Characterisation of L-Type Amino Acid Transporter 1 (LAT1) Expression in Human Skeletal Muscle by Immunofluorescent Microscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mouse Skeletal Muscle Sample Collection and Preparation
2.2. Human Skeletal Muscle Sample Collection and Preparation
2.3. Immunofluorescent Staining
2.4. Image Capture
2.5. Immunoblotting
2.6. Statistical Analysis
3. Results
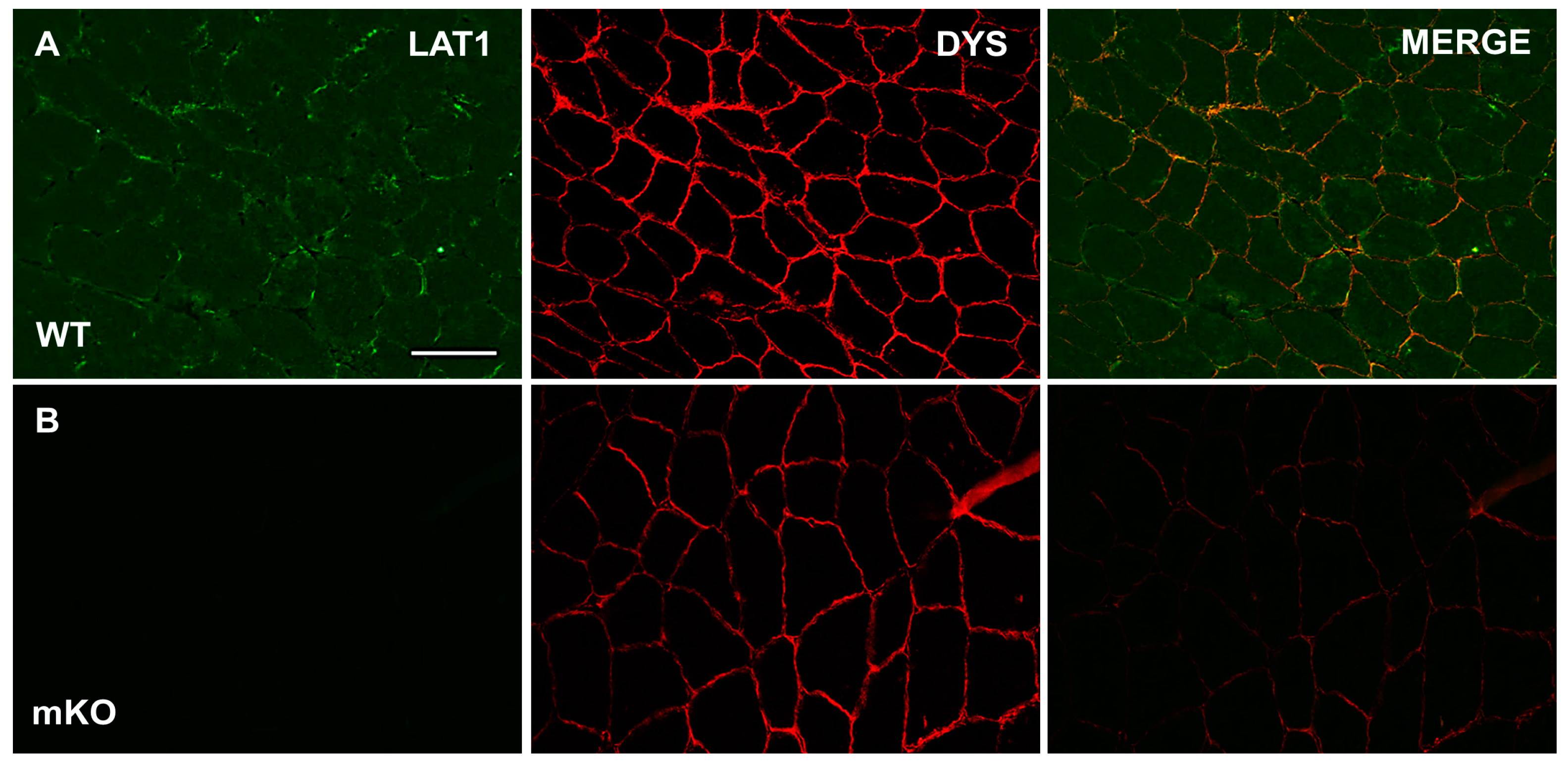
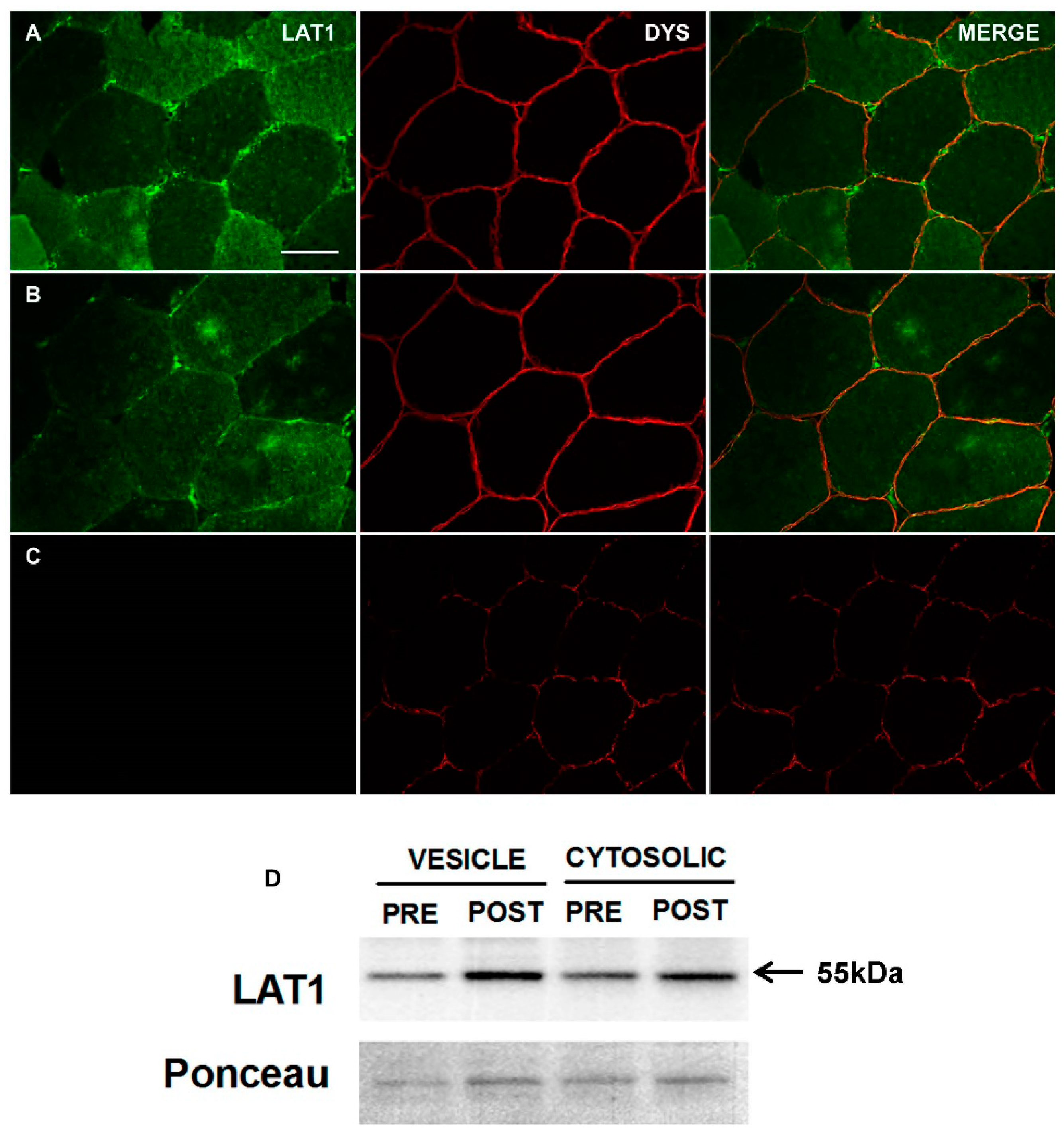
3.1. Characterisation and Validation of LAT1 Immunofluorescence Labelling Approaches
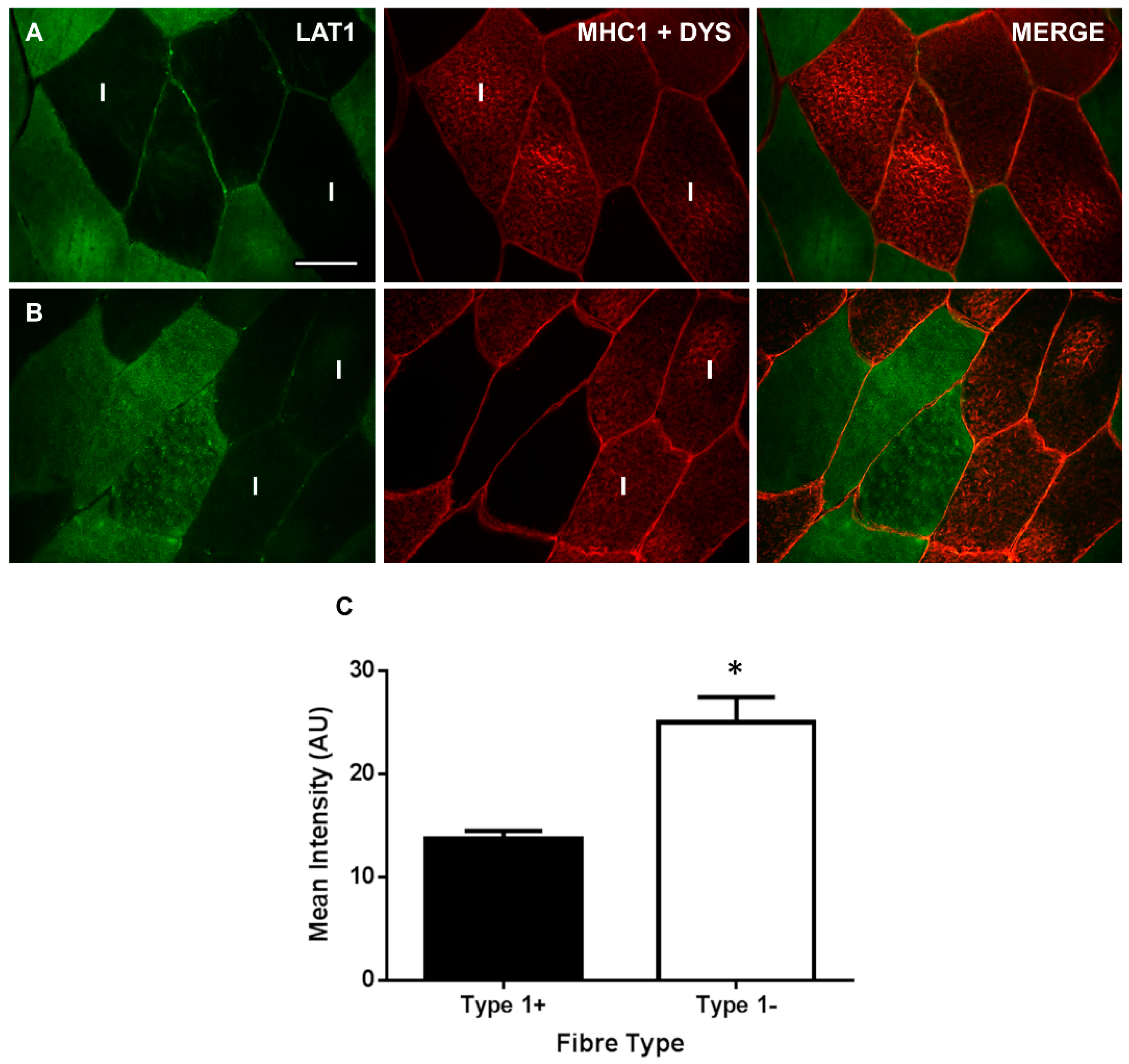
3.2. LAT1 Protein Content Is Greater in Fast-Twitch Skeletal Muscle Fibres
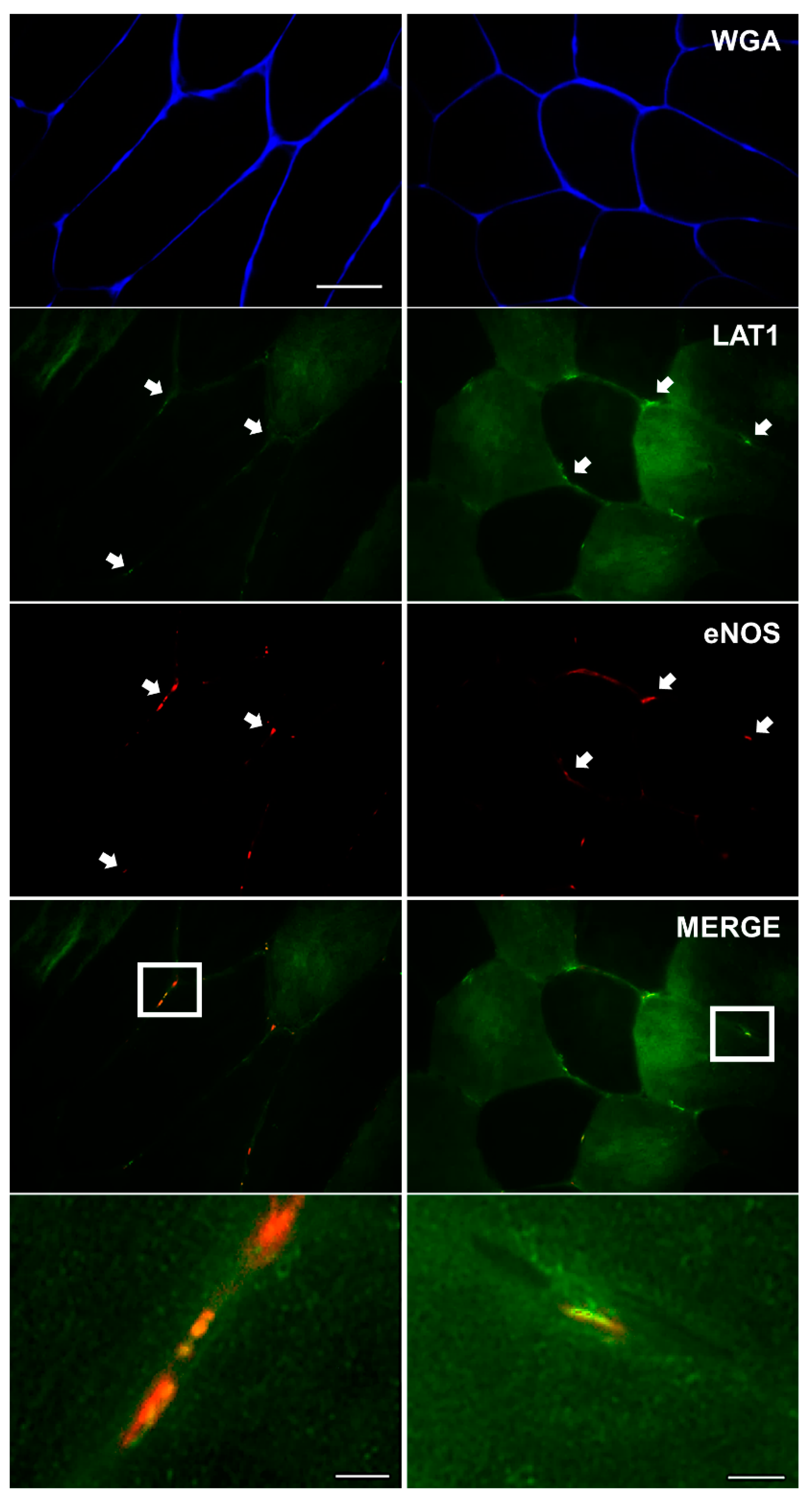
3.3. LAT1 Localises Close to the Microvasculature
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kilberg, M.S.; Pan, Y.X.; Chen, H.; Leung-Pineda, V. Nutritional control of gene expression: How mammalian cells respond to amino acid limitation. Annu. Rev. Nutr. 2005, 25, 59–85. [Google Scholar] [CrossRef] [PubMed]
- Biolo, G.; Tipton, K.D.; Klein, S.; Wolfe, R.R. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am. J. Physiol. 1997, 273, E122–E129. [Google Scholar] [CrossRef] [PubMed]
- Tipton, K.D.; Gurkin, B.E.; Matin, S.; Wolfe, R.R. Nonessential amino acids are not necessary to stimulate net muscle protein synthesis in healthy volunteers. J. Nutr. Biochem. 1999, 10, 89–95. [Google Scholar] [CrossRef]
- Dudani, A.K.; Prasad, R. Amino acid transport: Its role in cell division and growth of Saccharomyces cerevisiae cells. Biochem. Int. 1983, 7, 15–22. [Google Scholar] [PubMed]
- Gazzola, G.C.; Dall’Asta, V.; Guidotti, G.G. Adaptive regulation of amino acid transport in cultured human fibroblasts. Sites and mechanism of action. J. Biol. Chem. 1981, 256, 3191–3198. [Google Scholar] [PubMed]
- Holsbeeks, I.; Lagatie, O.; Van Nuland, A.; Van de Velde, S.; Thevelein, J.M. The eukaryotic plasma membrane as a nutrient-sensing device. Trends Biochem. Sci. 2004, 29, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Hyde, R.; Cwiklinski, E.L.; MacAulay, K.; Taylor, P.M.; Hundal, H.S. Distinct sensor pathways in the hierarchical control of SNAT2, a putative amino acid transceptor, by amino acid availability. J. Biol. Chem. 2007, 282, 19788–19798. [Google Scholar] [CrossRef] [PubMed]
- Hundal, H.S.; Taylor, P.M. Amino acid transceptors: Gate keepers of nutrient exchange and regulators of nutrient signaling. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E603–E613. [Google Scholar] [CrossRef] [PubMed]
- Yanagida, O.; Kanai, Y.; Chairoungdua, A.; Kim, D.K.; Segawa, H.; Nii, T.; Cha, S.H.; Matsuo, H.; Fukushima, J.; Fukasawa, Y.; et al. Human L-type amino acid transporter 1 (LAT1): Characterization of function and expression in tumor cell lines. Biochim. Biophys. Acta 2001, 1514, 291–302. [Google Scholar] [CrossRef]
- Dickinson, J.M.; Drummond, M.J.; Coben, J.R.; Volpi, E.; Rasmussen, B.B. Aging differentially affects human skeletal muscle amino acid transporter expression when essential amino acids are ingested after exercise. Clin. Nutr. 2013, 32, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Drummond, M.J.; Fry, C.S.; Glynn, E.L.; Timmerman, K.L.; Dickinson, J.M.; Walker, D.K.; Gundermann, D.M.; Volpi, E.; Rasmussen, B.B. Skeletal muscle amino acid transporter expression is increased in young and older adults following resistance exercise. J. Appl. Physiol. (1985) 2011, 111, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Drummond, M.J.; Glynn, E.L.; Fry, C.S.; Timmerman, K.L.; Volpi, E.; Rasmussen, B.B. An increase in essential amino acid availability upregulates amino acid transporter expression in human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E1011–E1018. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Yoshinaga, M. Induction of amino acid transporters expression by endurance exercise in rat skeletal muscle. Biochem. Biophys. Res. Commun. 2013, 439, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Poncet, N.; Mitchell, F.E.; Ibrahim, A.F.; McGuire, V.A.; English, G.; Arthur, J.S.; Shi, Y.B.; Taylor, P.M. The catalytic subunit of the system L1 amino acid transporter (slc7a5) facilitates nutrient signalling in mouse skeletal muscle. PLoS ONE 2014, 9, e89547. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.K.; Drummond, M.J.; Dickinson, J.M.; Borack, M.S.; Jennings, K.; Volpi, E.; Rasmussen, B.B. Insulin increases mRNA abundance of the amino acid transporter SLC7A5/LAT1 via an mTORC1-dependent mechanism in skeletal muscle cells. Physiol. Rep. 2014, 2, e00238. [Google Scholar] [CrossRef] [PubMed]
- Atherton, P.J.; Smith, K.; Etheridge, T.; Rankin, D.; Rennie, M.J. Distinct anabolic signalling responses to amino acids in C2C12 skeletal muscle cells. Amino Acids 2010, 38, 1533–1539. [Google Scholar] [CrossRef] [PubMed]
- Churchward-Venne, T.A.; Burd, N.A.; Mitchell, C.J.; West, D.W.; Philp, A.; Marcotte, G.R.; Baker, S.K.; Baar, K.; Phillips, S.M. Supplementation of a suboptimal protein dose with leucine or essential amino acids: Effects on myofibrillar protein synthesis at rest and following resistance exercise in men. J. Physiol. 2012, 590, 2751–2765. [Google Scholar] [CrossRef] [PubMed]
- Churchward-Venne, T.A.; Breen, L.; Di Donato, D.M.; Hector, A.J.; Mitchell, C.J.; Moore, D.R.; Stellingwerff, T.; Breuille, D.; Offord, E.A.; Baker, S.K.; et al. Leucine supplementation of a low-protein mixed macronutrient beverage enhances myofibrillar protein synthesis in young men: A double-blind, randomized trial. Am. J. Clin. Nutr. 2014, 99, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Baird, F.E.; Bett, K.J.; MacLean, C.; Tee, A.R.; Hundal, H.S.; Taylor, P.M. Tertiary active transport of amino acids reconstituted by coexpression of System A and L transporters in Xenopus oocytes. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E822–E829. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, M.G.; Hamilton, D.L.; Murray, J.T.; Taylor, P.M.; Baar, K. mVps34 is activated following high-resistance contractions. J. Physiol. 2009, 587, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Bodoy, S.; Martin, L.; Zorzano, A.; Palacin, M.; Estevez, R.; Bertran, J. Identification of LAT4, a novel amino acid transporter with system L activity. J. Biol. Chem. 2005, 280, 12002–12011. [Google Scholar] [CrossRef] [PubMed]
- Verrey, F.; Closs, E.I.; Wagner, C.A.; Palacin, M.; Endou, H.; Kanai, Y. CATs and HATs: The SLC7 family of amino acid transporters. Pflügers Arch. 2004, 447, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Cleal, J.K.; Glazier, J.D.; Ntani, G.; Crozier, S.R.; Day, P.E.; Harvey, N.C.; Robinson, S.M.; Cooper, C.; Godfrey, K.M.; Hanson, M.A.; et al. Facilitated transporters mediate net efflux of amino acids to the fetus across the basal membrane of the placental syncytiotrophoblast. J. Physiol. 2011, 589, 987–997. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.M.; Reyna, S.V.; Ensenat, D.; Peyton, K.J.; Wang, H.; Schafer, A.I.; Durante, W. Platelet-derived growth factor stimulates LAT1 gene expression in vascular smooth muscle: Role in cell growth. FASEB J. 2004, 18, 768–770. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, B.B.; Tipton, K.D.; Miller, S.L.; Wolf, S.E.; Wolfe, R.R. An oral essential amino acid-carbohydrate supplement enhances muscle protein anabolism after resistance exercise. J. Appl. Physiol. (1985) 2000, 88, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Park, K.J.; Park, J.R.; Kanai, Y.; Endou, H.; Park, J.C.; Kim, D.K. The RNA interference of amino acid transporter LAT1 inhibits the growth of KB human oral cancer cells. Anticancer Res. 2006, 26, 2943–2948. [Google Scholar] [PubMed]
- Song, Z.; Moore, D.R.; Hodson, N.; Ward, C.; Dent, J.R.; O’Leary, M.F.; Shaw, A.M.; Hamilton, D.L.; Sarkar, S.; Gangloff, Y.-G.; et al. Resistance exercise initiates mechanistic target of rapamycin (mTOR) translocation and protein complex co-localisation in human skeletal muscle. Sci. Rep. 2017, 7, 5028. [Google Scholar] [CrossRef] [PubMed]
- Bradley, N.S.; Snook, L.A.; Jain, S.S.; Heigenhauser, G.J.; Bonen, A.; Spriet, L.L. Acute endurance exercise increases plasma membrane fatty acid transport proteins in rat and human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E183–E189. [Google Scholar] [CrossRef] [PubMed]
- Perez-Schindler, J.; Esparza, M.C.; McKendry, J.; Breen, L.; Philp, A.; Schenk, S. Overload-mediated skeletal muscle hypertrophy is not impaired by loss of myofiber STAT3. Am. J. Physiol. Cell. Physiol. 2017, 313, C257–C261. [Google Scholar] [CrossRef] [PubMed]
- Cocks, M.; Shepherd, S.O.; Shaw, C.S.; Achten, J.; Costa, M.L.; Wagenmakers, A.J. Immunofluorescence microscopy to assess enzymes controlling nitric oxide availability and microvascular blood flow in muscle. Microcirculation 2012, 19, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef] [PubMed]
- Koonen, D.P.; Coumans, W.A.; Arumugam, Y.; Bonen, A.; Glatz, J.F.; Luiken, J.J. Giant membrane vesicles as a model to study cellular substrate uptake dissected from metabolism. Mol. Cell. Biochem. 2002, 239, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, J.; Paglialunga, S.; Smith, B.K.; Miotto, P.M.; Simnett, G.; Robson, H.L.; Jain, S.S.; Herbst, E.A.F.; Desjardins, E.M.; Dyck, D.J.; et al. Ablating TBC1D1 impairs contraction-induced sarcolemmal glucose transporter type 4 redistribution, but not insulin-mediated responses in rats. J. Biol. Chem. 2017. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.M. Role of amino acid transporters in amino acid sensing. Am. J. Clin. Nutr. 2014, 99, 223S–230S. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, R.; Rossier, G.; Spindler, B.; Meier, C.; Kühn, L.; Verrey, F. Amino acid transport of y+L-type by heterodimers of 4F2hc/CD98 and members of the glycoprotein-associated amino acid transporter family. EMBO J. 1999, 18, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Warren, A.P.; Patel, K.; McConkey, D.J.; Palacios, R. CD98: A type II transmembrane glycoprotein expressed from the beginning of primitive and definitive hematopoiesis may play a critical role in the development of hematopoietic cells. Blood 1996, 87, 3676–3687. [Google Scholar] [PubMed]
- Fenczik, C.A.; Zent, R.; Dellos, M.; Calderwood, D.A.; Satriano, J.; Kelly, C.; Ginsberg, M.H. Distinct domains of CD98hc regulate integrins and amino acid transport. J. Biol. Chem. 2001, 276, 8746–8752. [Google Scholar] [CrossRef] [PubMed]
- Gaccioli, F.; Aye, I.L.; Roos, S.; Lager, S.; Ramirez, V.I.; Kanai, Y.; Powell, T.L.; Jansson, T. Expression and functional characterisation of System L amino acid transporters in the human term placenta. Reprod. Biol. Endocrinol. 2015, 13, 57. [Google Scholar] [CrossRef] [PubMed]
- Nikkuni, O.; Kaira, K.; Toyoda, M.; Shino, M.; Sakakura, K.; Takahashi, K.; Tominaga, H.; Oriuchi, N.; Suzuki, M.; Iijima, M.; et al. Expression of Amino Acid Transporters (LAT1 and ASCT2) in Patients with Stage III/IV Laryngeal Squamous Cell Carcinoma. Pathol. Oncol. Res. 2015, 21, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Holst, J. L-type amino acid transport and cancer: Targeting the mTORC1 pathway to inhibit neoplasia. Am. J. Cancer Res. 2015, 5, 1281–1294. [Google Scholar] [PubMed]
- Ohno, C.; Nakanishi, Y.; Honma, T.; Henmi, A.; Sugitani, M.; Kanai, Y.; Nemoto, N. Significance of system L amino acid transporter 1 (LAT-1) and 4F2 heavy chain (4F2hc) expression in human developing intestines. Acta Histochem. Cytochem. 2009, 42, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, K.; Ogata, S.; Nakanishi, K.; Ozeki, Y.; Hiroi, S.; Tominaga, S.; Aida, S.; Matsuo, H.; Sakata, T.; Kawai, T. LAT1 expression in non-small-cell lung carcinomas: Analyses by semiquantitative reverse transcription-PCR (237 cases) and immunohistochemistry (295 cases). Lung Cancer 2010, 68, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Kaji, M.; Kabir-Salmani, M.; Anzai, N.; Jin, C.J.; Akimoto, Y.; Horita, A.; Sakamoto, A.; Kanai, Y.; Sakurai, H.; Iwashita, M. Properties of L-type amino acid transporter 1 in epidermal ovarian cancer. Int. J. Gynecol. Cancer 2010, 20, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.C.; Kim, Y.S.; Shadchehr, A.; Garrido, A.; Macgregor, I.L.; Sleisenger, M.H. Protein digestion and absorption in human small intestine. Gastroenterology 1979, 76, 1415–1421. [Google Scholar] [PubMed]
- Roberts, L.M.; Black, D.S.; Raman, C.; Woodford, K.; Zhou, M.; Haggerty, J.E.; Yan, A.T.; Cwirla, S.E.; Grindstaff, K.K. Subcellular localization of transporters along the rat blood–brain barrier and blood-cerebral-spinal fluid barrier by in vivo biotinylation. Neuroscience 2008, 155, 423–438. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, G.; Mohacsik, P.; Balkhi, M.Y.; Gereben, B.; Lechan, R.M. Endotoxin-induced inflammation down-regulates L-type amino acid transporter 1 (LAT1) expression at the blood–brain barrier of male rats and mice. Fluids Barriers CNS 2015, 12, 21. [Google Scholar] [CrossRef] [PubMed]
- Van Herck, S.L.; Delbaere, J.; Bourgeois, N.M.; McAllan, B.M.; Richardson, S.J.; Darras, V.M. Expression of thyroid hormone transporters and deiodinases at the brain barriers in the embryonic chicken: Insights into the regulation of thyroid hormone availability during neurodevelopment. Gen. Comp. Endocrinol. 2015, 214, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Kaisto, T.; Rahkila, P.; Marjomaki, V.; Parton, R.G.; Metsikko, K. Endocytosis in skeletal muscle fibers. Exp. Cell Res. 1999, 253, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, J.M.; Gundermann, D.M.; Walker, D.K.; Reidy, P.T.; Borack, M.S.; Drummond, M.J.; Arora, M.; Volpi, E.; Rasmussen, B.B. Leucine-enriched amino acid ingestion after resistance exercise prolongs myofibrillar protein synthesis and amino acid transporter expression in older men. J. Nutr. 2014, 144, 1694–1702. [Google Scholar] [CrossRef] [PubMed]
- Koopman, R.; Zorenc, A.H.; Gransier, R.J.; Cameron-Smith, D.; van Loon, L.J. Increase in S6K1 phosphorylation in human skeletal muscle following resistance exercise occurs mainly in type II muscle fibers. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E1245–E1252. [Google Scholar] [CrossRef] [PubMed]
- Milkereit, R.; Persaud, A.; Vanoaica, L.; Guetg, A.; Verrey, F. LAPTM4b recruits the LAT1-4F2hc Leu transporter to lysosomes and promotes mTORC1 activation. Nat. Commun. 2015, 6, 7250. [Google Scholar] [CrossRef] [PubMed]
- Zoncu, R.; Bar-Peled, L.; Efeyan, A.; Wang, S.; Sancak, Y.; Sabatini, D.M. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science 2011, 334, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.; Crombleholme, T.; Habli, M. Regulation of amino acid transporters by adenoviral-mediated human insulin-like growth factor-1 in a mouse model of placental insufficiency in vivo and the human trophoblast line BeWo in vitro. Placenta 2014, 35, 132–138. [Google Scholar] [CrossRef] [PubMed]
| Primary Antibody | Source | Dilution | Secondary Antibody | Dilution |
|---|---|---|---|---|
| Rabbit polyclonal anti-Solute Carrier family 7 member 5 (SLC7A5) antibody isotype IgG | Abcam, ab85226 | 1:100 | Goat anti-rabbit IgG(H+L) Alexa®488 | 1:200 |
| SLC7A5 peptide | Abcam, ab192836 | 1:10 | N/A | N/A |
| Mouse monoclonal anti-Dystrophin antibody, isotype IgG2a | DSHB, MANDYS1 3B7 | 1:200 | Goat anti-mouse IgG2a Alexa®594 | 1:200 |
| Mouse monoclonal anti-Myosin Heavy Chain 1 (MHC1) antibody, isotype IgM | DSHB, A4.480 | 1:500 | Goat anti-mouse IgM Alexa®594 | 1:200 |
| Mouse monoclonal anti-endothelial nitrate oxide synthase (eNOS) antibody, isotype IgG1 | BD Transduction, #610297 | 1:200 | Goat anti-mouse IgG1 Alexa®594 | 1:200 |
| Wheat Germ Agglutinin-350 | W11263, Invitrogen | 1:20 | Alexa Fluor® 350 Conjugated | N/A |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hodson, N.; Brown, T.; Joanisse, S.; Aguirre, N.; West, D.W.D.; Moore, D.R.; Baar, K.; Breen, L.; Philp, A. Characterisation of L-Type Amino Acid Transporter 1 (LAT1) Expression in Human Skeletal Muscle by Immunofluorescent Microscopy. Nutrients 2018, 10, 23. https://doi.org/10.3390/nu10010023
Hodson N, Brown T, Joanisse S, Aguirre N, West DWD, Moore DR, Baar K, Breen L, Philp A. Characterisation of L-Type Amino Acid Transporter 1 (LAT1) Expression in Human Skeletal Muscle by Immunofluorescent Microscopy. Nutrients. 2018; 10(1):23. https://doi.org/10.3390/nu10010023
Chicago/Turabian StyleHodson, Nathan, Thomas Brown, Sophie Joanisse, Nick Aguirre, Daniel W. D. West, Daniel R. Moore, Keith Baar, Leigh Breen, and Andrew Philp. 2018. "Characterisation of L-Type Amino Acid Transporter 1 (LAT1) Expression in Human Skeletal Muscle by Immunofluorescent Microscopy" Nutrients 10, no. 1: 23. https://doi.org/10.3390/nu10010023





