Association between Diet-Quality Scores, Adiposity, Total Cholesterol and Markers of Nutritional Status in European Adults: Findings from the Food4Me Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Dietary Assessment
2.4. Biochemical Analysis
2.5. Diet-Quality Scores
2.5.1. Healthy Eating Index-2010 (HEI)
2.5.2. Alternate Healthy Eating Index (Ahei)
2.5.3. Predimed Mediterranean Diet Score (P-Mds)
2.5.4. Meddietscore (MDS)
2.5.5. Dutch Healthy Diet Index (Dhdi)
2.6. Statistical Analysis
3. Results
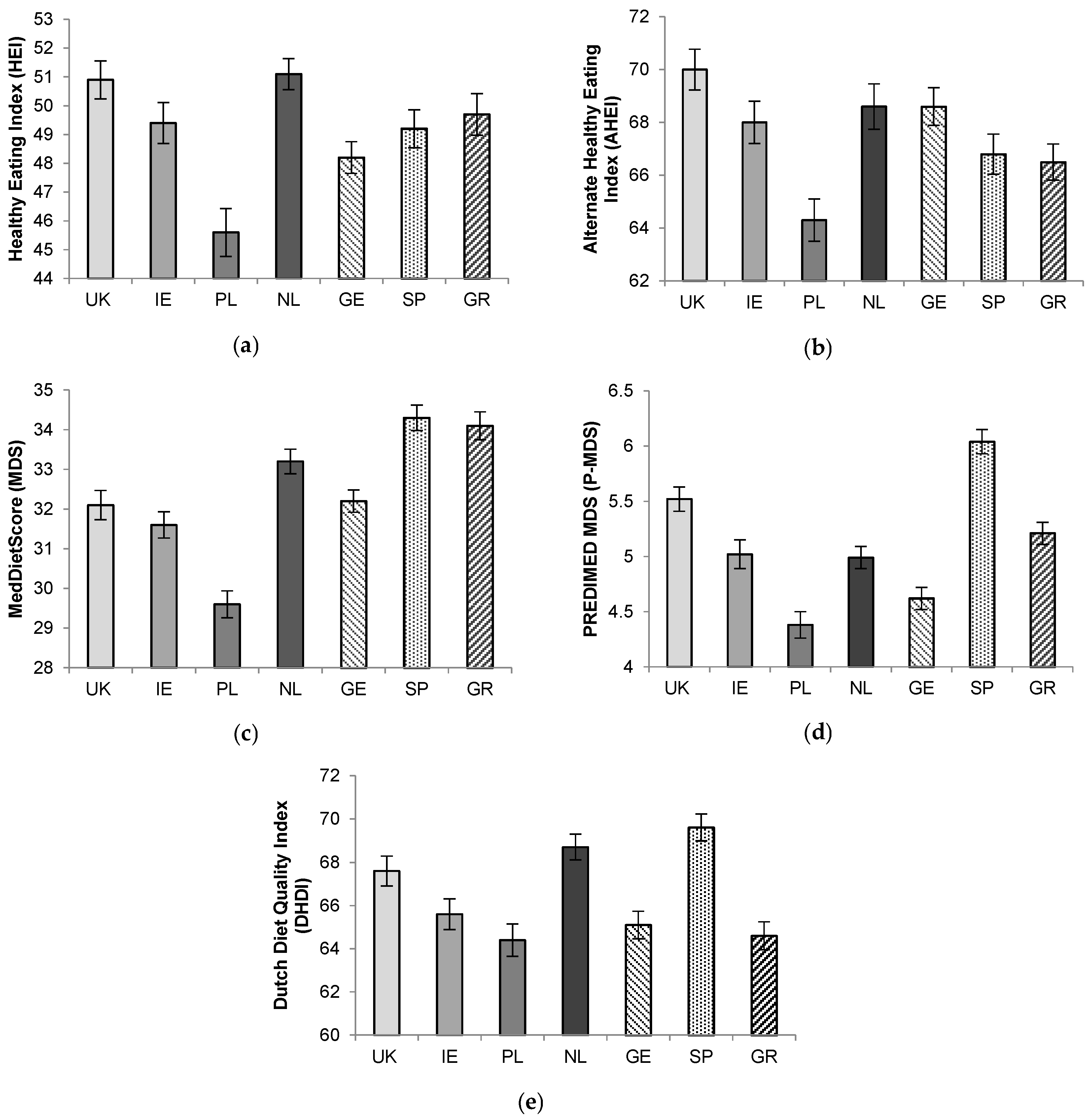
3.1. Comparison of Diet-Quality Scores across 7 European Countries
3.2. Association between Diet-Quality Scores and Markers of Cardiometabolic Risk
4. Discussion
4.1. Diet Quality Scores across Europe
4.2. Comparison with Previous Work
4.3. Implications
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fung, T.T.; Willett, W.C.; Stampfer, M.J.; Manson, J.E.; Hu, F.B. Dietary patterns and the risk of coronary heart disease in women. Arch. Intern. Med. 2001, 161, 1857–1862. [Google Scholar] [CrossRef] [PubMed]
- Micha, R.; Peñalvo, J.L.; Cudhea, F.; Imamura, F.; Rehm, C.D.; Mozaffarian, D. Association between dietary factors and mortality from heart disease, stroke, and type 2 diabetes in the united states. JAMA 2017, 317, 912–924. [Google Scholar] [CrossRef] [PubMed]
- Haveman-Nies, A.; Burema, J.; Cruz, J.A.A.; Osler, M.; van Staveren, W.A. Dietary quality and lifestyle factors in relation to 10-year mortality in older europeans the seneca study. Am. J. Epidemiol. 2002, 156, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Huijbregts, P.; Feskens, E.; Räsänen, L.; Fidanza, F.; Nissinen, A.; Menotti, A.; Kromhout, D. Dietary pattern and 20 year mortality in elderly men in finland, italy, and the netherlands: Longitudinal cohort study. BMJ 1997, 315, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Guenther, P.M.; Casavale, K.O.; Reedy, J.; Kirkpatrick, S.I.; Hiza, H.A.B.; Kuczynski, K.J.; Kahle, L.L.; Krebs-Smith, S.M. Update of the healthy eating index: Hei-2010. J. Acad. Nutr. Diet. 2013, 113, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Warden, B.; Paeratakul, S.; Bray, G. Healthy eating index and obesity. Eur. J. Clin. Nutr. 2004, 58, 1580–1586. [Google Scholar] [CrossRef] [PubMed]
- Drewnowski, A.; Fiddler, E.C.; Dauchet, L.; Galan, P.; Hercberg, S. Diet quality measures and cardiovascular risk factors in france: Applying the healthy eating index to the su. Vi. Max study. J. Am. Coll. Nutr. 2009, 28, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Chiuve, S.E.; Fung, T.T.; Rimm, E.B.; Hu, F.B.; McCullough, M.L.; Wang, M.; Stampfer, M.J.; Willett, W.C. Alternative dietary indices both strongly predict risk of chronic disease. J. Nutr. 2012, 142, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Hoffmann, G. Diet quality as assessed by the healthy eating index, the alternate healthy eating index, the dietary approaches to stop hypertension score, and health outcomes: A systematic review and meta-analysis of cohort studies. J. Acad. Nutr. Diet. 2015, 115, 780–800. [Google Scholar] [CrossRef] [PubMed]
- McCullough, M.L.; Willett, W.C. Evaluating adherence to recommended diets in adults: The alternate healthy eating index. Public Health Nutr. 2006, 9, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Knoops, K.T.; de Groot, L.C.; Kromhout, D.; Perrin, A.-E.; Moreiras-Varela, O.; Menotti, A.; Van Staveren, W.A. Mediterranean diet, lifestyle factors, and 10-year mortality in elderly european men and women: The hale project. JAMA 2004, 292, 1433–1439. [Google Scholar] [CrossRef] [PubMed]
- Panagiotakos, D.B.; Pitsavos, C.; Arvaniti, F.; Stefanadis, C. Adherence to the mediterranean food pattern predicts the prevalence of hypertension, hypercholesterolemia, diabetes and obesity, among healthy adults; the accuracy of the meddietscore. Prev. Med. 2007, 44, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; García-Arellano, A.; Toledo, E.; Salas-Salvado, J.; Buil-Cosiales, P.; Corella, D.; Covas, M.I.; Schröder, H.; Arós, F.; Gómez-Gracia, E. A 14-item mediterranean diet assessment tool and obesity indexes among high-risk subjects: The predimed trial. PLoS ONE 2012, 7, e43134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Lee, L.; Geelen, A.; van Huysduynen, E.J.H.; de Vries, J.H.; van’t Veer, P.; Feskens, E.J. The dutch healthy diet index (dhd-index): An instrument to measure adherence to the dutch guidelines for a healthy diet. Nutr. J. 2012, 11, 49. [Google Scholar] [CrossRef] [PubMed]
- Van Lee, L.; Feskens, E.J.; van Huysduynen, E.J.H.; de Vries, J.H.; van’t Veer, P.; Geelen, A. The dutch healthy diet index as assessed by 24 h recalls and ffq: Associations with biomarkers from a cross-sectional study. J. Nutr. Sci. 2013, 2, e40. [Google Scholar] [CrossRef] [PubMed]
- Celis-Morales, C.; Livingstone, K.M.; Marsaux, C.F.; Forster, H.; O’Donovan, C.B.; Woolhead, C.; Macready, A.L.; Fallaize, R.; Navas-Carretero, S.; San-Cristobal, R. Design and baseline characteristics of the food4me study: A web-based randomised controlled trial of personalised nutrition in seven european countries. Genes Nutr. 2015, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Forster, H.; Walsh, M.C.; O’Donovan, C.B.; Woolhead, C.; McGirr, C.; O’Riordan, R.; Celis-Morales, C.; Fallaize, R.; Macready, A.L.; Marsaux, C.F.; et al. A dietary feedback system for the delivery of consistent personalized dietary advice in the web-based multicenter food4me study. J. Med. Internet Res. 2016, 18, e150. [Google Scholar] [CrossRef] [PubMed]
- Forster, H.; Fallaize, R.; Gallagher, C.; O’Donovan, C.B.; Woolhead, C.; Walsh, M.C.; Macready, A.L.; Lovegrove, J.A.; Mathers, J.C.; Gibney, M.J. Online dietary intake estimation: The food4me food frequency questionnaire. J. Med. Internet Res. 2014, 16, e150. [Google Scholar] [CrossRef] [PubMed]
- Fallaize, R.; Forster, H.; Macready, A.L.; Walsh, M.C.; Mathers, J.C.; Brennan, L.; Gibney, E.R.; Gibney, M.J.; Lovegrove, J.A. Online dietary intake estimation: Reproducibility and validity of the food4me food frequency questionnaire against a 4-day weighed food record. J. Med. Internet Res. 2014, 16, e190. [Google Scholar] [CrossRef] [PubMed]
- Baecke, J.A.; Burema, J.; Frijters, J.E. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am. J. Clin. Nutr. 1982, 36, 936–942. [Google Scholar] [PubMed]
- Montoye, H.J.; Kemper, H.C.; Saris, W.H.; Washburn, R.A. Measuring Physical Activity and Energy Expenditure; Human Kinetics: Champaign, IL, USA, 1996. [Google Scholar]
- Philippaerts, R.M.; Westerterp, K.R.; Lefevre, J. Doubly labelled water validation of three physical activity questionnaires. Int. J. Sports Med. 1999, 20, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Bonomi, A.G.; Plasqui, G.; Goris, A.H.; Westerterp, K.R. Estimation of free-living energy expenditure using a novel activity monitor designed to minimize obtrusiveness. Obesity 2010, 18, 1845–1851. [Google Scholar] [CrossRef] [PubMed]
- Marsaux, C.F.; Celis-Morales, C.; Fallaize, R.; Macready, A.L.; Kolossa, S.; Woolhead, C.; O’Donovan, C.B.; Forster, H.; Navas-Carretero, S.; San-Cristobal, R. Effects of a web-based personalized intervention on physical activity in european adults: A randomized controlled trial. J. Med. Internet Res. 2015, 17, e230. [Google Scholar] [CrossRef] [PubMed]
- Holen, T.; Norheim, F.; Gundersen, T.; Mitry, P.; Linseisen, J.; Iversen, P.; Drevon, C. Biomarkers for nutrient intake with focus on alternative sampling techniques. Genes Nutr. 2016, 11, 12. [Google Scholar] [CrossRef] [PubMed]
- Markussen, M.S.; Veierød, M.B.; Sakhi, A.K.; Ellingjord-Dale, M.; Blomhoff, R.; Ursin, G.; Andersen, L.F. Evaluation of dietary patterns among norwegian postmenopausal women using plasma carotenoids as biomarkers. Br. J. Nutr. 2015, 113, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S.; von Schacky, C. The omega-3 index: A new risk factor for death from coronary heart disease? Prev. Med. 2004, 39, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Albani, V.; Celis-Morales, C.; Marsaux, C.F.; Forster, H.; O’donovan, C.B.; Woolhead, C.; Macready, A.L.; Fallaize, R.; Navas-Carretero, S.; San-Cristobal, R. Exploring the association of dairy product intake with the fatty acids c15: 0 and c17: 0 measured from dried blood spots in a multipopulation cohort: Findings from the food4me study. Mol. Nutr. Food Res. 2016, 60, 834–845. [Google Scholar] [CrossRef] [PubMed]
- Celis-Morales, C.; Livingstone, K.M.; Marsaux, C.F.; Macready, A.L.; Fallaize, R.; O’Donovan, C.B.; Woolhead, C.; Forster, H.; Walsh, M.C.; Navas-Carretero, S. Effect of personalized nutrition on health-related behaviour change: Evidence from the food4me european randomized controlled trial. Int. J. Epidemiol. 2016, 46, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Schröder, H.; Fitó, M.; Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventós, R.; Ros, E.; Salaverría, I.; Fiol, M. A short screener is valid for assessing mediterranean diet adherence among older spanish men and women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, K.M.; Celis-Morales, C.; Navas-Carretero, S.; San-Cristobal, R.; Macready, A.L.; Fallaize, R.; Forster, H.; Woolhead, C.; O’Donovan, C.B.; Marsaux, C.F. Effect of an internet-based, personalized nutrition randomized trial on dietary changes associated with the mediterranean diet: The food4me study. Am. J. Clin. Nutr. 2016, 104, 288–297. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Waist Circumference and Waist-Hip Ratio: Report of a Who Expert Consultation; World Health Organisation: Geneva, Switzerlands, 2008. [Google Scholar]
- San-Cristobal, R.; Navas-Carretero, S.; Celis-Morales, C.; Brennan, L.; Walsh, M.; Lovegrove, J.A.; Daniel, H.; Saris, W.H.; Traczyk, I.; Manios, Y. Analysis of dietary pattern impact on weight status for personalised nutrition through on-line advice: The food4me spanish cohort. Nutrients 2015, 7, 9523–9537. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.T.; McCullough, M.L.; Newby, P.; Manson, J.E.; Meigs, J.B.; Rifai, N.; Willett, W.C.; Hu, F.B. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am. J. Clin. Nutr. 2005, 82, 163–173. [Google Scholar] [PubMed]
- Reedy, J.; Krebs-Smith, S.M.; Miller, P.E.; Liese, A.D.; Kahle, L.L.; Park, Y.; Subar, A.F. Higher diet quality is associated with decreased risk of all-cause, cardiovascular disease, and cancer mortality among older adults. J. Nutr. 2014, 144, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Kant, A.K.; Graubard, B.I. A comparison of three dietary pattern indexes for predicting biomarkers of diet and disease. J. Am. Coll. Nutr. 2005, 24, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Lauretani, F.; Semba, R.D.; Dayhoff-Brannigan, M.; Corsi, A.M.; Di Iorio, A.; Buiatti, E.; Bandinelli, S.; Guralnik, J.M.; Ferrucci, L. Low total plasma carotenoids are independent predictors of mortality among older persons. Eur. J. Nutr. 2008, 47, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Hann, C.; Rock, C.; King, I.; Drewnowski, A. Validation of the healthy eating index with use of plasma biomarkers in a clinical sample of women. Am. J. Clin. Nutr. 2001, 74, 479. [Google Scholar] [PubMed]
- Campbell, D.R.; Gross, M.D.; Martini, M.C.; Grandits, G.A.; Slavin, J.L.; Potter, J.D. Plasma carotenoids as biomarkers of vegetable and fruit intake. Cancer Epidemiol. Biomarkers Prev. 1994, 3, 493–500. [Google Scholar] [PubMed]
- Sakhi, A.K.; Bastani, N.E.; Ellingjord-Dale, M.; Gundersen, T.E.; Blomhoff, R.; Ursin, G. Feasibility of self-sampled dried blood spot and saliva samples sent by mail in a population-based study. BMC Cancer 2015, 15, 265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Delaimy, W.K.; Van Kappel, A.L.; Ferrari, P.; Slimani, N.; Steghens, J.-P.; Bingham, S.; Johansson, I.; Wallström, P.; Overvad, K.; Tjønneland, A. Plasma levels of six carotenoids in nine european countries: Report from the european prospective investigation into cancer and nutrition (epic). Public Health Nutr. 2004, 7, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Pitsavos, C.; Panagiotakos, D.B.; Chrysohoou, C.; Stefanadis, C. Epidemiology of cardiovascular risk factors in greece: Aims, design and baseline characteristics of the attica study. BMC Public Health 2003, 3, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, L.F.; Solvoll, K.; Drevon, C.A. Very-long-chain n-3 fatty acids as biomarkers for intake of fish and n-3 fatty acid concentrates. Am. J. Clin. Nutr. 1996, 64, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S. The omega-3 index as a risk factor for coronary heart disease. Am. J. Clin. Nutr. 2008, 87, 1997S–2002S. [Google Scholar] [PubMed]
- Von Schacky, C. Omega-3 index and cardiovascular health. Nutrients 2014, 6, 799–814. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S.; Polreis, J. Measurement of the omega-3 index in dried blood spots. Ann. Clin. Lab. Res. 2016, 4, 4. [Google Scholar] [CrossRef]
- Pate, R.R.; Heath, G.W.; Dowda, M.; Trost, S.G. Associations between physical activity and other health behaviors in a representative sample of us adolescents. Am. J. Public Health 1996, 86, 1577–1581. [Google Scholar] [CrossRef] [PubMed]
- Lakshmy, R.; Gupta, R.; Prabhakaran, D.; Snehi, U.; Reddy, K.S. Utility of Dried Blood Spots for Measurement of Cholesterol and Triglycerides in a Surveillance Study. J. Diabetes Sci. Technol. 2010, 4, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Miller, I.M.; Lacher, D.A.; Chen, T.-C.; Zipf, G.W.; Gindi, R.M.; Galinsky, A.M.; Nwankwo, T.; Terry, A.L. Collection and laboratory methods for dried blood spots for hemoglobin a1c and total and high-density lipoprotein cholesterol in population-based surveys. Clin. Chim. Acta 2015, 445, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Celis-Morales, C.; Livingstone, K.M.; Woolhead, C.; Forster, H.; O’Donovan, C.B.; Macready, A.L.; Fallaize, R.; Marsaux, C.F.; Tsirigoti, L.; Efstathopoulou, E. How reliable is internet-based self-reported identity, socio-demographic and obesity measures in european adults? Genes Nutr. 2015, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Spencer, E.A.; Roddam, A.W.; Key, T.J. Accuracy of self-reported waist and hip measurements in 4492 epic–oxford participants. Public Health Nutr. 2004, 7, 723–727. [Google Scholar] [CrossRef] [PubMed]
| All | Men | Women | p Value | |
|---|---|---|---|---|
| Age (years) c | 40 ± 13 | 42 ± 13 | 39 ± 13 | <0.001 |
| Weight (kg) c | 74.7 ± 15.8 | 83.4 ± 13.4 | 68.5 ± 14.4 | <0.001 |
| Height (m) c | 1.71 ± 0.09 | 1.79 ± 0.07 | 1.66 ± 0.07 | <0.001 |
| BMI (kg/m2) c | 25.4 ± 4.8 | 26.1 ± 4.1 | 25.0 ± 5.3 | <0.001 |
| WC (cm) d | 86 ± 14 | 93 ± 12 | 81 ± 13 | <0.001 |
| WHtR d | 0.50 ± 0.08 | 0.52 ± 0.07 | 0.49 ± 0.08 | <0.001 |
| PAL e | 1.73 ± 0.18 | 1.75 ± 0.19 | 1.72 ± 0.17 | 0.007 |
| SB (min/day) e | 745 ± 77 | 739 ± 82 | 749 ± 216 | 0.022 |
| Light PA (min/day) e | 74 ± 30 | 74 ± 30 | 74 ± 31 | 0.92 |
| Moderate PA (min/day) e | 33 ± 20 | 37 ± 21 | 30 ± 19 | <0.001 |
| Vigorous PA (min/day) d | 12 ± 16 | 17 ± 18 | 8 ± 13 | <0.001 |
| Cholesterol (mmol/L) f | 4.6 ± 1.0 | 4.7 ± 1.0 | 4.5 ± 1.0 | <0.001 |
| Carotenoids (ug/L) g | 1.52 ± 0.68 | 1.41 ± 0.64 | 1.60 ± 0.70 | <0.001 |
| Omega-3 index h | 5.70 ± 1.21 | 5.72 ± 1.26 | 5.68 ± 1.17 | 0.45 |
| HEI (0–100) | 49.2 ± 9.9 | 47.6 ± 9.9 | 50.3 ± 9.7 | <0.001 |
| AHEI (0–110) | 64.0 ± 10.0 | 62.0 ± 10.3 | 65.4 ± 9.6 | <0.001 |
| MDS (0–55) | 32.4 ± 5.0 | 32.3 ± 4.9 | 32.5 ± 5.1 | 0.45 |
| P-MDS (0–14) | 5.11 ± 1.68 | 5.06 ± 1.76 | 5.16 ± 1.63 | 0.25 |
| DHDI (0–100) | 66.6 ± 9.2 | 66.5 ± 9.5 | 66.6 ± 9.0 | 0.73 |
| HEI | AHEI | MDS | P-MDS | DHDI | |
|---|---|---|---|---|---|
| HEI | 1.00 | ||||
| AHEI | 0.62 | 1.00 | |||
| MDS | 0.39 | 0.46 | 1.00 | ||
| P-MDS | 0.52 | 0.49 | 0.31 | 1.00 | |
| DHDI | 0.63 | 0.60 | 0.48 | 0.56 | 1.00 |
| Quintiles | Age (Years) | BMI (kg/m2) | WHtR | WC (cm) | TC (mmol/L) | Carotenoids (µM) | Omega-3 Index |
|---|---|---|---|---|---|---|---|
| HEI | |||||||
| Q1 | 37 ± 12 | 26.4 ± 5.3 | 0.52 ± 0.08 | 89 ± 15 | 4.5 ± 1.0 | 1.29 ± 0.56 | 5.30 ± 0.98 |
| Q3 | 40 ± 13 | 25.5 ± 4.7 | 0.50 ± 0.08 | 86 ± 13 | 4.6 ± 1.0 | 1.55 ± 0.63 | 5.59 ± 1.02 |
| Q5 | 42 ± 13 | 24.8 ± 4.2 | 0.49 ± 0.07 | 83 ± 12 | 4.5 ± 0.9 | 1.76 ± 0.77 | 6.27 ± 1.55 |
| p for trend | <0.001 | 0.002 | <0.001 | <0.001 | 0.20 | <0.001 | <0.001 |
| AHEI | |||||||
| Q1 | 38 ± 13 | 26.97± 5.4 | 0.52 ± 0.08 | 89 ± 15 | 4.5 ± 1.0 | 1.28 ± 0.54 | 5.37 ± 1.05 |
| Q3 | 40 ± 12 | 25.7 ± 4.8 | 0.51 ± 0.08 | 86 ± 13 | 4.6 ± 0.9 | 1.49 ± 0.68 | 5.72 ± 1.22 |
| Q5 | 42 ± 14 | 24.0 ± 3.9 | 0.48 ± 0.07 | 82 ± 12 | 4.6 ± 1.0 | 1.81 ± 0.77 | 6.08 ± 1.37 |
| p for trend | <0.001 | <0.001 | <0.001 | <0.001 | 0.39 | <0.001 | <0.001 |
| MDS | |||||||
| Q1 | 36 ± 13 | 26.0 ± 5.5 | 0.51 ± 0.08 | 86 ± 15 | 4.4 ± 0.9 | 1.43 ± 0.65 | 5.51 ± 1.18 |
| Q3 | 40 ± 13 | 25.0 ± 4.3 | 0.50 ± 0.07 | 85 ± 14 | 4.5 ± 0.9 | 1.56 ± 0.73 | 5.75 ± 1.28 |
| Q5 | 43 ± 13 | 25.2 ± 4.4 | 0.50 ± 0.07 | 86 ± 13 | 4.6 ± 0.9 | 1.57 ± 0.67 | 5.84 ± 1.23 |
| p for trend | <0.001 | <0.001 | 0.002 | 0.013 | 0.70 | 0.017 | 0.001 |
| P-MDS | |||||||
| Q1 | 39 ± 13 | 25.8 ± 5.0 | 0.51 ± 0.08 | 87 ± 14 | 4.6 ± 1.0 | 1.42 ± 0.60 | 5.44 ± 1.04 |
| Q3 | 40 ± 13 | 25.1 ± 4.8 | 0.49 ± 0.08 | 84 ± 14 | 4.5 ± 1.0 | 1.61 ± 0.76 | 5.79 ± 1.25 |
| Q5 | 42 ± 13 | 24.9 ± 4.2 | 0.49 ± 0.07 | 84 ± 14 | 4.6 ± 0.9 | 1.77 ± 0.87 | 6.44 ± 1.59 |
| p for trend | <0.001 | 0.002 | <0.001 | <0.001 | 0.16 | <0.001 | <0.001 |
| DHDI | |||||||
| Q1 | 38 ± 12 | 26.1 ± 5.1 | 0.51 ± 0.08 | 88 ± 14 | 4.5 ± 0.9 | 1.32 ± 0.55 | 5.27 ± 1.05 |
| Q3 | 41 ± 13 | 25.6 ± 4.3 | 0.50 ± 0.07 | 86 ± 13 | 4.6 ± 1.0 | 1.54 ± 0.67 | 5.66 ± 1.19 |
| Q5 | 42 ± 14 | 23.7 ± 4.5 | 0.47 ± 0.06 | 81 ± 11 | 4.4 ± 0.9 | 1.83 ± 0.79 | 6.23 ± 1.31 |
| p for trend | <0.001 | <0.001 | <0.001 | <0.001 | 0.32 | <0.001 | <0.001 |
| Quintiles | PAL | SB (min/day) | Light PA (min/day) | Moderate PA (min/day) | Vigorous PA (min/day) |
|---|---|---|---|---|---|
| HEI | |||||
| Q1 | 1.71 ± 0.17 | 746 ± 78 | 75 ± 32 | 32 ± 21 | 10 ± 15 |
| Q3 | 1.73 ± 0.18 | 750 ± 70 | 74 ± 29 | 33 ± 21 | 10 ± 14 |
| Q5 | 1.76 ± 0.18 | 727 ± 76 | 76 ± 33 | 34 ± 19 | 14 ± 19 |
| p for trend | <0.001 | 0.001 | 0.74 | <0.001 | <0.001 |
| AHEI | |||||
| Q1 | 1.71 ± 0.14 | 750 ± 76 | 74 ± 32 | 33 ±19 | 9 ± 12 |
| Q3 | 1.72 ± 0.18 | 748 ± 76 | 73 ± 29 | 32 ± 23 | 11 ± 14 |
| Q5 | 1.78 ± 0.21 | 735 ± 76 | 74 ± 30 | 36 ± 20 | 17 ± 20 |
| p for trend | <0.001 | 0.003 | 0.26 | 0.002 | <0.001 |
| MDS | |||||
| Q1 | 1.72 ± 0.17 | 745 ± 83 | 74 ± 32 | 32 ± 20 | 10 ± 14 |
| Q3 | 1.74 ± 0.18 | 743 ± 73 | 74 ± 32 | 34 ± 21 | 13 ± 17 |
| Q5 | 1.74 ± 0.18 | 744 ± 74 | 76 ± 31 | 34 ± 20 | 12 ± 15 |
| p for trend | 0.10 | 0.022 | 0.53 | 0.019 | 0.011 |
| P-MDS | |||||
| Q1 | 1.72 ± 0.16 | 745 ± 77 | 74 ± 30 | 34 ± 21 | 10 ± 12 |
| Q3 | 1.73 ± 0.17 | 747 ± 77 | 74 ± 32 | 32 ± 19 | 11 ± 16 |
| Q5 | 1.78 ± 0.87 | 730 ± 77 | 74 ± 30 | 36 ± 20 | 19 ± 20 |
| p for trend | <0.001 | 0.004 | 0.71 | 0.10 | <0.001 |
| DHDI | |||||
| Q1 | 1.67 ± 0.13 | 757 ± 74 | 70 ± 29 | 27 ± 16 | 7 ± 9 |
| Q3 | 1.73 ± 0.18 | 747 ± 78 | 74 ± 30 | 31 ± 20 | 10 ± 15 |
| Q5 | 1.85 ± 0.22 | 720 ± 70 | 80 ± 30 | 44 ± 22 | 22 ± 23 |
| p for trend | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fallaize, R.; Livingstone, K.M.; Celis-Morales, C.; Macready, A.L.; San-Cristobal, R.; Navas-Carretero, S.; Marsaux, C.F.M.; O’Donovan, C.B.; Kolossa, S.; Moschonis, G.; et al. Association between Diet-Quality Scores, Adiposity, Total Cholesterol and Markers of Nutritional Status in European Adults: Findings from the Food4Me Study. Nutrients 2018, 10, 49. https://doi.org/10.3390/nu10010049
Fallaize R, Livingstone KM, Celis-Morales C, Macready AL, San-Cristobal R, Navas-Carretero S, Marsaux CFM, O’Donovan CB, Kolossa S, Moschonis G, et al. Association between Diet-Quality Scores, Adiposity, Total Cholesterol and Markers of Nutritional Status in European Adults: Findings from the Food4Me Study. Nutrients. 2018; 10(1):49. https://doi.org/10.3390/nu10010049
Chicago/Turabian StyleFallaize, Rosalind, Katherine M. Livingstone, Carlos Celis-Morales, Anna L. Macready, Rodrigo San-Cristobal, Santiago Navas-Carretero, Cyril F. M. Marsaux, Clare B. O’Donovan, Silvia Kolossa, George Moschonis, and et al. 2018. "Association between Diet-Quality Scores, Adiposity, Total Cholesterol and Markers of Nutritional Status in European Adults: Findings from the Food4Me Study" Nutrients 10, no. 1: 49. https://doi.org/10.3390/nu10010049







