Desphospho-Uncarboxylated Matrix-Gla Protein Is Increased Postoperatively in Cardiovascular Risk Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Sampling and Storage
2.3. PIVKA-II
2.4. MGP
2.5. OC
2.6. Comorbidities
2.7. Gastrointestinal Recovery Time—GIRT
2.8. Statistics
3. Results
3.1. Patient Characteristics
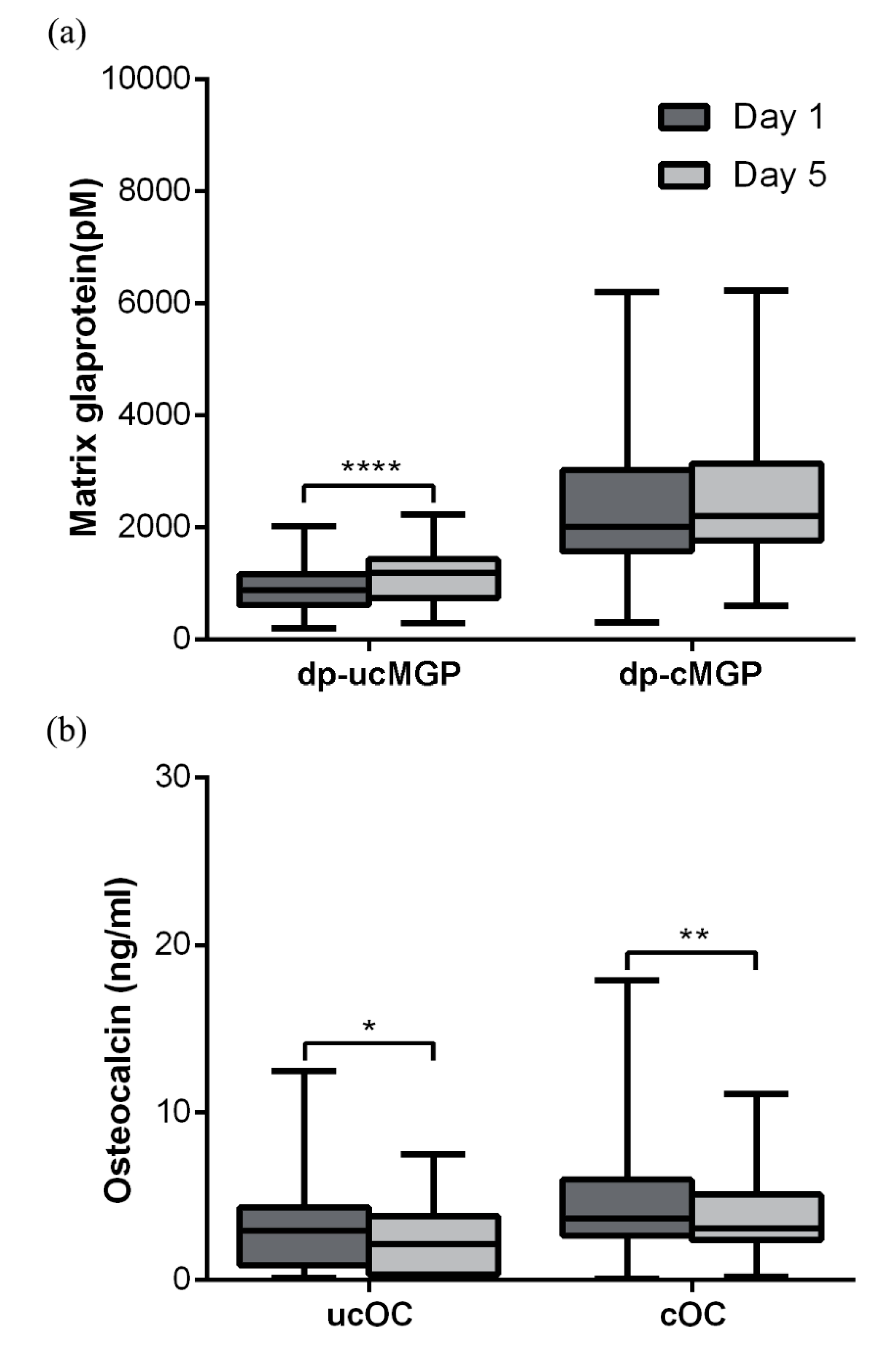
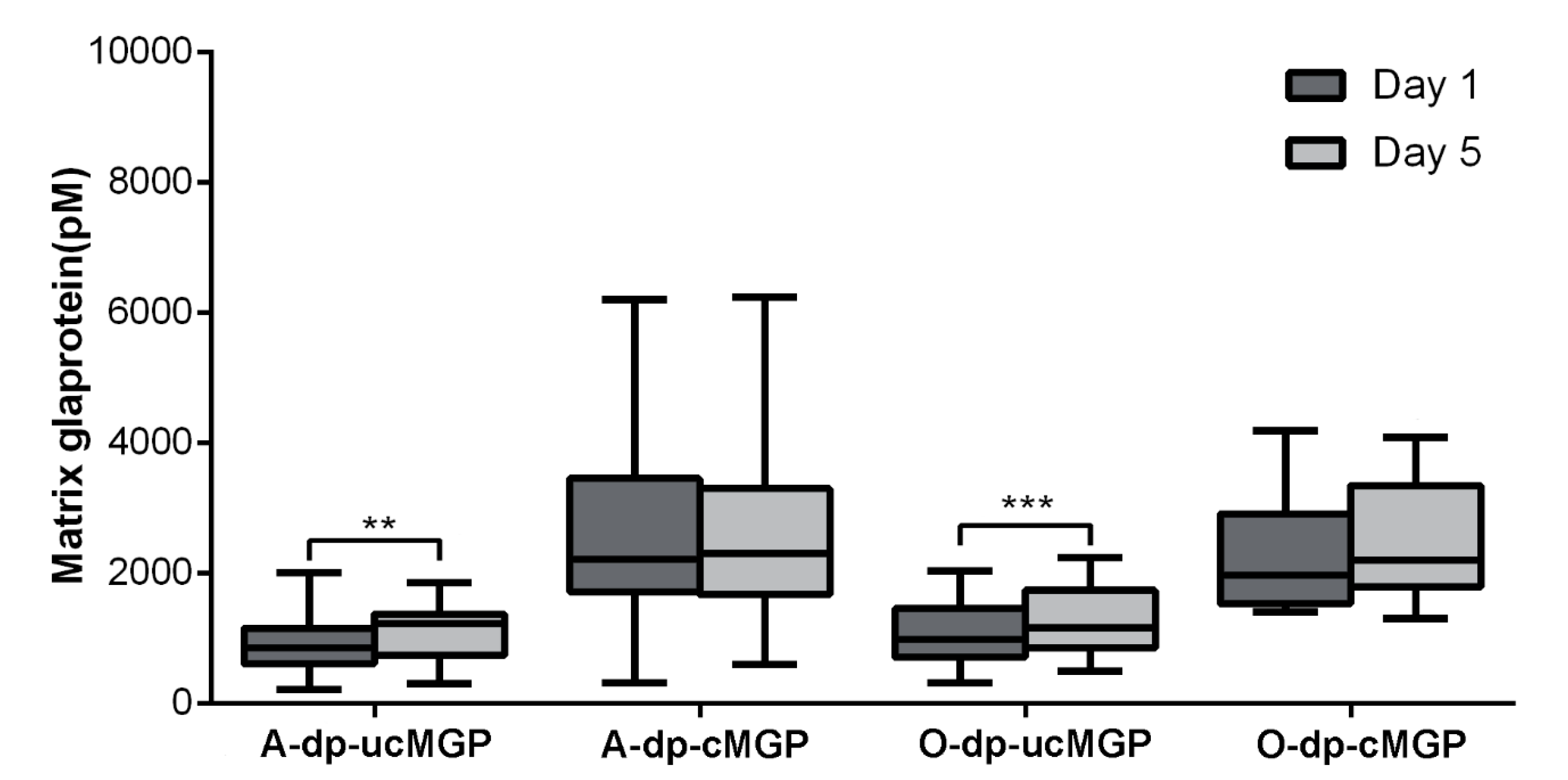
3.2. MGP
3.3. OC
3.4. Correlations
3.5. Comorbidities and MGP and OC Changes
3.6. Postoperative Complications and MGP and OC Changes
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cranenburg, E.C.; Schurgers, L.J.; Vermeer, C. Vitamin k: The coagulation vitamin that became omnipotent. Thromb. Haemost. 2007, 98, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Willems, B.A.; Vermeer, C.; Reutelingsperger, C.P.; Schurgers, L.J. The realm of vitamin k dependent proteins: Shifting from coagulation toward calcification. Mol. Nutr. Food Res. 2014, 58, 1620–1635. [Google Scholar] [CrossRef] [PubMed]
- Cancela, M.L.; Laize, V.; Conceicao, N. Matrix gla protein and osteocalcin: From gene duplication to neofunctionalization. Arch. Biochem. Biophys. 2014, 561, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Schurgers, L.J.; Cranenburg, E.C.; Vermeer, C. Matrix gla-protein: The calcification inhibitor in need of vitamin K. Thromb. Haemost. 2008, 100, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Ducy, P.; McKee, M.D.; Pinero, G.J.; Loyer, E.; Behringer, R.R.; Karsenty, G. Spontaneous calcification of arteries and cartilage in mice lacking matrix gla protein. Nature 1997, 386, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Murshed, M.; Schinke, T.; McKee, M.D.; Karsenty, G. Extracellular matrix mineralization is regulated locally; different roles of two gla-containing proteins. J. Cell Biol. 2004, 165, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Wajih, N.; Borras, T.; Xue, W.; Hutson, S.M.; Wallin, R. Processing and transport of matrix gamma-carboxyglutamic acid protein and bone morphogenetic protein-2 in cultured human vascular smooth muscle cells: Evidence for an uptake mechanism for serum fetuin. J. Biol. Chem. 2004, 279, 43052–43060. [Google Scholar] [CrossRef] [PubMed]
- Mayer, O., Jr.; Seidlerova, J.; Wohlfahrt, P.; Filipovsky, J.; Vanek, J.; Cifkova, R.; Windrichova, J.; Topolcan, O.; Knapen, M.H.; Drummen, N.E.; et al. Desphospho-uncarboxylated matrix gla protein is associated with increased aortic stiffness in a general population. J. Hum. Hypertens. 2016, 30, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Mayer, O., Jr.; Seidlerova, J.; Bruthans, J.; Filipovsky, J.; Timoracka, K.; Vanek, J.; Cerna, L.; Wohlfahrt, P.; Cifkova, R.; Theuwissen, E.; et al. Desphospho-uncarboxylated matrix gla-protein is associated with mortality risk in patients with chronic stable vascular disease. Atherosclerosis 2014, 235, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Dalmeijer, G.W.; van der Schouw, Y.T.; Magdeleyns, E.J.; Vermeer, C.; Verschuren, W.M.; Boer, J.M.; Beulens, J.W. Matrix gla protein species and risk of cardiovascular events in type 2 diabetic patients. Diabetes Care 2013, 36, 3766–3771. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.F.; Drummen, N.E.; Schutte, A.E.; Thijs, L.; Jacobs, L.; Petit, T.; Yang, W.Y.; Smith, W.; Zhang, Z.Y.; Gu, Y.M.; et al. Vitamin k dependent protection of renal function in multi-ethnic population studies. EBioMedicine 2016, 4, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Zoch, M.L.; Clemens, T.L.; Riddle, R.C. New insights into the biology of osteocalcin. Bone 2016, 82, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Dauti, F.; Hjaltalin Jonsson, M.; Hillarp, A.; Bentzer, P.; Schott, U. Perioperative changes in pivka-ii. Scand. J. Clin. Lab. Investig. 2015, 75, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Sokoll, L.J.; Sadowski, J.A. Comparison of biochemical indexes for assessing vitamin k nutritional status in a healthy adult population. Am. J. Clin. Nutr. 1996, 63, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Schlieper, G.; Westenfeld, R.; Kruger, T.; Cranenburg, E.C.; Magdeleyns, E.J.; Brandenburg, V.M.; Djuric, Z.; Damjanovic, T.; Ketteler, M.; Vermeer, C.; et al. Circulating nonphosphorylated carboxylated matrix gla protein predicts survival in esrd. J. Am. Soc. Nephrol. 2011, 22, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Torgersen, Z.; Balters, M. Perioperative nutrition. Surg. Clin. N. Am. 2015, 95, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Ferland, G.; Sadowski, J.A.; O’Brien, M.E. Dietary induced subclinical vitamin k deficiency in normal human subjects. J. Clin. Investig. 1993, 91, 1761–1768. [Google Scholar] [CrossRef] [PubMed]
- Grosley, B.M.; Hirschauer, C.; Chambrette, B.; Bezeaud, A.; Amiral, J. Specific measurement of hypocarboxylated prothrombin in plasma or serum and application to the diagnosis of hepatocellular carcinoma. J. Lab. Clin. Med. 1996, 127, 553–564. [Google Scholar] [CrossRef]
- Cranenburg, E.C.; Koos, R.; Schurgers, L.J.; Magdeleyns, E.J.; Schoonbrood, T.H.; Landewe, R.B.; Brandenburg, V.M.; Bekers, O.; Vermeer, C. Characterisation and potential diagnostic value of circulating matrix gla protein (mgp) species. Thromb. Haemost. 2010, 104, 811–822. [Google Scholar] [PubMed]
- Cranenburg, E.C.; Schurgers, L.J.; Uiterwijk, H.H.; Beulens, J.W.; Dalmeijer, G.W.; Westerhuis, R.; Magdeleyns, E.J.; Herfs, M.; Vermeer, C.; Laverman, G.D. Vitamin k intake and status are low in hemodialysis patients. Kidney Int. 2012, 82, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.P.; Gu, Y.M.; Thijs, L.; Knapen, M.H.; Salvi, E.; Citterio, L.; Petit, T.; Carpini, S.D.; Zhang, Z.; Jacobs, L.; et al. Inactive matrix gla protein is causally related to adverse health outcomes: A mendelian randomization study in a flemish population. Hypertension 2015, 65, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Theuwissen, E.; Magdeleyns, E.J.; Braam, L.A.; Teunissen, K.J.; Knapen, M.H.; Binnekamp, I.A.; van Summeren, M.J.; Vermeer, C. Vitamin K status in healthy volunteers. Food Funct. 2014, 5, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Ueland, T.; Dahl, C.P.; Gullestad, L.; Aakhus, S.; Broch, K.; Skardal, R.; Vermeer, C.; Aukrust, P.; Schurgers, L.J. Circulating levels of non-phosphorylated undercarboxylated matrix gla protein are associated with disease severity in patients with chronic heart failure. Clin. Sci. 2011, 121, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Caluwe, R.; Pyfferoen, L.; De Boeck, K.; De Vriese, A.S. The effects of vitamin k supplementation and vitamin k antagonists on progression of vascular calcification: Ongoing randomized controlled trials. Clin. Kidney J. 2016, 9, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Vossen, L.M.; Schurgers, L.J.; van Varik, B.J.; Kietselaer, B.L.; Vermeer, C.; Meeder, J.G.; Rahel, B.M.; van Cauteren, Y.J.; Hoffland, G.A.; Rennenberg, R.J.; et al. Menaquinone-7 supplementation to reduce vascular calcification in patients with coronary artery disease: Rationale and study protocol (vitak-cac trial). Nutrients 2015, 7, 8905–8915. [Google Scholar] [CrossRef] [PubMed]
- Hirota, Y.; Nakagawa, K.; Sawada, N.; Okuda, N.; Suhara, Y.; Uchino, Y.; Kimoto, T.; Funahashi, N.; Kamao, M.; Tsugawa, N.; et al. Functional characterization of the vitamin k2 biosynthetic enzyme ubiad1. PLoS ONE 2015, 10, e0125737. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Qureshi, A.R.; Parini, P.; Hurt-Camejo, E.; Ripsweden, J.; Brismar, T.B.; Barany, P.; Jaminon, A.M.; Schurgers, L.J.; Heimburger, O.; et al. Does statins promote vascular calcification in chronic kidney disease? Eur. J. Clin. Investig. 2017, 47, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Nimptsch, K.; Rohrmann, S.; Kaaks, R.; Linseisen, J. Dietary vitamin k intake in relation to cancer incidence and mortality: Results from the Heidelberg cohort of the European prospective investigation into cancer and nutrition (epic-heidelberg). Am. J. Clin. Nutr. 2010, 91, 1348–1358. [Google Scholar] [CrossRef] [PubMed]
- Juanola-Falgarona, M.; Salas-Salvado, J.; Martinez-Gonzalez, M.A.; Corella, D.; Estruch, R.; Ros, E.; Fito, M.; Aros, F.; Gomez-Gracia, E.; Fiol, M.; et al. Dietary intake of vitamin k is inversely associated with mortality risk. J. Nutr. 2014, 144, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Dahlberg, S.; Ede, J.; Schott, U. Vitamin k and cancer. Scand. J. Clin. Lab. Investig. 2017, 77, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Gheorghe, S.R.; Craciun, A.M. Matrix gla protein in tumoral pathology. Clujul Med. 2016, 89, 319–321. [Google Scholar] [CrossRef] [PubMed]
- Vermeer, C. Vitamin K: The effect on health beyond coagulation—An overview. Food Nutr. Res. 2012, 56. [Google Scholar] [CrossRef] [PubMed]
- Thomas, O.; Rein, H.; Strandberg, K.; Schott, U. Coagulative safety of epidural catheters after major upper gastrointestinal surgery: Advanced and routine coagulation analysis in 38 patients. Perioper. Med. 2016, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Van den Heuvel, E.G.; van Schoor, N.M.; Lips, P.; Magdeleyns, E.J.; Deeg, D.J.; Vermeer, C.; den Heijer, M. Circulating uncarboxylated matrix gla protein, a marker of vitamin k status, as a risk factor of cardiovascular disease. Maturitas 2014, 77, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Shea, M.K.; Booth, S.L.; Weiner, D.E.; Brinkley, T.E.; Kanaya, A.M.; Murphy, R.A.; Simonsick, E.M.; Wassel, C.L.; Vermeer, C.; Kritchevsky, S.B. Circulating vitamin K is inversely associated with incident cardiovascular disease risk among those treated for hypertension in the health, aging, and body composition study (health abc). J. Nutr. 2017, 147, 888–895. [Google Scholar] [CrossRef] [PubMed]

| Gla-prot | Dp-ucMGP.d1 | Dp-ucMGP.d5 | Dp-cMGP.d1 | Dp-cMGP.d5 | ucOC.d1 | ucOC.d5 | cOC.d1 | cOC.d5 | PIVKA-II.d1 | PIVKA-II.d5 |
|---|---|---|---|---|---|---|---|---|---|---|
| Dp-ucMGP.d1 | 1.00 | 0.79 | 0.50 | 0.49 | 0.07 | −0.04 | −0.18 | −0.13 | 0.31 | 0.38 |
| Dp-ucMGP.d5 | 0.79 | 1.00 | 0.29 | 0.48 | −0.11 | 0.04 | −0.19 | −0.06 | 0.15 | 0.44 |
| Dp-cMGP.d1 | 0.50 | 0.29 | 1.00 | 0.86 | 0.07 | −0.17 | 0.05 | −0.04 | 0.16 | −0.02 |
| Dp-cMGP.d5 | 0.49 | 0.48 | 0.86 | 1.00 | −0.04 | −0.06 | 0.14 | 0.17 | 0.10 | −0.07 |
| ucOC.d1 | 0.07 | −0.11 | 0.07 | −0.04 | 1.00 | 0.68 | 0.55 | 0.47 | −0.02 | −0.09 |
| ucOC.d5 | −0.04 | 0.04 | −0.17 | −0.06 | 0.68 | 1.00 | 0.36 | 0.44 | −0.06 | −0.10 |
| cOC.d1 | −0.18 | −0.19 | 0.05 | 0.14 | 0.55 | 0.36 | 1.00 | 0.84 | −0.11 | −0.12 |
| cOC.d5 | −0.13 | −0.06 | −0.04 | 0.17 | 0.47 | 0.44 | 0.84 | 1.00 | −0.24 | −0.20 |
| PIVKA-II.d1 | 0.31 | 0.15 | 0.16 | 0.10 | −0.02 | −0.06 | −0.11 | −0.24 | 1.00 | 0.52 |
| PIVKA-II.d5 | 0.38 | 0.44 | −0.02 | −0.07 | −0.09 | −0.10 | −0.12 | −0.20 | 0.52 | 1.00 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dahlberg, S.; Ede, J.; Schurgers, L.; Vermeer, C.; Kander, T.; Klarin, B.; Schött, U. Desphospho-Uncarboxylated Matrix-Gla Protein Is Increased Postoperatively in Cardiovascular Risk Patients. Nutrients 2018, 10, 46. https://doi.org/10.3390/nu10010046
Dahlberg S, Ede J, Schurgers L, Vermeer C, Kander T, Klarin B, Schött U. Desphospho-Uncarboxylated Matrix-Gla Protein Is Increased Postoperatively in Cardiovascular Risk Patients. Nutrients. 2018; 10(1):46. https://doi.org/10.3390/nu10010046
Chicago/Turabian StyleDahlberg, Sofia, Jacob Ede, Leon Schurgers, Cees Vermeer, Thomas Kander, Bengt Klarin, and Ulf Schött. 2018. "Desphospho-Uncarboxylated Matrix-Gla Protein Is Increased Postoperatively in Cardiovascular Risk Patients" Nutrients 10, no. 1: 46. https://doi.org/10.3390/nu10010046






