Dairy Products Intake and Endometrial Cancer Risk: A Meta-Analysis of Observational Studies
Abstract
:1. Introduction
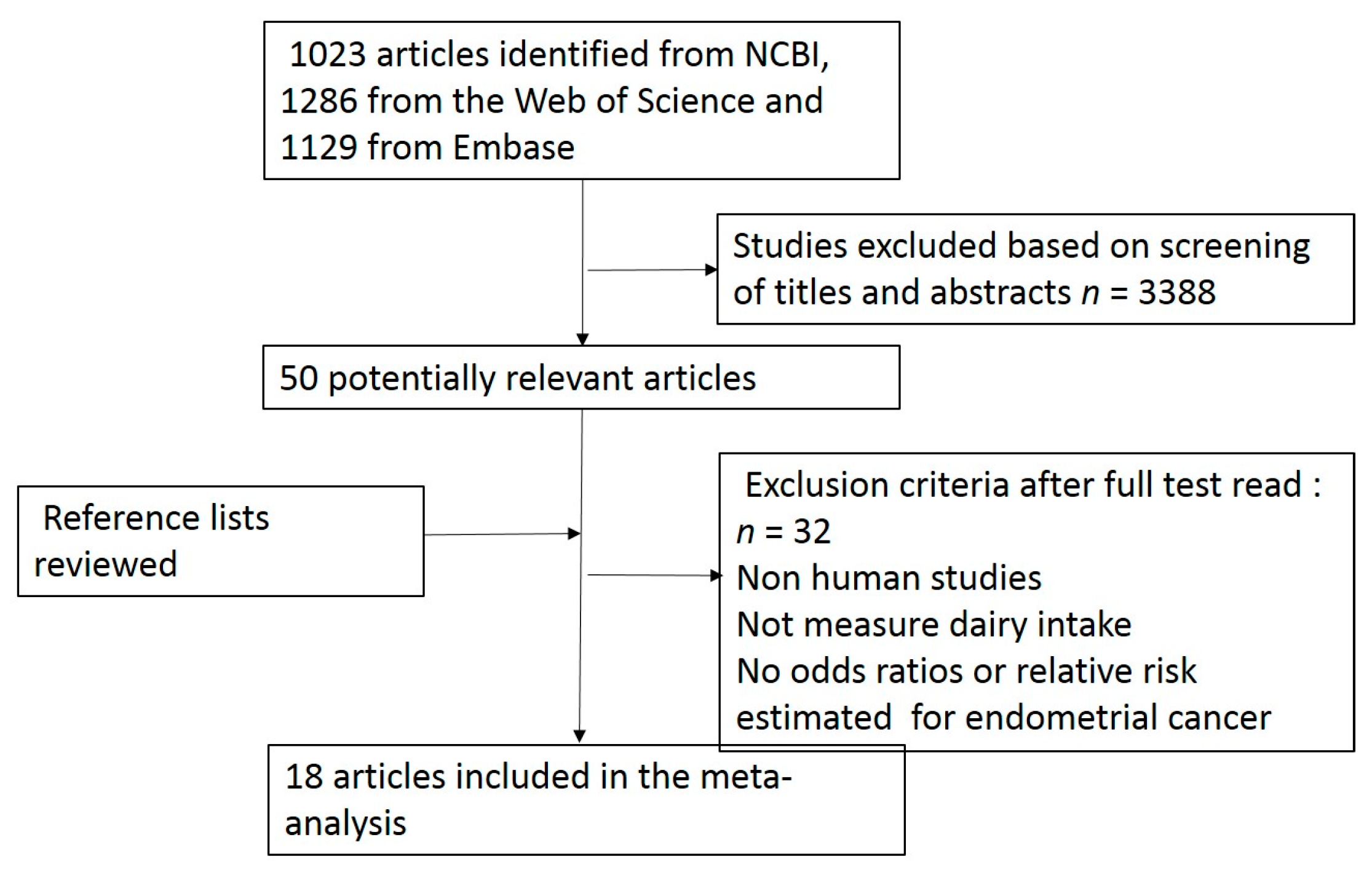
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction and Assessment of Study Quality
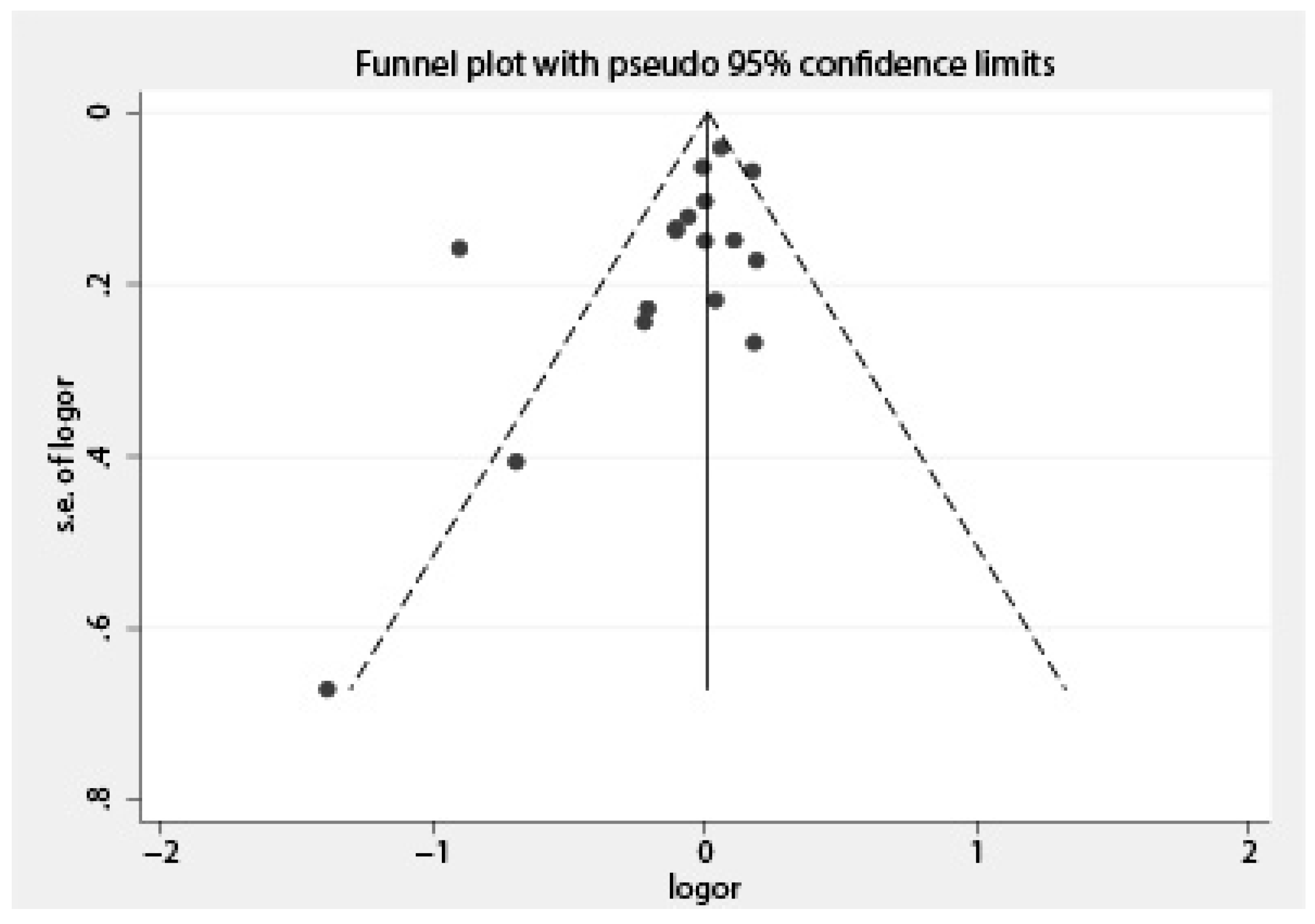
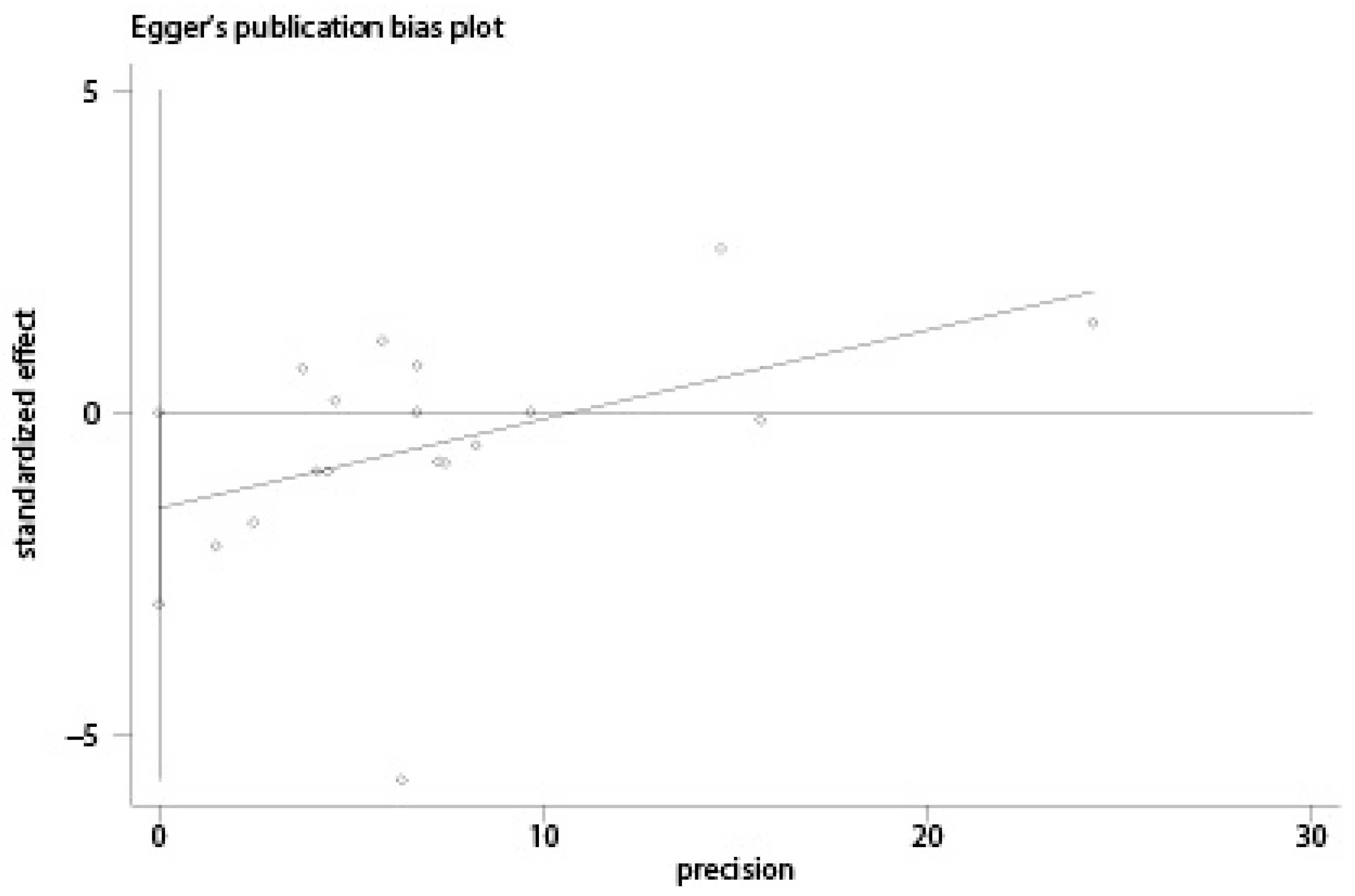
2.4. Statistical Analysis
3. Results
3.1. Study Characteristics
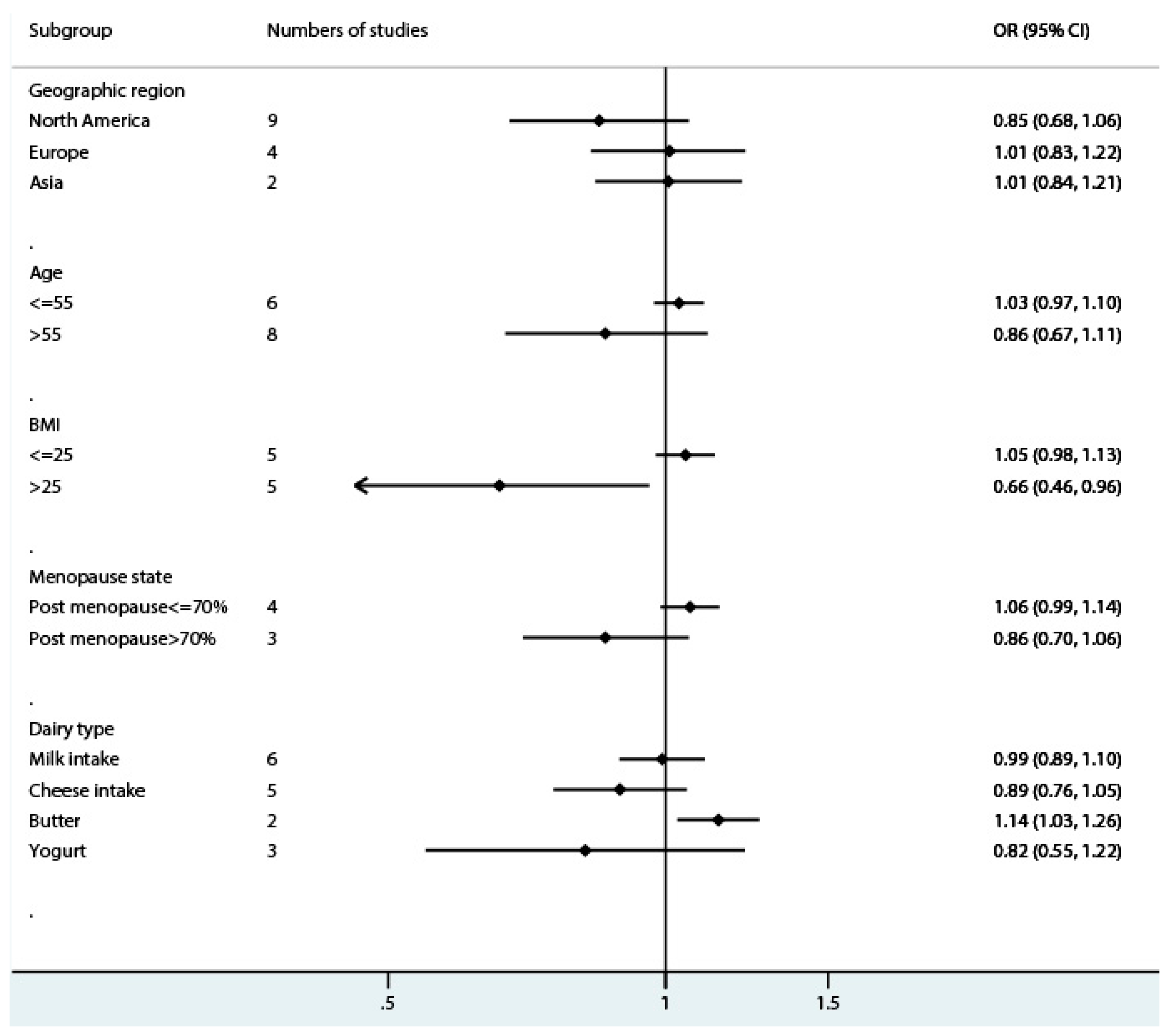
3.2. Total Dairy Intake and Endometrial Cancer Risk
3.3. Milk Intake and Endometrial Cancer Risk
3.4. Cheese Intake and Endometrial Cancer Risk
3.5. Butter Intake and Endometrial Cancer Risk
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in Globocan 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- GBD 2015 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: A systematic analysis for the global burden of disease study 2015. Lancet 2016, 388, 1659–1724. [Google Scholar]
- Miura, K.; Hughes, M.C.; Ungerer, J.P.; Green, A.C. Plasma eicosapentaenoic acid is negatively associated with all-cause mortality among men and women in a population-based prospective study. Nutr. Res. 2016, 36, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
- Majumder, B.; Wahle, K.W.; Moir, S.; Schofield, A.; Choe, S.-N.; Farquharson, A.; Grant, I.; Heys, S.D. Conjugated linoleic acids (CLAs) regulate the expression of key apoptotic genes in human breast cancer cells. FASEB J. 2002, 16, 1447–1449. [Google Scholar] [CrossRef] [PubMed]
- Hill, T.R.; O’Brien, M.M.; Lamberg-Allardt, C.; Jakobsen, J.; Kiely, M.; Flynn, A.; Cashman, K.D. Vitamin D status of 51–75-year-old Irish women: Its determinants and impact on biochemical indices of bone turnover. Public Health Nutr. 2006, 9, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Biel, R.K.; Csizmadi, I.; Cook, L.S.; Courneya, K.S.; Magliocco, A.M.; Friedenreich, C.M. Risk of endometrial cancer in relation to individual nutrients from diet and supplements. Public Health Nutr. 2011, 14, 1948–1960. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, X.; Zhang, X.; Liu, J.; Ye, S.; Xiao, S.; Chen, H.; Wang, H. Induction of apoptosis by c9, t11-CLA in human endometrial cancer RL 95-2 cells via ERalpha-mediated pathway. Chem. Phys. Lipids 2013, 175–176, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Khankari, N.K.; Bradshaw, P.T.; Steck, S.E.; He, K.; Olshan, A.F.; Shen, J.; Ahn, J.; Chen, Y.; Ahsan, H.; Terry, M.B.; et al. Dietary intake of fish, polyunsaturated fatty acids, and survival after breast cancer: A population-based follow-up study on Long Island, New York. Cancer 2015, 121, 2244–2252. [Google Scholar] [CrossRef] [PubMed]
- Zang, J.; Shen, M.; Du, S.; Chen, T.; Zou, S. The association between dairy intake and breast cancer in western and Asian populations: A systematic review and meta-analysis. J. Breast Cancer 2015, 18, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Ke, J.; Yang, Y.; Che, Q.; Jiang, F.; Wang, H.; Chen, Z.; Zhu, M.; Tong, H.; Zhang, H.; Yan, X.; et al. Prostaglandin E2 (PGE2) promotes proliferation and invasion by enhancing SUMO-1 activity via EP4 receptor in endometrial cancer. Tumour Biol. 2016, 37, 12203–12211. [Google Scholar] [CrossRef] [PubMed]
- Bandera, E.V.; Kushi, L.H.; Moore, D.F.; Gifkins, D.M.; McCullough, M.L. Consumption of animal foods and endometrial cancer risk: A systematic literature review and meta-analysis. Cancer Causes Control 2007, 18, 967–988. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Lyu, C.; Gao, J.; Du, L.; Shan, B.; Zhang, H.; Wang, H.Y.; Gao, Y. Dietary fat intake and endometrial cancer risk: A dose response meta-analysis. Medicine 2016, 95, e4121. [Google Scholar] [CrossRef] [PubMed]
- Mettlin, C.J.; Schoenfeld, E.R.; Natarajan, N. Patterns of milk consumption and risk of cancer. Nutr. Cancer 1990, 13, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Barbone, F.; Austin, H.; Partridge, E.E. Diet and endometrial cancer: A case-control study. Am. J. Epidemiol. 1993, 137, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Potischman, N.; Swanson, C.A.; Brinton, L.A.; McAdams, M.; Barrett, R.J.; Berman, M.L.; Mortel, R.; Twiggs, L.B.; Wilbanks, G.D.; Hoover, R.N. Dietary associations in a case-control study of endometrial cancer. Cancer Causes Control 1993, 4, 239–250. [Google Scholar] [PubMed]
- Tzonou, A.; Lipworth, L.; Kalandidi, A.; Trichopoulou, A.; Gamatsi, I.; Hsieh, C.C.; Notara, V.; Trichopoulos, D. Dietary factors and the risk of endometrial cancer: A case-control study in Greece. Br. J. Cancer 1996, 73, 1284–1290. [Google Scholar] [CrossRef] [PubMed]
- Hirose, K.; Tajima, K.; Hamajima, N.; Takezaki, T.; Inoue, M.; Kuroishi, T.; Kuzuya, K.; Nakamura, S.; Tokudome, S. Subsite (cervix/endometrium)-specific risk and protective factors in uterus cancer. Jpn. J. Cancer Res. 1996, 87, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.G.; Howe, G.R.; Rohan, T.E. Nutritional factors and endometrial cancer in Ontario, Canada. Cancer Control 2000, 7, 288–296. [Google Scholar] [CrossRef] [PubMed]
- McCann, S.E.; Freudenheim, J.L.; Marshall, J.R.; Brasure, J.R.; Swanson, M.K.; Graham, S. Diet in the epidemiology of endometrial cancer in western New York (United States). Cancer Causes Control 2000, 11, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Littman, A.J.; Beresford, S.A.; White, E. The association of dietary fat and plant foods with endometrial cancer (United States). Cancer Causes Control 2001, 12, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Petridou, E.; Kedikoglou, S.; Koukoulomatis, P.; Dessypris, N.; Trichopoulos, D. Diet in relation to endometrial cancer risk: A case-control study in Greece. Nutr. Cancer 2002, 44, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Terry, P.; Vainio, H.; Wolk, A.; Weiderpass, E. Dietary factors in relation to endometrial cancer: A nationwide case-control study in Sweden. Nutr. Cancer 2002, 42, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Martinez, E.; Lazcano-Ponce, E.; Sanchez-Zamorano, L.M.; Gonzalez-Lira, G.; Escudero, D.E.L.R.P.; Hernandez-Avila, M. Dietary factors and endometrial cancer risk. Results of a case-control study in Mexico. Int. J. Gynecol. Cancer 2005, 15, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.H.; Dai, Q.; Xiang, Y.B.; Zhao, G.M.; Zheng, W.; Gao, Y.T.; Ruan, Z.X.; Cheng, J.R.; Shu, X.O. Animal food intake and cooking methods in relation to endometrial cancer risk in Shanghai. Br. J. Cancer 2006, 95, 1586–1592. [Google Scholar] [CrossRef] [PubMed]
- Bravi, F.; Scotti, L.; Bosetti, C.; Zucchetto, A.; Talamini, R.; Montella, M.; Greggi, S.; Pelucchi, C.; Negri, E.; Franceschi, S.; et al. Food groups and endometrial cancer risk: A case-control study from Italy. Am. J. Obstet. Gynecol. 2009, 200, 293. [Google Scholar] [CrossRef] [PubMed]
- Chandran, U.; Bandera, E.V.; Williams-King, M.G.; Sima, C.; Bayuga, S.; Pulick, K.; Wilcox, H.; Zauber, A.G.; Olson, S.H. Adherence to the dietary guidelines for Americans and endometrial cancer risk. Cancer Causes Control 2010, 21, 1895–1904. [Google Scholar] [CrossRef] [PubMed]
- Ganmaa, D.; Cui, X.; Feskanich, D.; Hankinson, S.E.; Willett, W.C. Milk, dairy intake and risk of endometrial cancer: A 26-year follow-up. Int. J. Cancer 2012, 130, 2664–2671. [Google Scholar] [CrossRef] [PubMed]
- Merritt, M.A.; Tzoulaki, I.; Tworoger, S.S.; De Vivo, I.; Hankinson, S.E.; Fernandes, J.; Tsilidis, K.K.; Weiderpass, E.; Tjonneland, A.; Petersen, K.E.; et al. Investigation of dietary factors and endometrial cancer risk using a nutrient-wide association study approach in the EPIC and nurses’ health study (NHS) and NHSII. Cancer Epidemiol. Biomark. Prev. 2015, 24, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Filomeno, M.; Bosetti, C.; Bidoli, E.; Levi, F.; Serraino, D.; Montella, M.; La Vecchia, C.; Tavani, A. Mediterranean diet and risk of endometrial cancer: A pooled analysis of three Italian case-control studies. Br. J. Cancer 2015, 112, 1816–1821. [Google Scholar] [CrossRef] [PubMed]
- Plagens-Rotman, K.; Zak, E.; Pieta, B. Odds ratio analysis in women with endometrial cancer. Prz. Menopauzalny 2016, 15, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; La Valley, M.P.; Tucker, K.L. Prospective studies of dairy product and calcium intakes and prostate cancer risk: A meta-analysis. J. Natl. Cancer Inst. 2005, 97, 1768–1777. [Google Scholar] [CrossRef] [PubMed]
- Putnam, J.J.; Allshouse, J.E. Food Consumption, Prices, and Expenditures, 1970–97; United States Department of Agriculture Economic Research Service: Washington, DC, USA, 1999.
- Domestic Deliveries and Consumption of Selected Consumer Goods Per Capita in 2017. Available online: http://stat.gov.pl/files/gfx/portalinformacyjny/en/defaultaktualnosci/3285/9/6/1/domestic_deliveries_and_consumption_of_selected_consumer_goods_per_capita_in_2016.pdf (accessed on 13 December 2017).
- Cassandro, M. Status of milk production and market in Italy. Agric. Conspec. Sci. (ACS) 2003, 68, 65–69. [Google Scholar]
- Emons, G.; Grundker, C.; Hanf, V. Are estrogens carcinogens? Zentralbl. Gynakol. 2002, 124, 559–565. [Google Scholar] [PubMed]
- Finucane, O.M.; Lyons, C.L.; Murphy, A.M.; Reynolds, C.M.; Klinger, R.; Healy, N.P.; Cooke, A.A.; Coll, R.C.; McAllan, L.; Nilaweera, K.N.; et al. Monounsaturated fatty acid-enriched high-fat diets impede adipose NLRP3 inflammasome-mediated IL-1beta secretion and insulin resistance despite obesity. Diabetes 2015, 64, 2116–2128. [Google Scholar] [CrossRef] [PubMed]
- Bainbridge, M.L.; Egolf, E.; Barlow, J.W.; Alvez, J.P.; Roman, J.; Kraft, J. Milk from cows grazing on cool-season pastures provides an enhanced profile of bioactive fatty acids compared to those grazed on a monoculture of pearl millet. Food Chem. 2017, 217, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Cline, M.; Maxwell, L.G.; Berrigan, D.; Rodriguez, G.; Warri, A.; Hilakivi-Clarke, L. Dietary vitamin d exposure prevents obesity-induced increase in endometrial cancer in Pten+/− mice. Cancer Prev. Res. 2010, 3, 1246–1258. [Google Scholar] [CrossRef] [PubMed]
- Brevik, A.; Veierod, M.B.; Drevon, C.A.; Andersen, L.F. Evaluation of the odd fatty acids 15:0 and 17:0 in serum and adipose tissue as markers of intake of milk and dairy fat. Eur. J. Clin. Nutr. 2005, 59, 1417–1422. [Google Scholar] [CrossRef] [PubMed]
- Forouhi, N.G.; Koulman, A.; Sharp, S.J.; Imamura, F.; Kroger, J.; Schulze, M.B.; Crowe, F.L.; Huerta, J.M.; Guevara, M.; Beulens, J.W.; et al. Differences in the prospective association between individual plasma phospholipid saturated fatty acids and incident type 2 diabetes: The epic-interact case-cohort study. Lancet Diabetes Endocrinol. 2014, 2, 810–818. [Google Scholar] [CrossRef]
- Khaw, K.-T.; Friesen, M.D.; Riboli, E.; Luben, R.; Wareham, N. Plasma phospholipid fatty acid concentration and incident coronary heart disease in men and women: The EPIC-Norfolk prospective study. PLoS Med. 2012, 9, e1001255. [Google Scholar] [CrossRef] [PubMed]
- Crowe, F.L.; Allen, N.E.; Appleby, P.N.; Overvad, K.; Aardestrup, I.V.; Johnsen, N.F.; Tjonneland, A.; Linseisen, J.; Kaaks, R.; Boeing, H.; et al. Fatty acid composition of plasma phospholipids and risk of prostate cancer in a case-control analysis nested within the European prospective investigation into cancer and nutrition. Am. J. Clin. Nutr. 2008, 88, 1353–1363. [Google Scholar] [PubMed]
- Murali, R.; Soslow, R.A.; Weigelt, B. Classification of endometrial carcinoma: More than two types. Lancet Oncol. 2014, 15, e268–e278. [Google Scholar] [CrossRef]
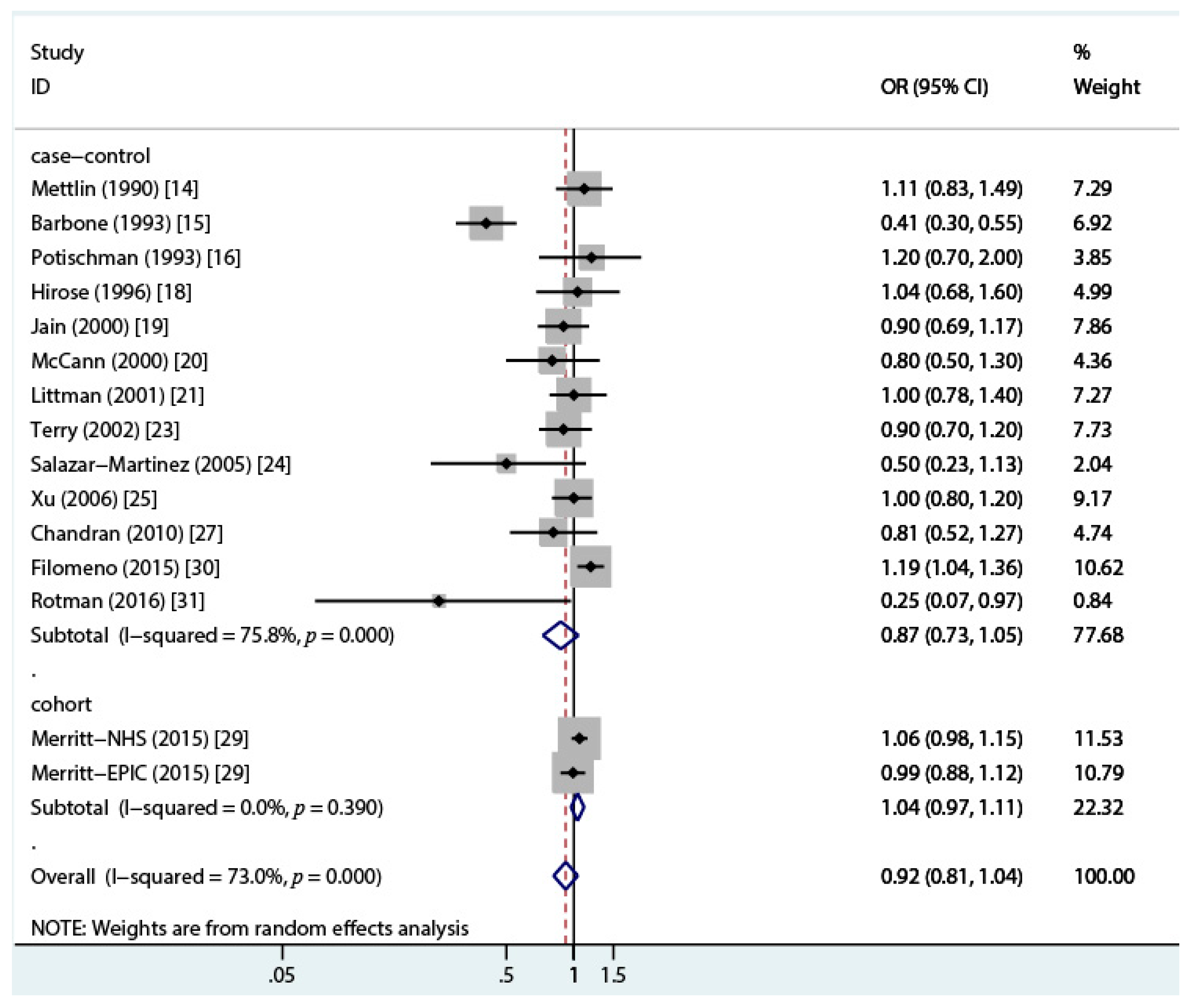
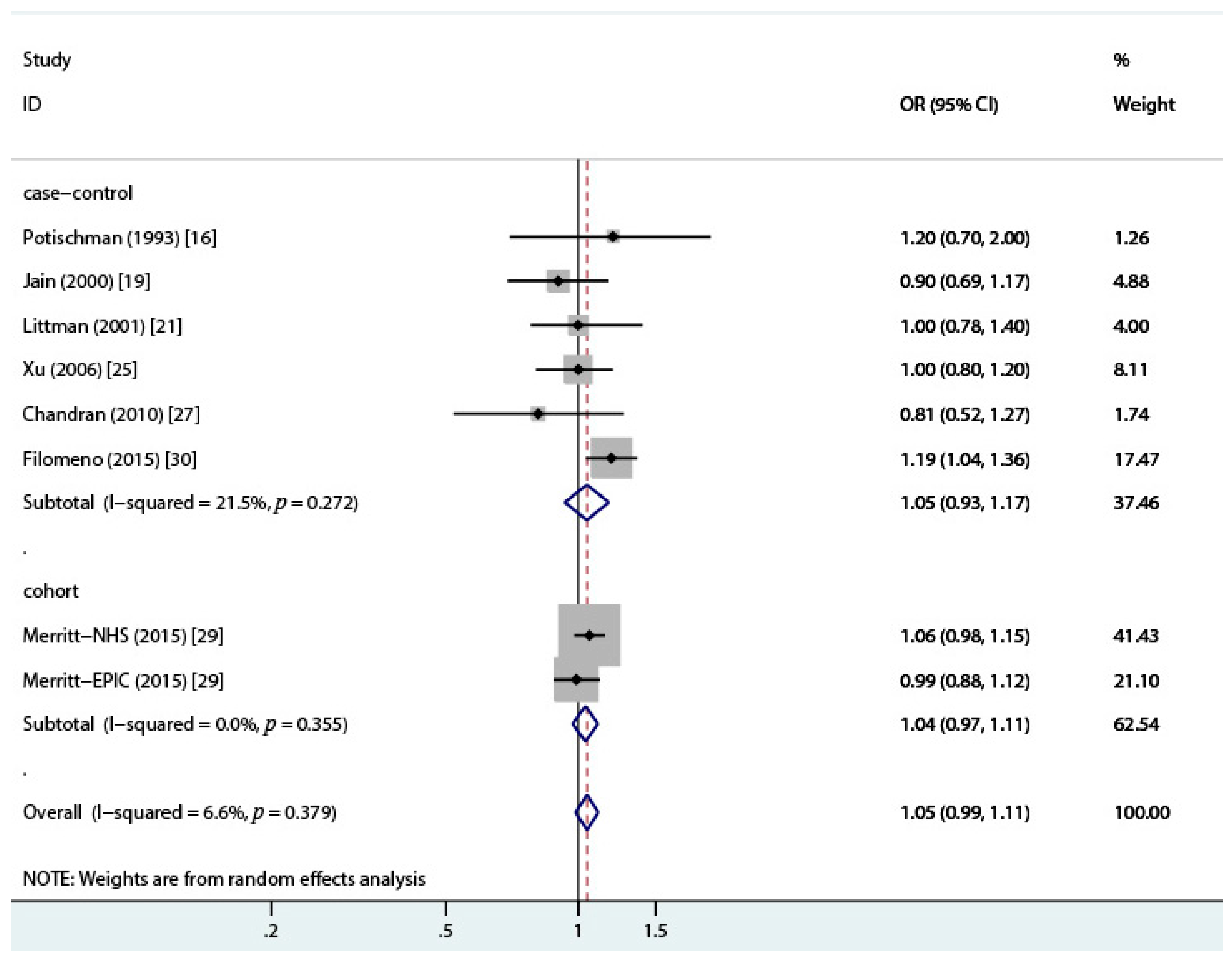
| Reference | Geographic Region | Case/Control (Cohort) | Age (Case) | Exclusion of Hysterectomy | Time Frame for Dietary Assessment § | Dairy Source | OR (95% CI) | Covariates ‖ | NOS Stars |
|---|---|---|---|---|---|---|---|---|---|
| Cohort | |||||||||
| Merritt, 2015 [29] | Europe | 1303/301,107 | 25–70 | yes | dietary questionnaire EPIC (1992–2000) | Butter: >10.3 vs. 0 g/day Yogurt: >145.3 vs. 0 g/day Cheese: >74.7 vs. 0 g/day Milk: >292.7 vs. 0 g/day | 1.23 (1.03, 1.47) 1.15 (0.98, 1.36) 0.83 (0.69, 1.01) 0.97 (0.81, 1.15) | B.E.S.H.R | 8 |
| Merritt, 2015 [29] | US | 1531/205,863 | 30–55/25–42 | yes | every 4 years NHS I 1976 (1980–2010) NHSII1989 (1991–2011) | Butter: 5.0 vs. 0 g/day Cheese: 28.0 vs. 2 g/day Yogurt: 105.4 vs. 0 g/day | 1.10 (0.97, 1.24) 0.98 (0.82, 1.16) 1.06 (0.93, 1.22) | B.E.S.H.R | 7 |
| Population-based Case-control | |||||||||
| Potischman, 1992 [16] | US | 399/296 | 20–74 | yes | few years | Dairy: 17.6 vs. 6.0 times/week | 1.2 (0.70, 2.00) | A.B.E.H.S.R | 7 |
| McCann, 2000 [20] | US | 232/639 | 40–85 | yes | 2 years | Dairy: >56 vs. ≤32 times/month | 0.8 (0.5, 1.3) | A.B.H.S.R | 6 |
| Jain, 2000 [19] | Canada | 552/562 | 30–79 | yes | - | Milk: 413 vs. 84 g/day Cheese: 29.1 vs. 5.3 g/day | 0.86 (0.59, 1.24) 0.94 (0.65, 1.37) | A.B.E.H.S.R | 7 |
| Littman, 2001 [21] | US | 679/944 | 45–74 | yes | 5 years | Dairy: >2.4 vs. <1.2 servings/day | 1.0 (0.78, 1.40) | A.B.E.H.S | 8 |
| Terry, 2002 [23] | Sweden | 709/2887 | 50–74 | yes | 1 year | Dairy: median intake 35 vs. 5 serving/week | 0.90 (0.70, 1.20) | A.B.S | 6 |
| Xu, 2006 [25] | China | 1204/1212 | 30–69 | yes | 5 years | Milk: ever vs. never | 1.0 (0.80, 1.20) | A.B.E | 7 |
| Chandran et al., 2010 [27] | US | 417/395 | >21 | yes | 6 months | Dairy: ≥0.99 vs. <0.26 cups/day/1000 kcal) | 0.81 (0.52, 1.27) | A.B.E.S.H.R | 7 |
| Hospital-based Case-control | |||||||||
| Mettlin, 1990 [14] | US | 231/1300 | 19–97 | - | habits before | Whole Milk: daily vs. none 2% Milk: daily vs. none Skim milk: daily vs. none | 1.50 (1.0, 2.40) 1.0 (0.7, 1.40) 0.90 (0.50, 1.50) | A.S | 5 |
| Barbone, 1993 [15] | US | 103/236 | mean 64 | yes | 1 year | Skim milk: ≥1 vs. <1/month sour cream: ≥1 vs. <1/month yogurt: ≥1 vs. <1/month cheese: ≥1 vs. <1/month | 0.60 (0.30, 1.0) 0.40 (0.2, 0.70) 0.30 (0.20, 0.60) 0.40 (0.20, 0.90) | A.E.H.S.R | 5 |
| Tzonou, 1996 [17] | Greece | 145/298 | - | not mention | 1 year | Dairy: per quartile | 0.94 (0.74, 1.19) | A.B.E.H.S.R | 6 |
| Hirose, 1996 [18] | Japan | 145/26,751 | >20 | not mention | - | Milk: daily vs. occasional/none | 1.04 (0.68, 1.60) | B.S.R | 5 |
| Petridou, 2002 [22] | Greece | 84/84 | - | yes | 12 months | Dairy: per quartile | 1.21 (0.86, 1.69) | (A).B.E.R | 7 |
| Salazar-Martinez, 2005 [24] | Mexico | 85/629 | 22–79 | yes | 1 year | Dairy: tertile3 vs. tertile1 | 0.5 (0.23, 1.13) | A.B.E.R | 6 |
| Bravi, 2009 [26] | Italy | 454/908 | 18–79 | yes | 2 years | Milk and yogurt: >10.50 vs. <1 servings/week cheese: >6.32 vs. <2.4 servings/week | 1.24 (0.8, 1.93) 1.24 (0.80, 1.93) | B.E.H.R | 6 |
| Filomeno, 2015 [30] | Italy | 1411/3668 | median 61 | - | 2 years | Dairy: >median vs. <median | 1.19 (1.04, 1.36) | A.B.E.S.H.R | 5 |
| Rotman, 2016 [31] | Poland | 68/480 | 40–84 | - | - | Dairy: 250 vs. 0 g/day | 0.25 (0.07, 0.97) | - | 4 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zhao, J.; Li, P.; Gao, Y. Dairy Products Intake and Endometrial Cancer Risk: A Meta-Analysis of Observational Studies. Nutrients 2018, 10, 25. https://doi.org/10.3390/nu10010025
Li X, Zhao J, Li P, Gao Y. Dairy Products Intake and Endometrial Cancer Risk: A Meta-Analysis of Observational Studies. Nutrients. 2018; 10(1):25. https://doi.org/10.3390/nu10010025
Chicago/Turabian StyleLi, Xiaofan, Jing Zhao, Peiqin Li, and Ying Gao. 2018. "Dairy Products Intake and Endometrial Cancer Risk: A Meta-Analysis of Observational Studies" Nutrients 10, no. 1: 25. https://doi.org/10.3390/nu10010025





