Small Intestinal Absorption of Methylsulfonylmethane (MSM) and Accumulation of the Sulfur Moiety in Selected Tissues of Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Their Care
2.2. Small Intestinal Absorption of MSM
2.3. Tissue Accumulation of MSM
2.4. Statistics
3. Results
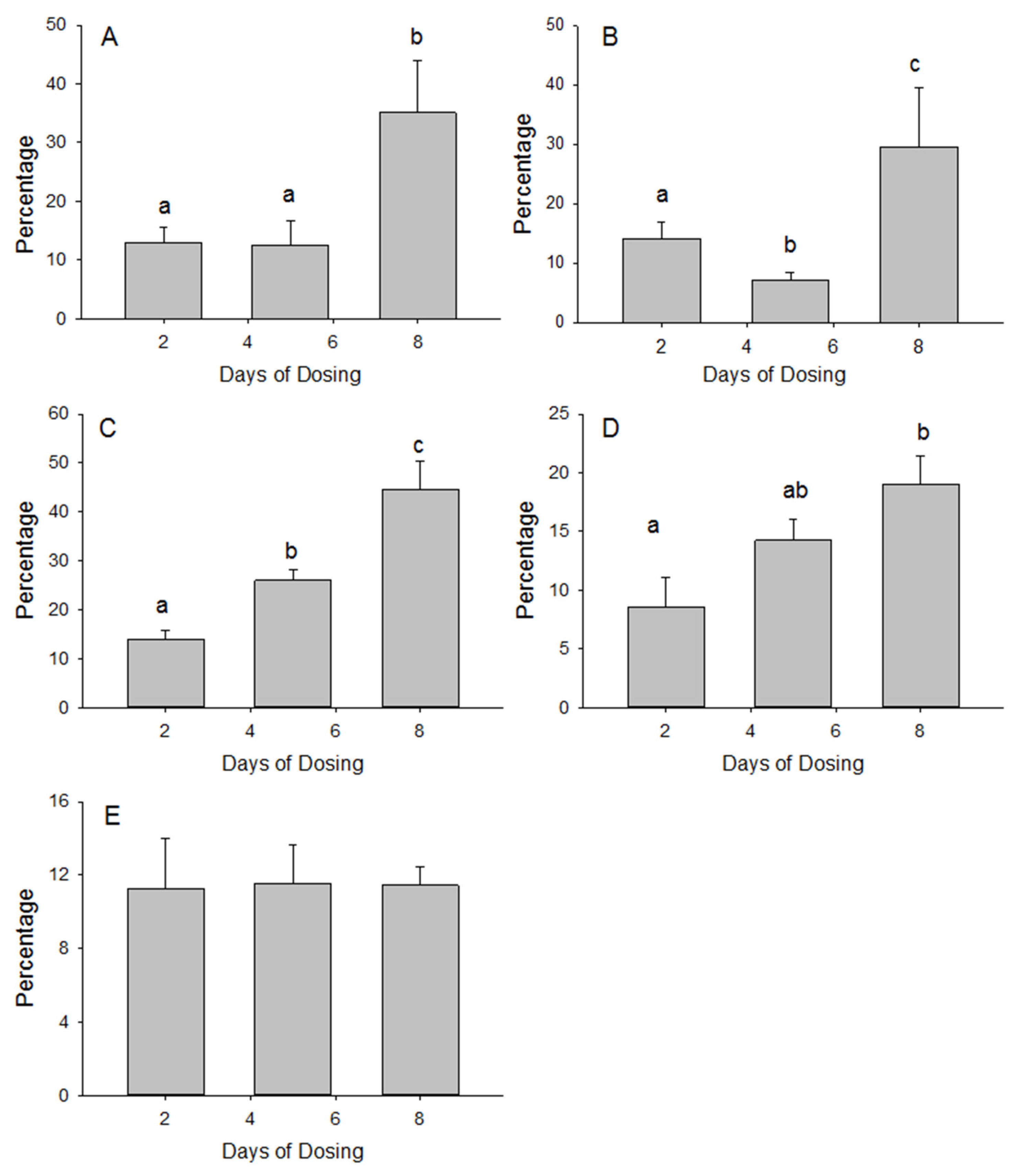
3.1. Small Intestine MSM Absorption
3.2. Tissue Accumulation of MSM
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Van de Poll, M.C.; Dejong, C.H.; Soeters, P.B. Adequate range for sulfur-containing amino acids and biomarkers for their excess: Lessons from enteral and parenteral nutrition. J. Nutr. 2006, 136 (Suppl. 6), 1694S–1700S. [Google Scholar] [PubMed]
- Nimni, M.E.; Han, B.; Cordoba, F. Are we getting enough sulfur in our diet? Nutr. Metab. 2007, 6. [Google Scholar] [CrossRef] [PubMed]
- Butawan, M.; Benjamin, R.L.; Bloomer, R.J. Methylsulfonylmethane: Applications and Safety of a Novel Dietary Supplement. Nutrients 2017, 16, E290. [Google Scholar] [CrossRef] [PubMed]
- Herschler, R.J. Inventor and Assignee. Use of methylsulfonylmethane to Enhance the Diet of an Animal. U.S. Patent 5,071,878, 10 December 1991. [Google Scholar]
- Herschler, R.J. Inventor and Assignee. Methylsulfonylmethane in Dietary Products. U.S. Patent 4616039, 29 April 1985. [Google Scholar]
- Available online: https://www.credenceresearch.com/press/global-methylsulfonylmethane-market (accessed on 10 December 2017).
- Ingenbleek, Y.; McCully, K.S. Vegetarianism produces subclinical malnutrition, hyperhomocysteinemia and atherogenesis. Nutrition 2012, 28, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Ingenbleek, Y.; Kimura, H. Nutritional essentiality of sulfur in health and disease. Nutr. Rev. 2013, 71, 413–432. [Google Scholar] [CrossRef] [PubMed]
- Dawson, P.A.; Richard, K.; Perkins, A.; Zhang, Z.; Simmons, D.G. Review: Nutrient sulfate supply from mother to fetus: Placental adaptive responses during human and animal gestation. Placenta 2017, 54, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Langford, R.; Hurrion, E.; Dawson, P.A. Genetics and pathophysiology of mammalian sulfate biology. J. Genet. Genom. 2017, 44, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Nguy, C.H.; Shic, F.; Ross, B.D. Accumulation of methylsulfonylmethane in the human brain: Identification by multinuclear magnetic resonance spectroscopy. Toxicol. Lett. 2001, 123, 169–177. [Google Scholar] [CrossRef]
- Richmond, V.L. Incorporation of methylsulfonylmethane sulfur into guinea pig serum proteins. Life Sci. 1986, 39, 263–268. [Google Scholar] [CrossRef]
- Daniels, J.L.; Bloomer, R.J.; van der Merwe, M.; Davis, S.L.; Buddington, K.K.; Buddington, R.K. Intestinal adaptations to a combination of different diets with and without endurance exercise. J. Int. Soc. Sports Nutr. 2016, 13. [Google Scholar] [CrossRef] [PubMed]
- Magnuson, B.A.; Appleton, J.; Ames, G.B. Pharmacokinetics and distribution of [35S]methylsulfonylmethane following oral administration to rats. J. Agric. Food Chem. 2007, 55, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- Layman, D.L.; Jacob, S.W. The absorption, metabolism and excretion of dimethyl sulfoxide by rhesus monkeys. Life Sci. 1985, 37, 2431–2437. [Google Scholar] [CrossRef]
- Curno, R.; Magee, E.A.; Edmond, L.M.; Cummings, J.H. Studies of a urinary biomarker of dietary inorganic sulphur in subjects on diets containing 1–38 mmol sulphur/day and of the half-life of ingested 34SO4(2-). Eur. J. Clin. Nutr. 2008, 62, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Beck, L.; Silve, C. Molecular aspects of renal tubular handling and regulation of inorganic sulfate. Kidney Int. 2001, 59, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Otsuki, S.; Qian, W.; Ishihara, A.; Kabe, T. Elucidation of dimethylsulfone metabolism in rat using a 35S radioisotope tracer method. Nutr. Res. 2002, 22, 313–322. [Google Scholar] [CrossRef]
- Magee, E.A.; Curno, R.; Edmond, L.M.; Cummings, J.H. Contribution of dietary protein and inorganic sulfur to urinary sulfate: Toward a biomarker of inorganic sulfur intake. Am. J. Clin. Nutr. 2004, 80, 137–142. [Google Scholar] [PubMed]
- He, X.; Slupsky, C.M. Metabolic fingerprint of dimethyl sulfone (DMSO2) in microbial-mammalian co-metabolism. J. Proteome Res. 2014, 13, 5281–5292. [Google Scholar] [CrossRef] [PubMed]
- Metges, C.C.; Eberhard, M.; Petzke, K.J. Synthesis and absorption of intestinal microbial lysine in humans and non-ruminant animals and impact on human estimated average requirement of dietary lysine. Curr. Opin. Clin. Nutr. Metab. Care 2006, 9, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Carbonero, F.; Benefiel, A.C.; Alizadeh-Ghamsari, A.H.; Gaskins, H.R. Microbial pathways in colonic sulfur metabolism and links with health and disease. Front. Physiol. 2012, 3, 448. [Google Scholar] [CrossRef] [PubMed]
- Tobisawa, Y.; Imai, Y.; Fukuda, M.; Kawashima, H. Sulfation of colonic mucins by N-acetylglucosamine 6-O-sulfotransferase-2 and its protective function in experimental colitis in mice. J. Biol. Chem. 2010, 285, 6750–6760. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.S.; Wang, C.C.; Chen, B.H.; Hou, Y.H.; Hung, K.S.; Mao, Y.C. Tyrosine sulfation as a protein post-translational modification. Molecules 2015, 20, 2138–2164. [Google Scholar] [CrossRef] [PubMed]
- Alnouti, Y.; Klaassen, C.D. Tissue distribution and ontogeny of sulfotransferase enzymes in mice. Toxicol. Sci. 2006, 93, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Gregus, Z.; Kim, H.J.; Madhu, C.; Liu, Y.; Rozman, P.; Klaassen, C.D. Sulfation of acetaminophen and acetaminophen-induced alterations in sulfate and 3′-phosphoadenosine 5′-phosphosulfate homeostasis in rats with deficient dietary intake of sulfur. Drug Metab. Dispos. 1994, 22, 725–730. [Google Scholar] [PubMed]
- Raouf, A.H.; Tsai, H.H.; Parker, N.; Hoffman, J.; Walker, R.J.; Rhodes, J.M. Sulphation of colonic and rectal mucin in inflammatory bowel disease: Reduced sulphation of rectal mucus in ulcerative colitis. Clin. Sci. 1992, 83, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Denko, C.W.; Petricevic, M.; Whitehouse, M.W. 35s incorporation in rats in relation to deprivation of copper and zinc in the diet. Int. J. Tissue React. 1981, 3, 121–125. [Google Scholar] [PubMed]
- Bohlooli, S.; Mohammadi, S.; Amirshahrokhi, K.; Mirzanejad-Asl, H.; Yosefi, M.; Mohammadi-Nei, A.; Chinifroush, M.M. Effect of Methylsulfonylmethane Pretreatment on Aceta-minophen Induced Hepatotoxicity in Rats. Iran J. Basic Med. Sci. 2013, 16, 896–900. [Google Scholar] [PubMed]
- Pecora, F.; Gualeni, B.; Forlino, A.; Superti-Furga, A.; Tenni, R.; Cetta, G.; Rossi, A. In vivo contribution of amino acid sulfur to cartilage proteoglycan sulfation. Biochem. J. 2006, 398, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Amirshahrokhi, K.; Bohlooli, S. Effect of methylsulfonylmethane on paraquat-induced acute lung and liver injury in mice. Inflammation 2013, 36, 1111–1121. [Google Scholar] [CrossRef] [PubMed]
- Nakhostin-Roohi, B.; Barmaki, S.; Khoshkhahesh, F.; Bohlooli, S. Effect of chronic supplementation with methylsulfonylmethane on oxidative stress following acute exercise in untrained healthy men. J. Pharm. Pharmacol. 2011, 63, 1290–1294. [Google Scholar] [CrossRef] [PubMed]
- Marañón, G.; Muñoz-Escassi, B.; Manley, W.; García, C.; Cayado, P.; de la Muela, M.S.; Olábarri, B.; León, R.; Vara, E. The effect of methyl sulphonyl methane supplementation on biomarkers of oxidative stress in sport horses following jumping exercise. Acta Vet. Scand. 2008, 50, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amirshahrokhi, K.; Bohlooli, S.; Chinifroush, M.M. The effect of methylsulfonylmethane on the experimental colitis in the rat. Toxicol. Appl. Pharmacol. 2011, 253, 197–202. [Google Scholar] [CrossRef] [PubMed]
| Region | 50 mmol | Tracer (0.03 mmol) |
|---|---|---|
| Proximal | 12.7 ± 0.6 | 0.0063 ± 0.0004 |
| Distal | 11.6 ± 0.7 | 0.0068 ± 0.0005 |
| p values for region comparisons | 0.30 | 0.52 |
| Days | Serum | Blood Cells | Liver | Small Intestine | Skeletal Muscle |
|---|---|---|---|---|---|
| Homogenate radioactivity | |||||
| 2 | 140.1 ± 17.7 a | 0.98 ± 0.42 ab | 2.70 ± 1.03 | 4.31 ± 1.65 ab | 3.38 ± 1.13 |
| 5 | 132.2 ± 11.4 a | 1.50 ± 0.29 b | 3.22 ± 0.47 | 5.77 ± 0.42 a | 4.65 ± 0.73 |
| 8 | 70.9 ± 12.8 b | 0.78 ± 0.12 a | 2.78 ± 0.24 | 3.71 ± 0.39 b | 3.47 ± 0.47 |
| Radioactivity in the gel | |||||
| 2 | 8.55 ± 1.20 a | 0.14 ± 0.06 | 0.14 ± 0.07 a | 0.27 ± 0.05 a | 0.31 ± 0.07 a |
| 5 | 7.99 ± 2.11 a | 0.10 ± 0.02 | 0.55 ± 0.07 b | 0.79 ± 0.08 b | 0.48 ± 0.02 b |
| 8 | 11.02 ± 2.12 b | 0.24 ± 0.11 | 0.81 ± 0.07 c | 0.71 ± 0.12 b | 0.39 ± 0.04 ab |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, T.; Bloomer, R.J.; Benjamin, R.L.; Buddington, R.K. Small Intestinal Absorption of Methylsulfonylmethane (MSM) and Accumulation of the Sulfur Moiety in Selected Tissues of Mice. Nutrients 2018, 10, 19. https://doi.org/10.3390/nu10010019
Wong T, Bloomer RJ, Benjamin RL, Buddington RK. Small Intestinal Absorption of Methylsulfonylmethane (MSM) and Accumulation of the Sulfur Moiety in Selected Tissues of Mice. Nutrients. 2018; 10(1):19. https://doi.org/10.3390/nu10010019
Chicago/Turabian StyleWong, Thomas, Richard J. Bloomer, Rodney L. Benjamin, and Randal K. Buddington. 2018. "Small Intestinal Absorption of Methylsulfonylmethane (MSM) and Accumulation of the Sulfur Moiety in Selected Tissues of Mice" Nutrients 10, no. 1: 19. https://doi.org/10.3390/nu10010019




