastric bypass patients to study alteration in taste functions after surgery. Surg. Endosc. 2016, 30, 892–898. [Google Scholar] [CrossRef] [PubMed]
- Miras, A.D.; Jackson, R.N.; Jackson, S.N.; Goldstone, A.P.; Olbers, T.; Hackenberg, T.; Spector, A.C.; le Roux, C.W. Gastric bypass surgery for obesity decreases the reward value of a sweet-fat stimulus as assessed in a progressive ratio task. Am. J. Clin. Nutr. 2012, 96, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, J.; Ernst, B.; Wilms, B.; Thurnheer, M.; Schultes, B. Roux-en Y gastric bypass surgery reduces hedonic hunger and improves dietary habits in severely obese subjects. Obes. Surg. 2013, 23, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Shin, A.C.; Zheng, H.; Pistell, P.J.; Berthoud, H.R. Roux-en-Y gastric bypass surgery changes food reward in rats. Int. J. Obes. 2011, 35, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Hajnal, A.; Kovacs, P.; Ahmed, T.; Meirelles, K.; Lynch, C.J.; Cooney, R.N. Gastric bypass surgery alters behavioral and neural taste functions for sweet taste in obese rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G967–G979. [Google Scholar] [CrossRef] [PubMed]
- Bueter, M.; Miras, A.D.; Chichger, H.; Fenske, W.; Ghatei, M.A.; Bloom, S.R.; Unwin, R.J.; Lutz, T.A.; Spector, A.C.; le Roux, C.W. Alterations of sucrose preference after Roux-en-Y gastric bypass. Physiol. Behav. 2011, 104, 709–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pepino, M.Y.; Bradley, D.; Eagon, J.C.; Sullivan, S.; Abumrad, N.A.; Klein, S. Changes in taste perception and eating behavior after bariatric surgery-induced weight loss in women. Obesity 2014, 22, E13–E20. [Google Scholar] [CrossRef] [PubMed]
- Ammon, B.S.; Bellanger, D.E.; Geiselman, P.J.; Primeaux, S.D.; Yu, Y.; Greenway, F.L. Short-term pilot study of the effect of sleeve gastrectomy on food preference. Obes. Surg. 2015, 25, 1094–1097. [Google Scholar] [CrossRef] [PubMed]
- Primeaux, S.D.; Tzeng, T.H.; Allerton, T.D.; Chiang, M.C.; Cosentino, G.; Dubin, R.L.; Varughese, A.; Moore, R.; Geiselman, P.J.; Greenway, F.L.; et al. Differences in short-term food preferences following vertical sleeve gastrectomy and Roux-en-Y gastric bypass surgery. Obes. Res. Clin. Pract. 2015, 9, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Gero, D.; Dib, F.; Ribeiro-Parenti, L.; Arapis, K.; Chosidow, D.; Marmuse, J.-P. Desire for core tastes decreases after sleeve gastrectomy: A single-center longitudinal observational study with 6-month follow-up. Obes. Surg. 2017, 27, 2919–2926. [Google Scholar] [CrossRef] [PubMed]
- Van Vuuren, M.A.J.; Strodl, E.; White, K.M.; Lockie, P.D. Taste, enjoyment, and desire of flavors change after sleeve gastrectomy-short term results. Obes. Surg. 2017, 27, 1466–1473. [Google Scholar] [CrossRef] [PubMed]
- Naseer, F.; Lim, S.L.; So, J.B.; Lomanto, D.; Er, P.S.; Shen, L.; Kim, G.; Shabbir, A. The early effect of laporascopic sleeve gastrectomy on taste change in a multiethnic asian cohort. Ann. Acad. Med. Singap. 2017, 46, 254–255. [Google Scholar] [PubMed]
- Gero, D.; Steinert, R.E.; le Roux, C.W.; Bueter, M. Do food preferences change after bariatric surgery? Curr. Atheroscler. Rep. 2017, 19, 38. [Google Scholar] [CrossRef] [PubMed]
- Pepino, M.Y.; Finkbeiner, S.; Beauchamp, G.K.; Mennella, J.A. Obese women have lower monosodium glutamate taste sensitivity and prefer higher concentrations than do normal-weight women. Obesity 2010, 18, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Bartoshuk, L.M.; Duffy, V.B.; Green, B.G.; Hoffman, H.J.; Ko, C.-W.; Lucchina, L.A.; Marks, L.E.; Snyder, D.J.; Weiffenbach, J.M. Valid across-group comparisons with labeled scales: The gLMS versus magnitude matching. Physiol. Behav. 2004, 82, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Coldwell, S.E.; Mennella, J.A.; Duffy, V.B.; Pelchat, M.L.; Griffith, J.W.; Smutzer, G.; Cowart, B.J.; Breslin, P.A.S.; Bartoshuk, L.M.; Hastings, L.; et al. Gustation assessment using the NIH Toolbox. Neurology 2013, 80, S20–S24. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, G.K.; Vazquez de Vaquera, M.; Pearson, P.B. Dietary Status of Human Infants and Their Sensory Responses to Amino Acid Flavor; Kawamura, Y., Kare, M.R., Eds.; Umami: A Basic Taste; Marcel Dekker: New York, NY, USA, 1987; pp. 125–138. [Google Scholar]
- Van Strien, T.; Frijters, J.E.R.; Bergers, G.P.A.; Defares, P.B. The Dutch Eating Behavior Questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. Int. J. Eat. Disord. 1986, 5, 295–315. [Google Scholar] [CrossRef]
- White, M.A.; Whisenhunt, B.L.; Williamson, D.A.; Greenway, F.L.; Netemeyer, R.G. Development and validation of the food-craving inventory. Obes. Res. 2002, 10, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar]
- Pepino, M.Y.; Mennella, J.A. Effects of cigarette smoking and family history of alcoholism on sweet taste perception and food cravings in women. Alcohol. Clin. Exp. Res. 2007, 31, 1891–1899. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.K.; O’Neil, P.M.; Pawlow, L. Changes in food cravings during low-calorie and very-low-calorie diets. Obesity 2006, 14, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.K.; Rosenbaum, D.; Han, H.M.; Geiselman, P.J.; Wyatt, H.R.; Hill, J.O.; Brill, C.; Bailer, B.; Miller-III, B.V.; Stein, R.; et al. Change in food cravings, food preferences, and appetite during a low-carbohydrate and low-fat diet. Obesity 2011, 19, 1963–1970. [Google Scholar] [CrossRef] [PubMed]
- Wilson-Pérez, H.E.; Chambers, A.P.; Sandoval, D.A.; Stefater, M.A.; Woods, S.C.; Benoit, S.C.; Seeley, R.J. The effect of vertical sleeve gastrectomy on food choice in rats. Int. J. Obes. 2013, 37, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Burgess, B.; Rao, S.P.; Tepper, B.J. Changes in liking for sweet and fatty foods following weight loss in women are related to prop phenotype but not to diet. Obesity 2016, 24, 1867–1873. [Google Scholar] [CrossRef] [PubMed]
- Peterli, R.; Wölnerhanssen, B.; Peters, T.; Devaux, N.; Kern, B.; Christoffel-Courtin, C.; Drewe, J.; von Flüe, M.; Beglinger, C. Improvement in glucose metabolism after bariatric surgery: Comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: A prospective randomized trial. Ann. Surg. 2009, 250, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Karamanakos, S.N.; Vagenas, K.; Kalfarentzos, F.; Alexandrides, T. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy: A prospective, double blind study. Ann. Surg. 2008, 247, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Goldstone, A.P.; Miras, A.D.; Scholtz, S.; Jachson, S.; Neff, K.J.; Pénicaud, L.; Geoghegan, J.; Chhina, N.; Durighel, G.; Meillon, J.D.B.S.; et al. Link between increased satiety gut hormones and reduced food reward after gastric bypass surgery for obesity. J. Clin. Endocrinol. Metab. 2016, 101, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, C.W.; Bueter, M.; Theis, N.; Werling, M.; Ashrafian, H.; Löwenstein, C.; Athanasiou, T.; Bloom, S.R.; Spector, A.C.; Olbers, T.; et al. Gastric bypass reduces fat intake and preference. Am. J. Phys. Regul. Integr. Comp. Physiol. 2011, 301, R1057–R1066. [Google Scholar] [CrossRef] [PubMed]
- Mathes, C.M.; Letourneau, C.; Blonde, G.D.; le Roux, C.W.; Spector, A.C. Roux-en-Y gastric bypass in rats progressively decreases the proportion of fat calories selected from a palatable cafeteria diet. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 310, R952–R959. [Google Scholar] [CrossRef] [PubMed]
- Chambers, A.P.; Wilson-Perez, H.E.; McGrath, S.; Grayson, B.E.; Ryan, K.K.; D’Alessio, D.A.; Woods, S.C.; Sandowal, D.A.; Seeley, R.J. Effect of vertical sleeve gastrectomy on food selection and satiation in rats. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E1076–E1084. [Google Scholar] [CrossRef] [PubMed]
- Hankir, M.K.; Seyfried, F.; Hintschich, C.A.; Diep, T.-A.; Kleberg, K.; Kranz, M.; Deuther-Conrad, W.; Tellez, L.A.; Rullmann, M.; Patt, M.; et al. Gastric bypass surgery recruits a gut PPAR-alpha-Striatal D1R pathway to reduce fat appetite in obese rats. Cell Metab. 2017, 25, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Han, W.F.; Tellez, L.A.; Niu, J.J.; Medina, S.; Ferreira, T.; Zhang, X.B.; Su, J.S.; Tong, J.; Schwartz, G.J.; van del Pol, A.; et al. Striatal dopamine links gastrointestinal rerouting to altered sweet appetite. Cell Metab. 2016, 23, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Cavin, J.B.; Couvelard, A.; Lebtahi, R.; Ducroc, R.; Arapis, K.; Voitellier, E.; Cuzeaud, F.; Gillard, L.; Hourseau, M.; Mikail, N.; et al. Differences in alimentary glucose absorption and intestinal disposal of blood glucose after Roux-en-Y gastric bypass vs. sleeve gastrectomy. Gastroenterology 2016, 150, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Beekley, A.; Kjorstad, R.; Sebesta, J. Socioeconomic disparities in eligibility and access to bariatric surgery: A national population-based analysis. Surg. Obes. Relat. Dis. 2010, 6, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Mack, I.; Ölschläger, S.; Sauer, H.; von Feilitzsch, M.; Weimer, K.; Junne, F.; Peeraully, R.; Enck, P.; Zipfel, S.; Teufel, M. Does laparoscopic sleeve gastrectomy improve depression, stress and eating behaviour? A 4-year follow-up study. Obes. Surg. 2016, 26, 2967–2973. [Google Scholar] [CrossRef] [PubMed]
- De Zwaan, M.; Hilbert, A.; Swan-Kremeier, L.; Simonich, H.; Lancaster, K.; Howell, M.; Monson, T.; Crosby, R.D.; Mitchell, J.E. Comprehensive interview assessment of eating behavior 18–35 months after gastric bypass surgery for morbid obesity. Surg. Obes. Relat. Dis. 2010, 6, 79–85. [Google Scholar] [CrossRef] [PubMed]
| Variables | RYGB (n = 23) | SG (n = 8) | p Value |
|---|---|---|---|
| Age (years) | 43.0 ± 9.6 | 36.6 ± 9.9 | 0.45 |
| Race (%) | |||
| White | 78.3 | 75.0 | |
| Black | 13.0 | 25.0 | 0.54 |
| Other/Mixed | 8.7 | 0.0 | |
| Gender (%) | |||
| Female | 87.0 | 87.5 | 0.97 |
| Male | 13.0 | 12.5 | |
| Body Weight (kg) | |||
| Before surgery | 129.8 ± 26.1 | 143.3 ± 16.2 | 0.14 |
| After surgery | 104.6 ± 24.1 | 115.6 ± 12.8 | 0.20 |
| % Weight Loss | 19.8 ± 3.7 | 19.3 ± 1.8 | 0.77 |
| Days to Achieve ~20% Weight Loss | 99.2 ± 44.3 | 141.3 ± 27.7 | 0.02 |
| BMI (kg/m2) | |||
| Before surgery | 46.9 ± 7.5 | 53.3 ± 8.7 | 0.31 |
| After surgery | 37.6 ± 6.7 | 43.0 ± 7.2 | 0.13 |
| Variable | RYGB (n = 23) | SG (n = 8) | ||||
|---|---|---|---|---|---|---|
| Before Surgery | After Surgery | Before Surgery | After Surgery | p Value (Time) | p Value (Group X Time) | |
| Detection Thresholds (mmol/L) | ||||||
| Glucose | 27.6 ± 14.1 | 27.6 ± 18.5 | 34.1 ± 5.3 | 39.5 ± 11.8 | 0.61 | 0.89 |
| Sucrose | 7.5 ± 5.1 | 6.5 ± 1.9 | 8.8 ± 5.0 | 6.1 ± 2.1 | 0.92 | 0.40 |
| NaCl | 2.4 ± 1.4 | 1.8 ± 0.9 | 2.0 ± 2.3 | 1.8 ± 1.1 | 0.24 | 0.66 |
| MSG | 1.2 ± 0.8 | 1.3 ± 0.9 | 1.3 ± 0.8 | 1.3 ± 1.3 | 0.89 | 0.46 |
| Food Cravings | ||||||
| High Fat | 2.2 ± 0.7 | 1.8 ± 0.6 | 2.1 ± 0.6 | 1.6 ± 0.4 | <0.01 | 0.78 |
| Carbohydrates | 2.2 ± 0.8 | 1.9 ± 0.7 | 2.6 ± 0.6 | 1.8 ± 0.5 | <0.01 | 0.07 |
| Sweets | 2.3 ± 0.8 | 1.6 ± 0.6 | 2.5 ± 0.8 | 1.8 ± 0.6 | <0.01 | 0.86 |
| Fast Food | 2.9 ± 0.7 | 2.2 ± 0.6 | 2.9 ± 0.7 | 2.0 ± 0.6 | <0.01 | 0.40 |
| DEBQ | ||||||
| Restrained | 2.9 ± 0.6 | 3.0 ± 0.7 | 2.9 ± 0.5 | 3.6 ± 0.6 | 0.02 | 0.06 |
| Emotional | 2.6 ± 0.9 | 1.8 ± 0.7 | 2.8 ± 0.9 | 2.2 ± 0.6 | <0.01 | 0.57 |
| External | 3.0 ± 0.5 | 2.2 ± 0.5 | 3.1 ± 0.6 | 2.3 ± 0.3 | <0.01 | 0.44 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nance, K.; Eagon, J.C.; Klein, S.; Pepino, M.Y. Effects of Sleeve Gastrectomy vs. Roux-en-Y Gastric Bypass on Eating Behavior and Sweet Taste Perception in Subjects with Obesity. Nutrients 2018, 10, 18. https://doi.org/10.3390/nu10010018
Nance K, Eagon JC, Klein S, Pepino MY. Effects of Sleeve Gastrectomy vs. Roux-en-Y Gastric Bypass on Eating Behavior and Sweet Taste Perception in Subjects with Obesity. Nutrients. 2018; 10(1):18. https://doi.org/10.3390/nu10010018
Chicago/Turabian StyleNance, Katie, J. Christopher Eagon, Samuel Klein, and Marta Yanina Pepino. 2018. "Effects of Sleeve Gastrectomy vs. Roux-en-Y Gastric Bypass on Eating Behavior and Sweet Taste Perception in Subjects with Obesity" Nutrients 10, no. 1: 18. https://doi.org/10.3390/nu10010018




