Immune-Mediated Mechanisms of Action of Probiotics and Synbiotics in Treating Pediatric Intestinal Diseases
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Major Clinical Effects and Related Mechanisms of Action of Probiotics in Pediatric Intestinal Diseases
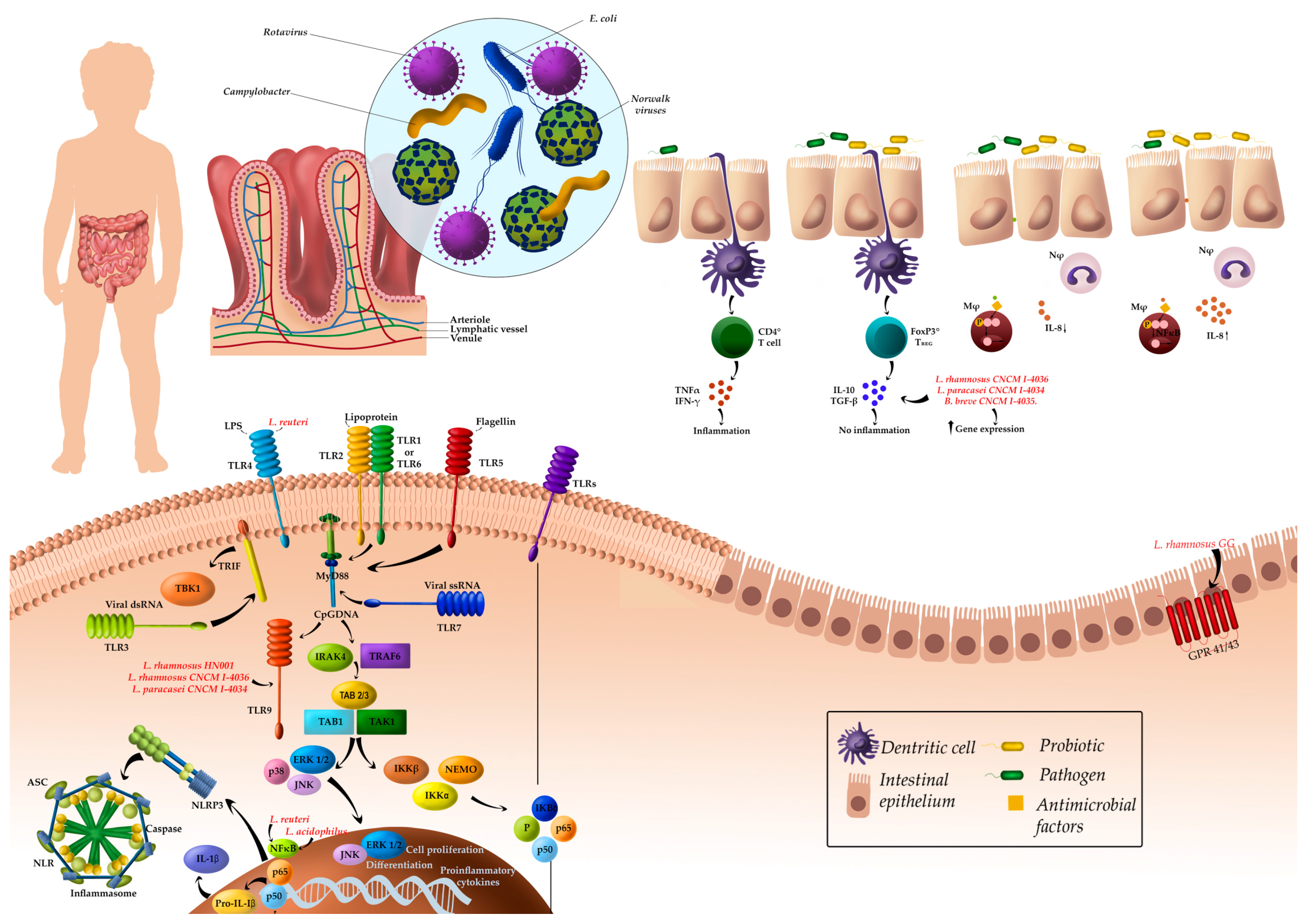
3.1.1. Gastrointestinal Infections
3.1.2. Antibiotic-Associated Diarrhea (AAD)
3.1.3. Clostridium difficile-Associated Diarrhea
3.1.4. Helicobacter pylori Gastritis and Peptic Ulcer
3.1.5. Necrotizing Enterocolitis
3.1.6. Inflammatory Bowel Diseases
3.1.7. Cystic Fibrosis
3.1.8. Other Studies
4. Conclusions
Acknowledgments
Authors Contributions
Conflicts of Interest
References
- Kvaerner, K.J.; Nafstad, P.; Jaakkola, J.J. Upper respiratory morbidity in preschool children: A cross-sectional study. Arch. Otolaryngol. Head Neck Surg. 2000, 126, 1201–1206. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Samuels, M.E.; Shi, L.; Baker, S.L.; Glover, S.H.; Sanders, J.M. Child day care risks of common infectious diseases revisited. Child Care Health Dev. 2004, 30, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Laursen, R.P.; Larnkjær, A.; Ritz, C.; Hauger, H.; Michaelsen, K.F.; Mølgaard, C. Probiotics and child care absence due to infections: A randomized controlled trial. Pediatrics 2017, 140, e20170735. [Google Scholar] [CrossRef] [PubMed]
- Nafstad, P.; Hagen, J.A.; Oie, L.; Magnus, P.; Jaakkola, J.J.K. Day care centers and respiratory health. Pediatrics 1999, 103, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Pickering, L.K.; Bartlett, A.V.; Woodward, W.E. Acute infectious diarrhea in day care: Epidemiology and control. Rev. Infect. Dis 1986, 8, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Binsztein, N.; Picandet, A.M.; Notario, R.; Patrito, E.; De Lesa, M.E.; De Petris, A.; Maurel, D.; Nader, O.; Rivas, M.; Szefner, M.; et al. Antimicrobial resistance among species of Salmonella, Shigella, Escherichia, and Aeromonas isolated from children with diarrhea in 7 Argentinian centers. Rev. Latinoam. Microbiol. 1999, 41, 121–126. [Google Scholar] [PubMed]
- Hudson, L.E.; Anderson, S.E.; Corbett, A.H.; Lamb, T.J. Gleaning insights from fecal microbiota transplantation and probiotic studies for the rational design of combination microbial therapies. Clin. Microbiol. Rev. 2017, 30, 191–231. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Umbrello, G.; Castellazzi, L.; Principi, N. Treatment of Clostridium difficile infection in pediatric patients. Expert Rev. Gastroenterol. Hepatol. 2015, 9, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Pacifico, L.; Osborn, J.F.; Bonci, E.; Romaggioli, S.; Baldini, R.; Chiesa, C. Probiotics for the treatment of Helicobacter pylori infection in children. World J. Gastroenterol. 2014, 20, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Soriano-Gabarró, M.; Mrukowicz, J.; Vesikari, T.; Verstraeten, T. Burden of rotavirus disease in European Union countries. Pediatr. Infect. Dis. J. 2006, 25, S7–S11. [Google Scholar] [CrossRef] [PubMed]
- Thielman, N.M.; Guerrant, R.L. Clinical practice. Acute infectious diarrhea. N. Engl. J. Med. 2004, 350, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; De Greef, E.; Devreker, T.; Veereman-Wauters, G.; Hauser, B. Probiotics and prebiotics in infants and children. Curr. Infect. Dis. Rep. 2013, 15, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Szajewska, H.; Dziechciarz, P. Gastrointestinal infections in the pediatric population. Curr. Opin. Gastroenterol. 2010, 26, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Caffarelli, C.; Cardinale, F.; Povesi-Dascola, C.; Dodi, I.; Mastrorilli, V.; Ricci, G. Use of probiotics in pediatric infectious diseases. Expert Rev. Anti-Infect. Ther. 2015, 13, 1517–1535. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Díaz, J.; Ruiz-Ojeda, F.J.; Vilchez-Padial, L.M.; Gil, A. Evidence of the anti-inflammatory effects of probiotics and synbiotics in intestinal chronic diseases. Nutrients 2017, 9, 555. [Google Scholar] [CrossRef] [PubMed]
- Di Nardo, G.; Oliva, S.; Menichella, A.; Pistelli, R.; De Biase, R.V.; Patriarchi, F.; Cucchiara, S.; Stronati, L. Lactobacillus reuteri ATCC55730 in cystic fibrosis. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Bellemare, S.; Hartling, L.; Wiebe, N.; Russell, K.; Craig, W.R.; McConnell, D.; Klassen, T.P. Oral rehydration versus intravenous therapy for treating dehydration due to gastroenteritis in children: A meta-analysis of randomised controlled trials. BMC Med. 2004, 2, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blum, L.S.; Oria, P.A.; Olson, C.K.; Breiman, R.F.; Ram, P.K. Examining the use of oral rehydration salts and other oral rehydration therapy for childhood diarrhea in Kenya. Am. J. Trop. Med. Hyg. 2011, 85, 1126–1233. [Google Scholar] [CrossRef] [PubMed]
- Desjeux, J.F.; Briend, A.; Butzner, J.D. Oral rehydration solution in the year 2000: Pathophysiology, efficacy and effectiveness. Bailliere’s Clin. Gastroenterol. 1997, 11, 509–527. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Díaz, J.; Fernandez-Caballero, J.Á.; Chueca, N.; Garcia, F.; Gómez-Llorente, C.; Sáez-Lara, M.J.; Fontana, L.; Gil, A. Pyrosequencing analysis reveals changes in intestinal microbiota of healthy adults who received a daily dose of immunomodulatory probiotic strains. Nutrients 2015, 7, 3999–4015. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Díaz, J.; Robles-Sánchez, C.; Abadía-Molina, F.; Morón-Calvente, V.; Sáez-Lara, M.J.; Ruiz-Bravo, A.; Jiménez-Valera, M.; Gil, A.; Gómez-Llorente, C.; Fontana, L. Adamdec1, Ednrb and Ptgs1/Cox1, inflammation genes upregulated in the intestinal mucosa of obese rats, are downregulated by three probiotic strains. Sci. Rep. 2017, 7, 1939. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Bermudez-Brito, M.; Plaza-Diaz, J.; Muñoz-Quezada, S.; Gil, A. Sources, isolation, characterisation and evaluation of probiotics. Br. J. Nutr. 2013, 109, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Szajewska, H.; Guarino, A.; Hojsak, I.; Indrio, F.; Kolacek, S.; Shamir, R.; Vandenplas, Y.; Weizman, Z.; European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. Use of probiotics for management of acute gastroenteritis: A position paper by the ESPGHAN working group for probiotics and prebiotics. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Szajewska, H.; Skórka, A.; Ruszczyński, M.; Gieruszczak-Białek, D. Meta-analysis: Lactobacillus GG for treating acute gastroenteritis in children–updated analysis of randomised controlled trials. Aliment. Pharmacol. Ther. 2013, 38, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Binns, C.; Lee, M.K. The use of probiotics to prevent diarrhea in young children attending child care centers: A review. J. Exp. Clin. Med. 2010, 2, 269–273. [Google Scholar] [CrossRef]
- King, S.; Glanville, J.; Sanders, M.E.; Fitzgerald, A.; Varley, D. Effectiveness of probiotics on the duration of illness in healthy children and adults who develop common acute respiratory infectious conditions: A systematic review and meta-analysis. Br. J. Nutr. 2014, 112, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hu, P.; Du, X.; Zhou, T.; Pei, X. Lactobacillus rhamnosus GG supplementation for preventing respiratory infections in children: A meta-analysis of randomized, placebo controlled trials. Indian Pediatr. 2013, 50, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Hao, Q.; Dong, B.R.; Wu, T. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst. Rev. 2015, 2, CD006895. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Ge, T.; Xiao, Y.; Liao, Y.; Cui, Y.; Zhang, Y.; Ho, W.; Yu, G.; Zhang, T. Probiotics for prevention and treatment of respiratory tract infections in children: A systematic review and meta-analysis of randomized controlled trials. Medicine 2016, 95, e4509. [Google Scholar] [CrossRef] [PubMed]
- Williams, N.T. Probiotics. Am. J. Health Syst. Pharm. 2010, 67, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Bermudez-Brito, M.; Plaza-Díaz, J.; Muñoz-Quezada, S.; Gómez-Llorente, C.; Gil, A. Probiotic mechanisms of action. Ann. Nutr. Metab. 2012, 61, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Quezada, S.; Bermudez-Brito, M.; Chenoll, E.; Genovés, S.; Gomez-Llorente, C.; Plaza-Diaz, J.; Matencio, E.; Bernal, M.J.; Romero, F.; Ramón, D.; et al. Competitive inhibition of three novel bacteria isolated from faeces of breast milk-fed infants against selected enteropathogens. Br. J. Nutr. 2013, 109, S63–S69. [Google Scholar] [CrossRef] [PubMed]
- Pineiro, M.; Asp, N.G.; Reid, G.; Macfarlane, S.; Morelli, L.; Brunser, O.; Tuohy, K. FAO Technical meeting on prebiotics. J. Clin. Gastroenterol. 2008, 42, S156–S159. [Google Scholar] [CrossRef] [PubMed]
- Sáez-Lara, M.J.; Robles-Sanchez, C.; Ruiz-Ojeda, F.J.; Plaza-Diaz, J.; Gil, A. Effects of probiotics and synbiotics on obesity, insulin resistance syndrome, type 2 diabetes and non-alcoholic fatty liver disease: A review of human clinical trials. Int. J. Mol. Sci. 2016, 17, 928. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.W.; Greer, F.R.; American Academy of Pediatrics Committee on Nutrition; American Academy of Pediatrics Section on Gastroenterology, Hepatology, and Nutrition. Probiotics and prebiotics in pediatrics. Pediatrics 2010, 126, 1217–1231. [Google Scholar] [CrossRef] [PubMed]
- Guarner, F.; Khan, A.G.; Garisch, J.; Eliakim, R.; Gangl, A.; Thomson, A.; Krabshuis, J.; Lemair, T.; Kaufmann, P.; de Paula, J.A.; et al. World Gastroenterology Organisation global guidelines. Probiotics and prebiotics October 2011. J. Clin. Gastroenterol. 2012, 46, 468–481. [Google Scholar] [CrossRef] [PubMed]
- Marchand, V.; Canadian Paediatric Society, Nutrition and Gastroenterology Committee. Using probiotics in the paediatric population. Paediatr. Child. Health 2012, 17, 575. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.S.; Chiu, Y.H.; Lin, N.T.; Chu, C.H.; Huang, K.C.; Liao, K.W.; Peng, K.C. Different effects of probiotic species/strains on infections in preschool children: A double-blind, randomized, controlled study. Vaccine 2009, 27, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Hojsak, I.; Snovak, N.; Abdović, S.; Szajewska, H.; Misak, Z.; Kolacek, S. Lactobacillus GG in the prevention of gastrointestinal and respiratory tract infections in children who attend day care centers: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2010, 29, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Hojsak, I.; Abdović, S.; Szajewska, H.; Milosević, M.; Krznarić, Z.; Kolacek, S. Lactobacillus GG in the prevention of nosocomial gastrointestinal and respiratory tract infections. Pediatrics 2010, 125. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, K.N.; Sowmyanarayanan, T.V.; Paul, A.; Babji, S.; Ajjampur, S.S.; Priyadarshini, S.; Sarkar, R.; Balasubramanian, K.A.; Wanke, C.A.; Ward, H.D.; et al. Immune response and intestinal permeability in children with acute gastroenteritis treated with Lactobacillus rhamnosus GG: A randomized, double-blind, placebo-controlled trial. Clin. Infect. Dis. 2014, 58, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Bruzzese, E.; Fedele, M.C.; Bruzzese, D.; Viscovo, S.; Giannattasio, A.; Mandato, C.; Siani, P.; Guarino, A. Randomised clinical trial: A Lactobacillus GG and micronutrient-containing mixture is effective in reducing nosocomial infections in children, vs. placebo. Aliment. Pharmacol. Ther. 2016, 44, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Scalabrin, D.; Harris, C.; Johnston, W.H.; Berseth, C.L. Long-term safety assessment in children who received hydrolyzed protein formulas with Lactobacillus rhamnosus GG: A 5-year follow-up. Eur. J. Pediatr. 2017, 176, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, J.; Lara-Villoslada, F.; Sierra, S.; Sempere, L.; Gómez, M.; Rodriguez, J.M.; Boza, J.; Xaus, J.; Olivares, M. Safety and tolerance of the human milk probiotic strain Lactobacillus salivarius CECT5713 in 6-month-old children. Nutrition 2010, 26, 1082–1087. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, J.; Cañabate, F.; Sempere, L.; Vela, F.; Sánchez, A.R.; Narbona, E.; López-Huertas, E.; Geerlings, A.; Valero, A.D.; Olivares, M.; et al. Human milk probiotic Lactobacillus fermentum CECT5716 reduces the incidence of gastrointestinal and upper respiratory tract infections in infants. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Lobón, J.A.; Gil-Campos, M.; Maldonado, J.; López-Huertas, E.; Flores-Rojas, K.; Valero, A.D.; Rodríguez-Benítez, M.V.; Bañuelos, O.; Lara-Villoslada, F.; Fonollá, J.; et al. Long-term safety of early consumption of Lactobacillus fermentum CECT5716: A 3-year follow-up of a randomized controlled trial. Pharmacol. Res. 2015, 95–96, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Agustina, R.; Kok, F.J.; van de Rest, O.; Fahmida, U.; Firmansyah, A.; Lukito, W.; Feskens, E.J.; van den Heuvel, E.G.; Albers, R.; Bovee-Oudenhoven, I.M. Randomized trial of probiotics and calcium on diarrhea and respiratory tract infections in Indonesian children. Pediatrics 2012, 129. [Google Scholar] [CrossRef] [PubMed]
- Corsello, G.; Carta, M.; Marinello, R.; Picca, M.; De Marco, G.; Micillo, M.; Ferrara, D.; Vigneri, P.; Cecere, G.; Ferri, P. Preventive effect of cow’s milk fermented with Lactobacillus paracasei CBA L74 on common infectious diseases in children: A multicenter randomized controlled trial. Nutrients 2017, 9, 669. [Google Scholar] [CrossRef] [PubMed]
- Merenstein, D.; Murphy, M.; Fokar, A.; Hernandez, R.K.; Park, H.; Nsouli, H.; Sanders, M.E.; Davis, B.A.; Niborski, V.; Tondu, F.; et al. Use of a fermented dairy probiotic drink containing Lactobacillus casei (DN-114 001) to decrease the rate of illness in kids: The DRINK study. A patient-oriented, double-blind, cluster-randomized, placebo-controlled, clinical trial. Eur. J. Clin. Nutr. 2010, 64, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Wanke, M.; Szajewska, H. Lack of an effect of Lactobacillus reuteri DSM 17938 in preventing nosocomial diarrhea in children: A randomized, double-blind, placebo-controlled trial. J. Pediatr. 2012. [Google Scholar] [CrossRef] [PubMed]
- Prodeus, A.; Niborski, V.; Schrezenmeir, J.; Gorelov, A.; Shcherbina, A.; Rumyantsev, A. Fermented Milk Consumption and Common Infections in Children Attending Day-Care Centers: A Randomized Trial. J. Pediatr. Gastroenterol. Nutr. 2016, 63, 534–543. [Google Scholar] [CrossRef] [PubMed]
- İşlek, A.; Sayar, E.; Yılmaz, A.; Baysan, B.Ö.; Mutlu, D.; Artan, R. The role of Bifidobacterium lactis B94 plus inulin in the treatment of acute infectious diarrhea in children. Turk. J. Gastroenterol. 2014, 25, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Hojsak, I.; Tokić Pivac, V.; Močić Pavić, A.; Pasini, A.M.; Kolaček, S. Bifidobacterium animalis subsp. lactis fails to prevent common infections in hospitalized children: A randomized, double-blind, placebo-controlled study. Am. J. Clin. Nutr. 2015, 101, 680–684. [Google Scholar] [CrossRef] [PubMed]
- Taipale, T.J.; Pienihäkkinen, K.; Isolauri, E.; Jokela, J.T.; Söderling, E.M. Bifidobacterium animalis subsp. lactis BB-12 in reducing the risk of infections in early childhood. Pediatr. Res. 2016, 79, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, J.Z.; Lytvyn, L.; Steurich, J.; Parkin, P.; Mahant, S.; Johnston, B.C. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst. Rev. 2015, 12, CD004827. [Google Scholar] [CrossRef]
- Georgieva, M.; Pancheva, R.; Rasheva, N.; Usheva, N.; Ivanova, L.; Koleva, K. Use of the probiotic Lactobacillus reuteri DSM17938 in the prevention of antibiotic-associated infections in hospitalized bulgarian children: A randomized, controlled trial. J. IMAB Annu. Proc. 2015, 21. [Google Scholar] [CrossRef]
- Goldenberg, J.Z.; Ma, S.S.; Saxton, J.D.; Martzen, M.R.; Vandvik, P.O.; Thorlund, K.; Guyatt, G.H.; Johnston, B.C. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst. Rev. 2013, 5, CD006095. [Google Scholar] [CrossRef]
- Ahmad, K.; Fatemeh, F.; Mehri, N.; Maryam, S. Probiotics for the treatment of pediatric Helicobacter pylori infection: A randomized double blind clinical trial. Iran. J. Pediatr. 2013, 23, 79–84. [Google Scholar] [PubMed]
- Namkin, K.; Zardast, M.; Basirinejad, F. Saccharomyces Boulardii in Helicobacter pylori Eradication in children: A randomized trial from Iran. Iran. J. Pediatr. 2016, 26, e3768. [Google Scholar] [CrossRef] [PubMed]
- Ustundag, G.H.; Altuntas, H.; Soysal, Y.D.; Kokturk, F. The effects of synbiotic “Bifidobacterium lactis B94 plus inulin” addition on standard triple therapy of Helicobacter pylori eradication in children. Can. J. Gastroenterol. Hepatol. 2017, 2017, 8130596. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.R.; Wang, F.; Qiu, X.; McFarland, L.V.; Chen, P.F.; Zhou, R.; Liu, J.; Zhao, Q.; Li, J. Efficacy and safety of probiotic-supplemented triple therapy for eradication of Helicobacter pylori in children: A systematic review and network meta-analysis. Eur. J. Clin. Pharmacol. 2017, 65, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Claud, E.C.; Keegan, K.P.; Brulc, J.M.; Lu, L.; Bartels, D.; Glass, E.; Chang, E.B.; Meyer, F.; Antonopoulos, D.A. Bacterial community structure and functional contributions to emergence of health or necrotizing enterocolitis in preterm infants. Microbiome 2013, 1, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Alfaleh, K.; Anabrees, J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst. Rev. 2014, 4, CD005496. [Google Scholar] [CrossRef]
- Olsen, R.; Greisen, G.; Schrøder, M.; Brok, J. Prophylactic probiotics for preterm infants: A systematic review and meta-analysis of observational studies. Neonatology 2016, 109, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Copeland, D.R.; McVay, M.R.; Dassinger, M.S.; Jackson, R.J.; Smith, S.D. Probiotic fortified diet reduces bacterial colonization and translocation in a long-term neonatal rabbit model. J. Pediatr. Surg. 2009, 44, 1061–1064. [Google Scholar] [CrossRef] [PubMed]
- Alfaleh, K.; Anabrees, J.; Bassler, D. Probiotics reduce the risk of necrotizing enterocolitis in preterm infants: A meta-analysis. Neonatology 2010, 97, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Good, M.; Sodhi, C.P.; Ozolek, J.A.; Buck, R.H.; Goehring, K.C.; Thomas, D.L.; Vikram, A.; Bibby, K.; Morowitz, M.J.; Firek, B.; et al. Lactobacillus rhamnosus HN001 decreases the severity of necrotizing enterocolitis in neonatal mice and preterm piglets: Evidence in mice for a role of TLR9. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 306, G1021–G1032. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.E.; Versalovic, J. Probiotic Lactobacillus reuteri biofilms produce antimicrobial and anti-inflammatory factors. BMC Microbiol. 2009, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.P.; Thibodeaux, C.H.; Pena, J.A.; Ferry, G.D.; Versalovic, J. Probiotic Lactobacillus reuteri suppress proinflammatory cytokines via c-Jun. Inflamm. Bowel Dis. 2008, 14, 1068–1083. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fatheree, N.Y.; Mangalat, N.; Rhoads, J.M. Human-derived probiotic Lactobacillus reuteri strains differentially reduce intestinal inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G1087–G1096. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fatheree, N.Y.; Mangalat, N.; Rhoads, J.M. Lactobacillus reuteri strains reduce incidence and severity of experimental necrotizing enterocolitis via modulation of TLR4 and NF-κB signaling in the intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G608–G617. [Google Scholar] [CrossRef] [PubMed]
- McVay, M.R.; Boneti, C.; Habib, C.M.; Keller, J.E.; Kokoska, E.R.; Jackson, R.J.; Smith, S.D. Formula fortified with live probiotic culture reduces pulmonary and gastrointestinal bacterial colonization and translocation in a newborn animal model. J. Pediatr. Surg. 2008, 43, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Hoque, S.S.; Faruque, A.S.; Mahalanabis, D.; Hasnat, A. Infectious agents causing acute watery diarrhoea in infants and young children in Bangladesh and their public implications. J. Trop. Pediatr. 1994, 40, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Louie, S.; Shi, H.N.; Walker, W.A. Preinoculation with the probiotic Lactobacillus acidophilus early in life effectively inhibits murine Citrobacter rodentium colitis. Pediatr. Res. 2005, 58, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Foye, O.T.; Huang, I.F.; Chiou, C.C.; Walker, W.A.; Shi, H.N. Early administration of probiotic Lactobacillus acidophilus and/or prebiotic inulin attenuates pathogen-mediated intestinal inflammation and Smad 7 cell signaling. FEMS Immunol. Med. Microbiol. 2012, 65, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Diaz, J.; Gomez-Llorente, C.; Campaña-Martin, L.; Matencio, E.; Ortuño, I.; Martínez-Silla, R.; Gomez-Gallego, C.; Periago, M.J.; Ros, G.; Chenoll, E.; et al. Safety and immunomodulatory effects of three probiotic strains isolated from the feces of breast-fed infants in healthy adults: SETOPROB study. PLoS ONE 2013, 8, e78111. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Russell, W.M.; Douglas-Escobar, M.; Hauser, N.; Lopez, M.; Neu, J. Live and heat-killed Lactobacillus rhamnosus GG (LGG): Effects on pro and anti-inflammatory cyto/chemokines in gastrostomy-fed infant rats. Pediatr. Res. 2009, 66, 203e7. [Google Scholar] [CrossRef] [PubMed]
- Guarino, A.; Lo Vecchio, A.; Canani, R.B. Probiotics as prevention and treatment for diarrhea. Curr. Opin. Gastroenterol. 2009, 25, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Bloise, E.; Torricelli, M.; Novembri, R.; Borges, L.E.; Carrarelli, P.; Reis, F.M.; Petraglia, F. Heat-killed Lactobacillus rhamnosus GG modulates urocortin and cytokine release in primarytrophoblast cells. Placenta 2010, 31, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Cresci, G.A.M.; Mayor, P.C.; Thompson, S.A. Effect of butyrate and Lactobacillus GG on a butyrate receptor and transporter during Campylobacter jejuni exposure. FEMS Microbiol. Lett. 2017, 364. [Google Scholar] [CrossRef] [PubMed]
- Huang, I.F.; Lin, I.C.; Liu, P.F.; Cheng, M.F.; Liu, Y.C.; Hsieh, Y.D.; Chen, J.J.; Chen, C.L.; Chang, H.W.; Shu, C.W. Lactobacillus acidophilus attenuates Salmonella-induced intestinal inflammation via TGF-β signaling. BMC Microbiol. 2015, 15, 203. [Google Scholar] [CrossRef] [PubMed]
- Rojas, M.A.; Lozano, J.M.; Rojas, M.X.; Rodriguez, V.A.; Rondon, M.A.; Bastidas, J.A.; Perez, L.A.; Rojas, C.; Ovalle, O.; Garcia-Harker, J.E.; et al. Prophylactic probiotics to prevent death and nosocomial infection in preterm infants. Pediatrics 2012, 130, e1113–e1120. [Google Scholar] [CrossRef] [PubMed]
- Bermudez-Brito, M.; Muñoz-Quezada, S.; Gomez-Llorente, C.; Romero, F.; Gil, A. Lactobacillus rhamnosus and its cell-free culture supernatant differentially modulate inflammatory biomarkers in Escherichia coli-challenged human dendritic cells. Br. J. Nutr. 2014, 111, 1727–1737. [Google Scholar] [CrossRef] [PubMed]
- Bermudez-Brito, M.; Muñoz-Quezada, S.; Gomez-Llorente, C.; Matencio, E.; Bernal, M.J.; Romero, F.; Gil, A. Cell-free culture supernatant of Bifidobacterium. breve CNCM I-4035 decreases pro-inflammatory cytokines in human dendritic cells challenged with Salmonella typhi through TLR activation. PLoS ONE 2013, 8, e59370. [Google Scholar] [CrossRef] [PubMed]
- Bermudez-Brito, M.; Muñoz-Quezada, S.; Gomez-Llorente, C.; Matencio, E.; Bernal, M.J.; Romero, F.; Gil, A. Human intestinal dendritic cells decrease cytokine release against Salmonella infection in the presence of Lactobacillus paracasei upon TLR activation. PLoS ONE 2012, 7, e43197. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Diaz, J.; Gomez-Llorente, C.; Abadia-Molina, F.; Saez-Lara, M.J.; Campaña-Martin, L.; Muñoz-Quezada, S.; Romero, F.; Gil, A.; Fontana, L. Effects of Lactobacillus paracasei CNCM I-4034, Bifidobacterium. breve CNCM I-4035 and Lactobacillus rhamnosus CNCM I-4036 on hepatic steatosis in Zucker rats. PLoS ONE 2014, 9, e98401. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Díaz, J.; Robles-Sánchez, C.; Abadía-Molina, F.; Sáez-Lara, M.J.; Vilchez-Padial, L.M.; Gil, A.; Gómez-Llorente, C.; Fontana, L. Gene expression profiling in the intestinal mucosa of obese rats administered probiotic bacteria. Sci. Data 2017, 4, 170186. [Google Scholar] [CrossRef] [PubMed]
- Gil-Campos, M.; López, M.A.; Rodriguez-Benítez, M.V.; Romero, J.; Roncero, I.; Linares, M.D.; Maldonado, J.; López-Huertas, E.; Berwind, R.; Ritzenthaler, K.L.; et al. Lactobacillus fermentum CECT 5716 is safe and well tolerated in infants of 1–6 months of age: A randomized controlled trial. Pharmacol. Res. 2012, 65, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Tsilingiri, K.; Barbosa, T.; Penna, G.; Caprioli, F.; Sonzogni, A.; Viale, G.; Rescigno, M. Probiotic and postbiotic activity in health and disease: Comparison on a novel polarised ex-vivo organ culture model. Gut 2012, 61, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Buccigrossi, V.; Laudiero, G.; Russo, C.; Miele, E.; Sofia, M.; Monini, M.; Ruggeri, F.M.; Guarino, A. Chloride secretion induced by rotavirus is oxidative stress-dependent and inhibited by Saccharomyces boulardii in human enterocytes. PLoS ONE 2014, 9, e99830. [Google Scholar] [CrossRef] [PubMed]
| Reference | Participants | Probiotic Strain/Treatment | Time | Primary Outcome |
|---|---|---|---|---|
| Song-Lin et al., 2009 [39] | 986 children | L. casei rhamnosus, L. rhamnosus T cell-1, and a mixture of strains | 7 months | L. casei rhamnosus reduced respiratory infections, whereas multiple probiotic supplementation reduced the gastrointestinal disease. L. rhamnosus T cell-1 decreased the incidence of bacterial infection at 7 months |
| Hojsak et al., 2010 [40] | 281 children | L. rhamnosus GG | 3 months | Only prevention of upper respiratory tract infections |
| Hojsak et al., 2010 [41] | 742 children | L. rhamnosus GG | 1 week | L. rhamnosus GG treatment significantly reduced the risk for gastrointestinal infections, vomiting, and episodes of gastrointestinal infections |
| Kulandaipalayam et al., 2014 [42] | 124 children | L. rhamnosus GG | 1 month | L. rhamnosus GG decreased diarrheal episodes and restored normal intestinal permeability |
| Bruzzese et al., 2016 [43] | 90 children | L. rhamnosus GG plus vitamins B, C and zinc | 2 weeks | Treatment reduced incidence of gastrointestinal infections and length of hospitalization |
| Maldonado et al., 2010 [45] | 80 children | L. salivarius CECT5713 | 6 months | L. salivarius CECT5713 decreased incidence of diarrhea and respiratory infections |
| Maldonado et al., 2012 [46] | 215 children | L. fermentum CECT5716 plus GOS | 6 months | Synbiotic administration prevented community-acquired gastrointestinal infections in infants |
| Maldonado et al., 2015 [47] | 91 children | L. fermentum CECT5716 plus GOS | 3 years follow-up | All variables measured were similar compared with placebo |
| Scalabrin et al., 2017 [44] | 109 children | L. rhamnosus GG | 5 years follow-up | A decrease in the incidence of acute gastroenteritis was not detected |
| Agustina et al., 2012 [48] | 494 children | RCC plus L. casei CRL431, or RCC plus L. reuteri DSM17938 | 6 months | Incidence of all reported diarrhea and diarrhea incidence in children with a lower nutritional status were significantly lower in the L. reuteri group |
| Corsello et al., 2017 [49] | 126 children | L. paracasei CBA L74 | 3 months | Probiotic treatment decreased the number of episodes of acute gastroenteritis |
| Merenstein et al., 2010 [50] | 638 children | L. casei DN-114 001 | 3 months | L. casei DN-114 001 decreased gastrointestinal infections |
| Wanke et al., 2012 [51] | 106 children | L. reuteri DSM 17938 | 1 week | No effects |
| Prodeus et al., 2016 [52] | 599 children | L. casei CNCM I-1518 | 3 months | No effects |
| Islek et al., 2014 [53] | 156 children | B. lactis B94 plus inulin | 1 week | Synbiotic treatment decreased the duration of diarrhea |
| Hoksak et al., 2015 [54] | 727 children | B. animalis subsp. lactis BB-12 | 1 week | No effects |
| Taipale et al., 2016 [55] | 67 children | B. animalis subsp. lactis BB-12 | 2 years follow-up | No effects |
| Laursen et al., 2017 [3] | 290 children | B. animalis subsp. lactis and L. rhamnosus GG | 6 months | No effects |
| Reference | Animal Species | Probiotic Strain/Treatment | Type of Study | Time | Primary Outcome |
|---|---|---|---|---|---|
| Good et al., 2014 [68] | Newborn mice/premature piglets | L. rhamnosus HN001 | In vivo and ex vivo | 5 days | L. rhamnosus HN001 or its DNA could protect against the development of NEC in animals. This seems to require DNA receptor TLR9 activation |
| Liu et al., 2012 [72] | Newborn rats | L. reuteri | In vivo and ex vivo | 3 days | L. reuteri strains reduced intestinal inflammation by down-regulating the IL-6, TNF-α, TLR4, and NF-kB and up-regulating the IL-10 in rats with NEC |
| Liu et al., 2010 [71] | Newborn rats | L. reuteri | In vivo and in vitro (IPEC-J2 intestinal cell line) | 3 days | L. reuteri reduced the inflammation caused by LPS in intestinal epithelial cells and in the ileum. |
| Copeland et al., 2009 [66] | Neonatal rabbit model | L. lactis, E. cloacae | In vivo | 7 days | E. cloacae probiotic fortified diet was effective by reducing the colonization of pathogenic bacterium |
| Foye et al., 2012 [76] | Newborn mice | L. acidophilus | In vivo and in vitro (mouse intestinal epithelial cell line | 7 weeks | L. acidophilus, inulin, or synbiotic attenuate C. rodentium-induced intestinal inflammation through NF-κB and Smad 7 expression |
| Bloise et al., 2010 [80] | L. rhamnosus GG | In vitro (primary trophoblast cells from human placenta) | 3 h | L. rhamnosus GG provokes IL-4, IL-10 and urocortin expression and inhibits LPS-induced TNF-α in trophoblast cells from human term placenta |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plaza-Díaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Immune-Mediated Mechanisms of Action of Probiotics and Synbiotics in Treating Pediatric Intestinal Diseases. Nutrients 2018, 10, 42. https://doi.org/10.3390/nu10010042
Plaza-Díaz J, Ruiz-Ojeda FJ, Gil-Campos M, Gil A. Immune-Mediated Mechanisms of Action of Probiotics and Synbiotics in Treating Pediatric Intestinal Diseases. Nutrients. 2018; 10(1):42. https://doi.org/10.3390/nu10010042
Chicago/Turabian StylePlaza-Díaz, Julio, Francisco Javier Ruiz-Ojeda, Mercedes Gil-Campos, and Angel Gil. 2018. "Immune-Mediated Mechanisms of Action of Probiotics and Synbiotics in Treating Pediatric Intestinal Diseases" Nutrients 10, no. 1: 42. https://doi.org/10.3390/nu10010042






