Gelidium elegans Extract Ameliorates Type 2 Diabetes via Regulation of MAPK and PI3K/Akt Signaling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Approval of Animal Experiments
2.3. Animal Husbandry and Experimental Design
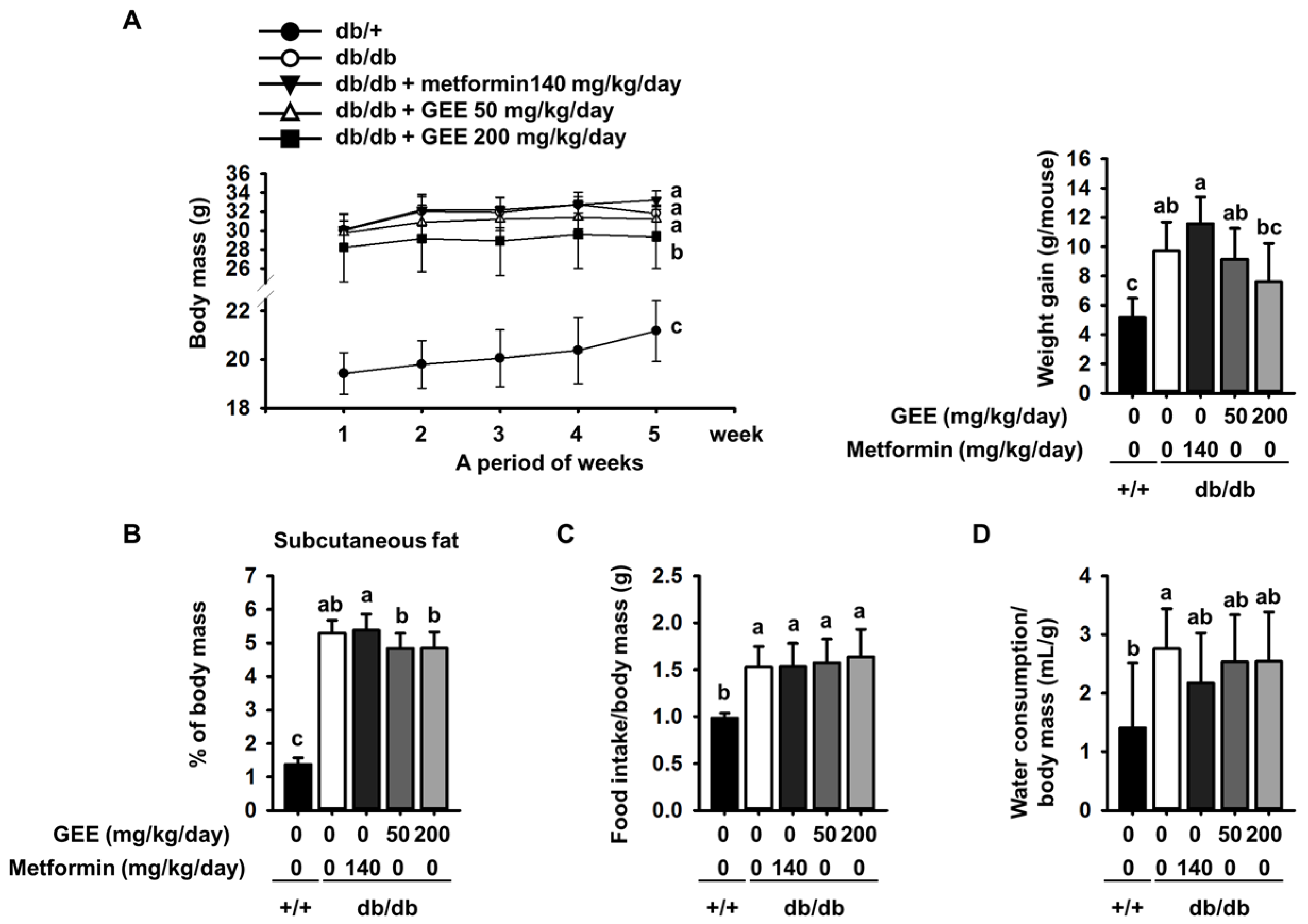
2.4. Body Mass, Food Intake, and Water Consumption
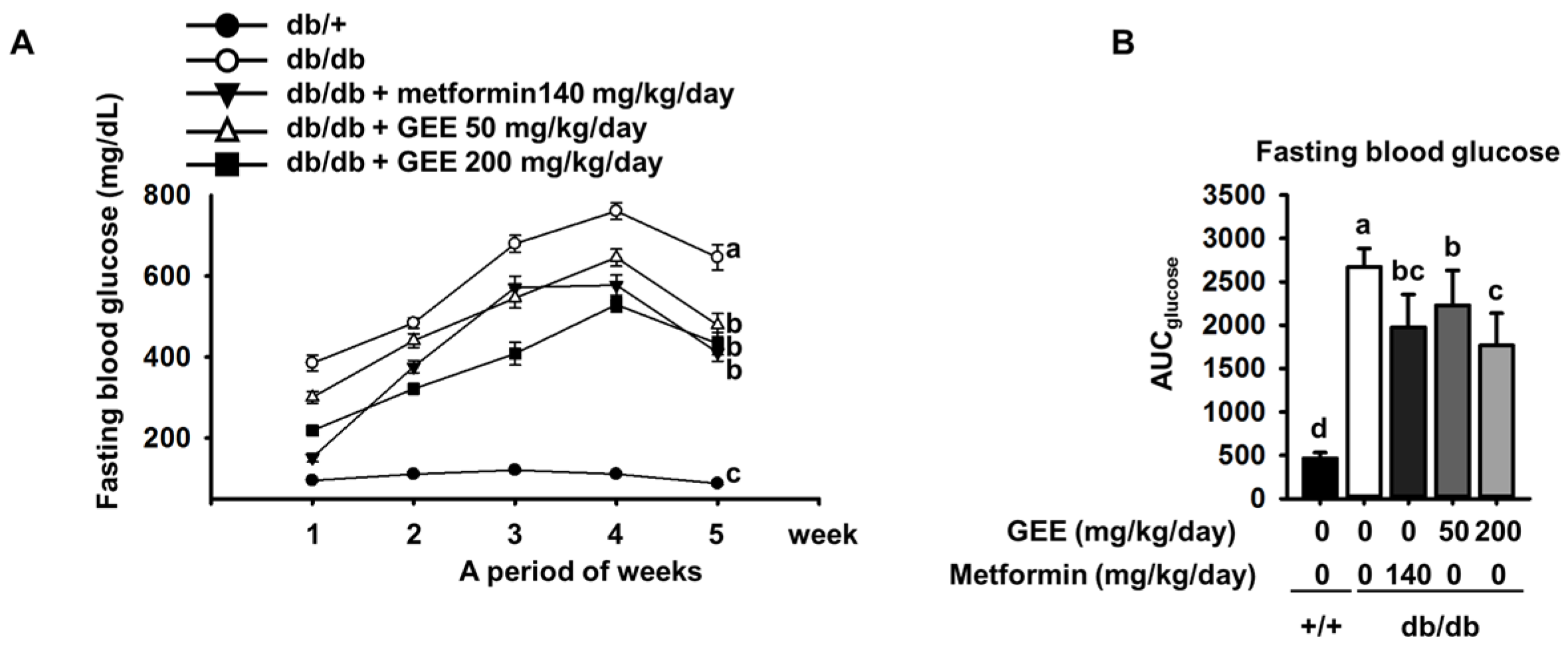
2.5. Fasting Blood Glucose Measurement
2.6. Blood Biochemistry
2.7. Organ Masses
2.8. Western Blot Analysis
2.9. Statistical Analysis
3. Results
3.1. Effect of GEE on Body Mass and Fasting Blood Glucose in db/db Mice
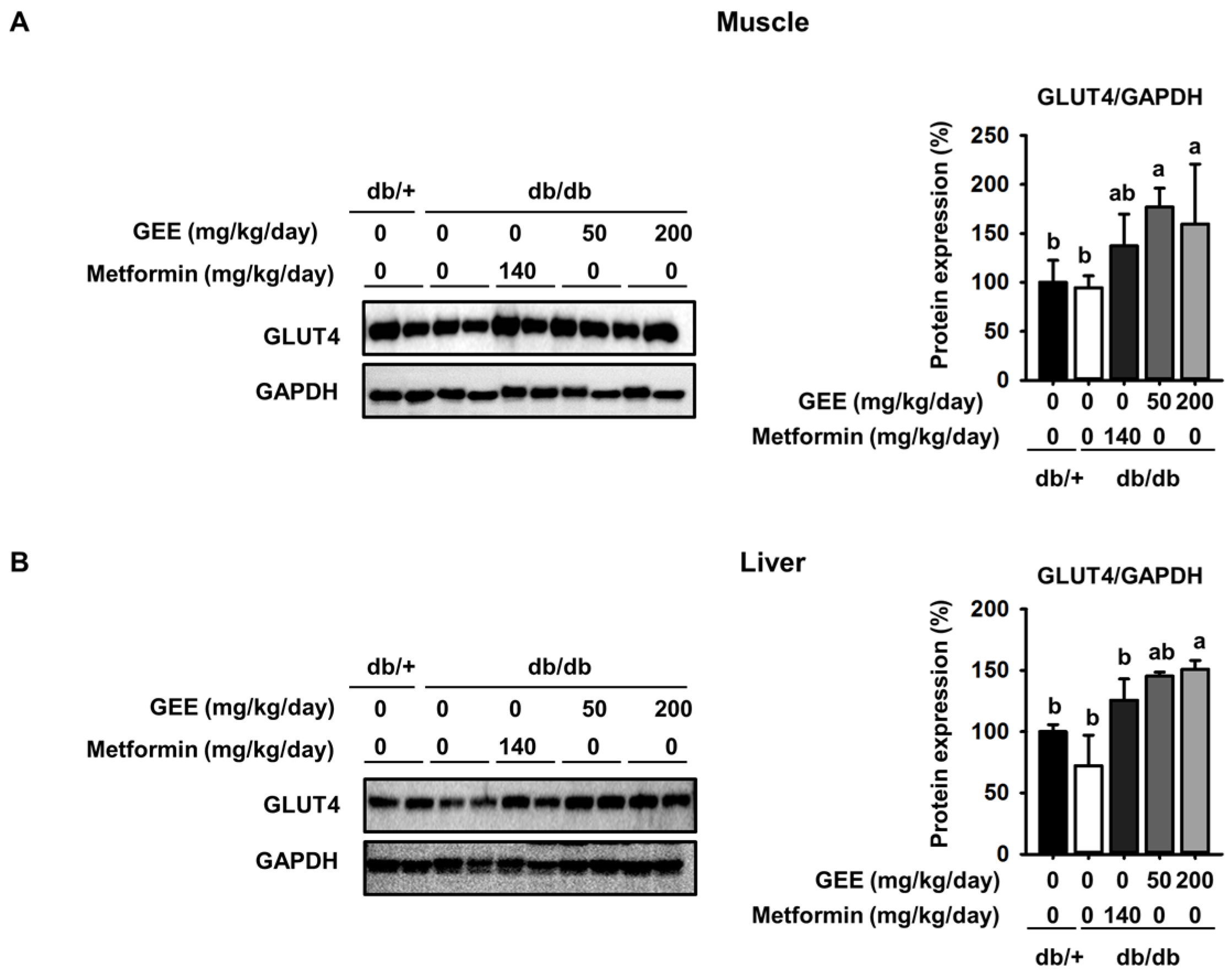
3.2. Effect of GEE on GLUT4 Protein Expression in the Skeletal Muscle and Liver of db/db Mice
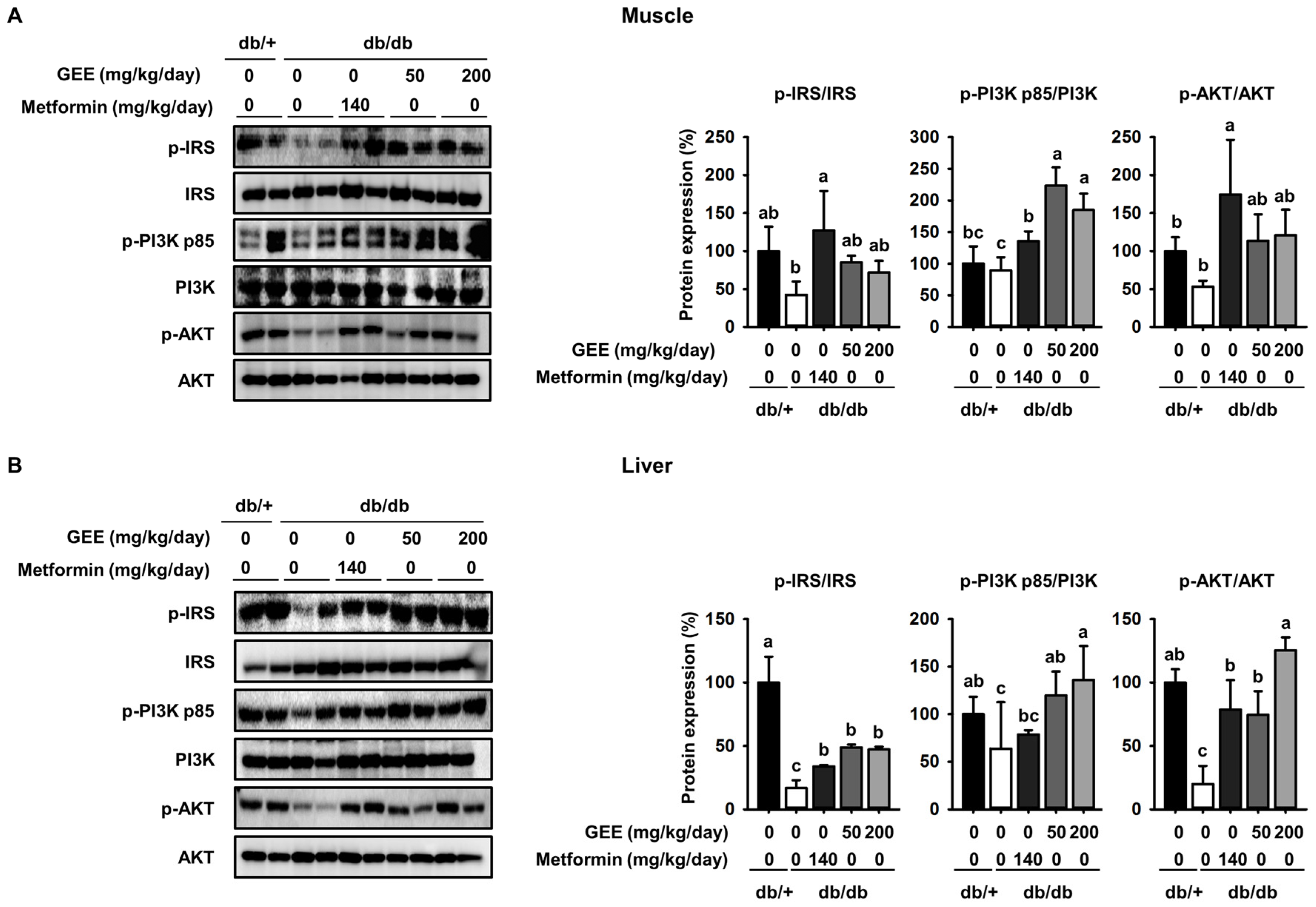
3.3. GEE Increases Phosphorylation of Intermediates in the PI3K/Akt Pathway in db/db Mice
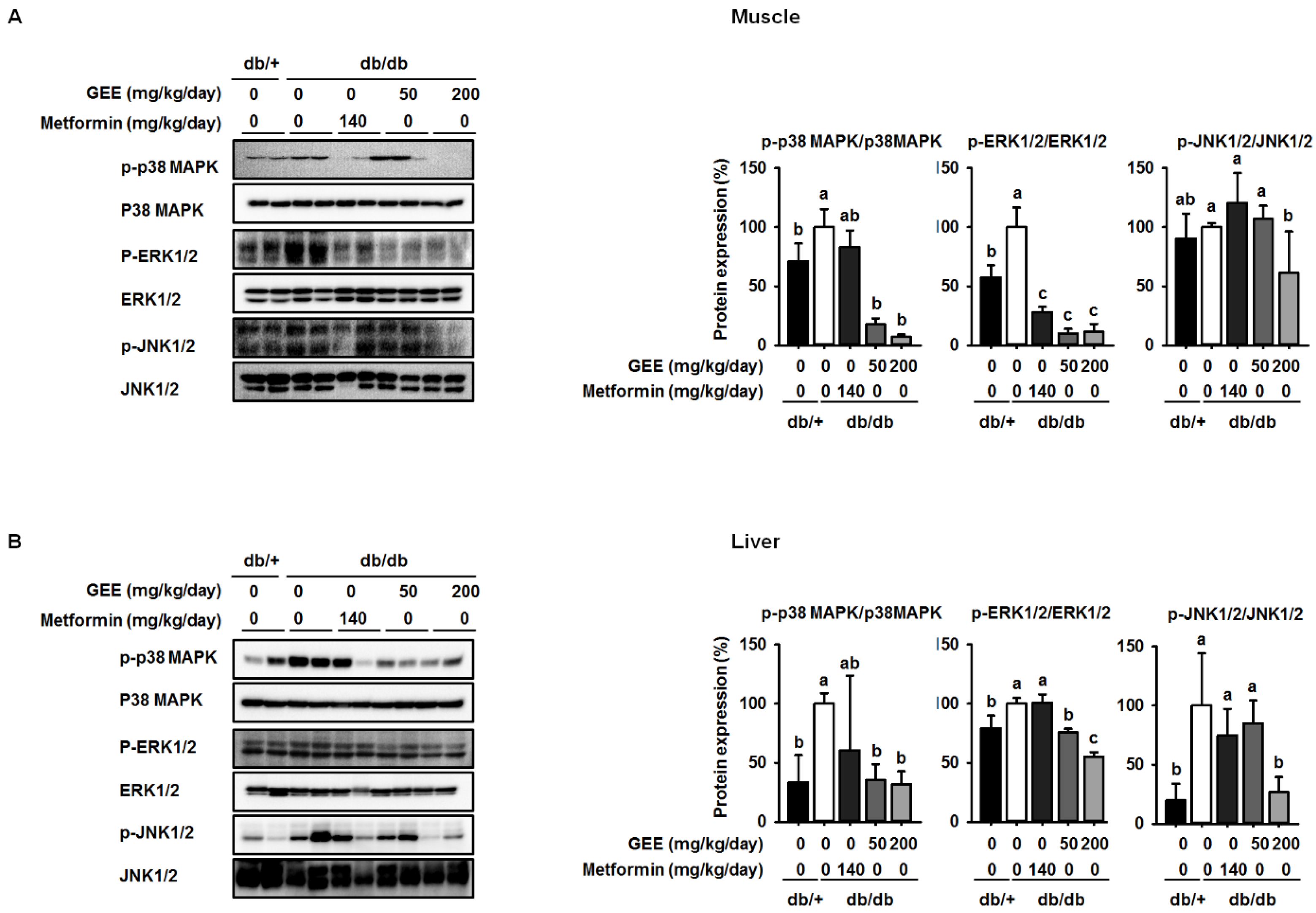
3.4. GEE Suppresses MAPK Pathway Activation in db/db Mice
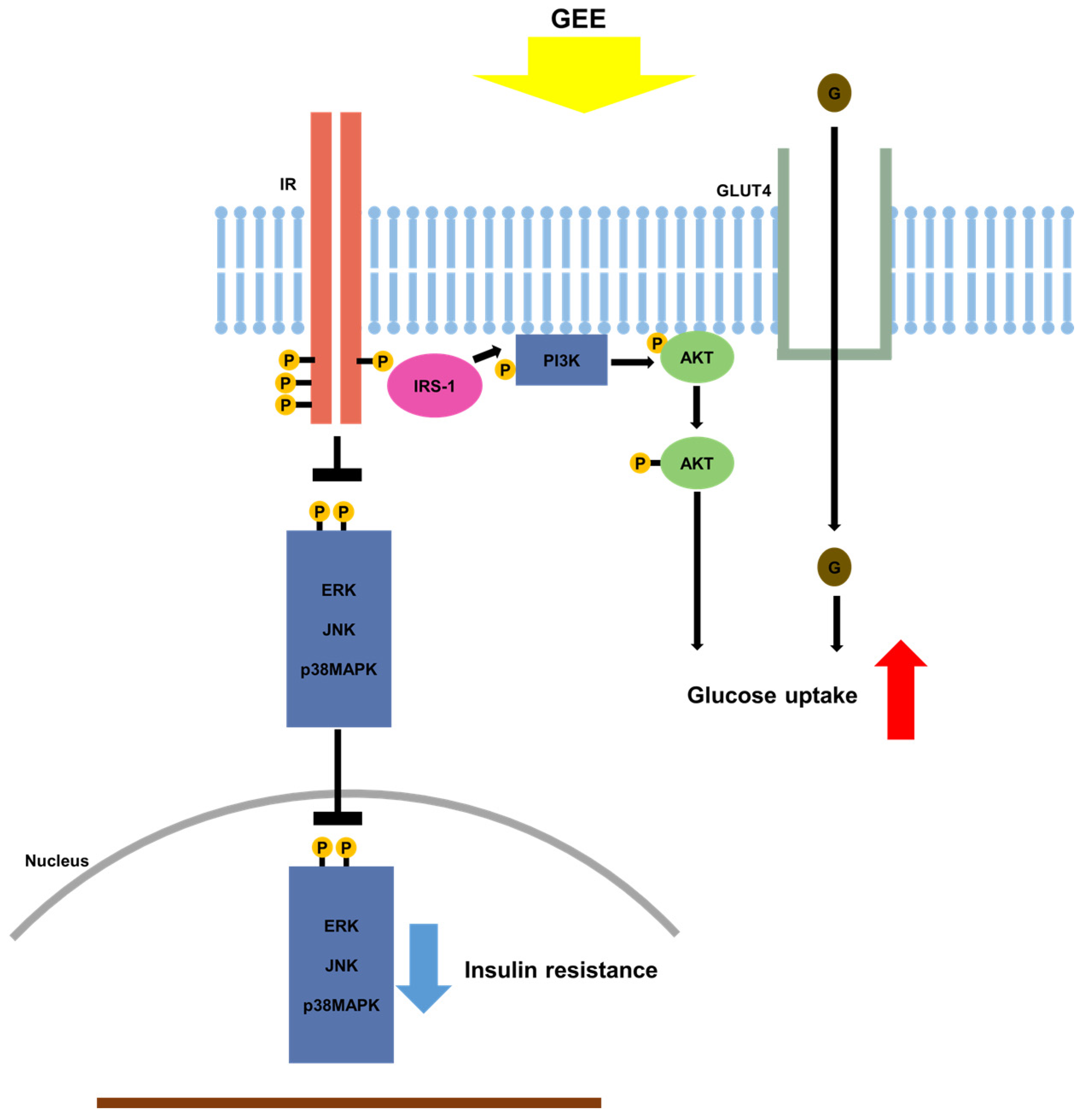
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Musselman, D.L.; Betan, E.; Larsen, H.; Phillips, L.S. Relationship of depression to diabetes types 1 and 2: Epidemiology, biology, and treatment. Biol. Psychiatry 2003, 54, 317–329. [Google Scholar] [CrossRef]
- Burge, M.R.; Schade, D.S. Diabetes and the Affordable Care Act; Mary Ann Liebert, Inc.: New Rochelle, NY, USA, 2014. [Google Scholar]
- Cnop, M.; Welsh, N.; Jonas, J.-C.; Jörns, A.; Lenzen, S.; Eizirik, D.L. Mechanisms of pancreatic β-cell death in type 1 and type 2 diabetes. Diabetes 2005, 54, S97–S107. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Ferrannini, E. Insulin resistance: A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care 1991, 14, 173–194. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.J.; Cobb, M.H. Mitogen-activated protein kinase pathways. Curr. Opin. Cell Biol. 1997, 9, 180–186. [Google Scholar] [CrossRef]
- Li, S.; Brown, M.S.; Goldstein, J.L. Bifurcation of insulin signaling pathway in rat liver: MTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 3441–3446. [Google Scholar] [CrossRef] [PubMed]
- Harrington, L.S.; Findlay, G.M.; Gray, A.; Tolkacheva, T.; Wigfield, S.; Rebholz, H.; Barnett, J.; Leslie, N.R.; Cheng, S.; Shepherd, P.R. The TSC1-2 tumor suppressor controls insulin–PI3K signaling via regulation of IRS proteins. J. Cell Biol. 2004, 166, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Khorami, S.A.H.; Movahedi, A.; Khaza’ai, H.; Mutalib, A.; Sokhini, M. PI3K/AKT pathway in modulating glucose homeostasis and its alteration in diabetes. AMBS 2015, 1, 46–55. [Google Scholar]
- Sano, H.; Eguez, L.; Teruel, M.N.; Fukuda, M.; Chuang, T.D.; Chavez, J.A.; Lienhard, G.E.; McGraw, T.E. Rab10, a target of the AS160 Rab GAP, is required for insulin-stimulated translocation of GLUT4 to the adipocyte plasma membrane. Cell Metab. 2007, 5, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Kraegen, E.W.; Clark, P.W.; Jenkins, A.B.; Daley, E.A.; Chisholm, D.J.; Storlien, L.H. Development of muscle insulin resistance after liver insulin resistance in high-fat–fed rats. Diabetes 1991, 40, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- Guilherme, A.; Virbasius, J.V.; Puri, V.; Czech, M.P. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat. Rev. Mol. Cell Biol. 2008, 9, 367–377. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Tripathy, D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 2009, 32 (Suppl. 2), S157–S163. [Google Scholar] [CrossRef] [PubMed]
- Aksamitiene, E.; Kiyatkin, A.; Kholodenko, B.N. Cross-Talk between Mitogenic Ras/MAPK and Survival PI3K/Akt Pathways: A Fine Balance; Portland Press Limited: London, UK, 2012. [Google Scholar]
- Miyoshi, H.; Kato, K.; Iwama, H.; Maeda, E.; Sakamoto, T.; Fujita, K.; Toyota, Y.; Tani, J.; Nomura, T.; Mimura, S. Effect of the anti-diabetic drug metformin in hepatocellular carcinoma in vitro and in vivo. Int. J. Oncol. 2014, 45, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Hampp, C.; Borders-Hemphill, V.; Moeny, D.G.; Wysowski, D.K. Use of antidiabetic drugs in the US, 2003–2012. Diabetes Care 2014, 37, 1367–1374. [Google Scholar] [CrossRef] [PubMed]
- Doran, E.; Halestrap, A.P. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem. J. 2000, 348, 607–614. [Google Scholar]
- Kramer, D.; Shapiro, R.; Adler, A.; Bush, E.; Rondinone, C. Insulin-sensitizing effect of rosiglitazone (BRL-49653) by regulation of glucose transporters in muscle and fat of Zucker rats. Metabolism 2001, 50, 1294–1300. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, Y.; Taketomi, S.; Shimura, Y.; Ikeda, H.; Fujita, T. Effects of pioglitazone on glucose and lipid metabolism in Wistar fatty rats. Arzneimittelforschung 1990, 40, 263–267. [Google Scholar] [PubMed]
- Singh, S.; Loke, Y.K.; Furberg, C.D. Long-term risk of cardiovascular events with rosiglitazone: A meta-analysis. JAMA 2007, 298, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Schuster, D.P.; Duvuuri, V. Diabetes mellitus. Clin. Podiatr. Med. Surg. 2002, 19, 79–107. [Google Scholar] [CrossRef]
- Tunnicliffe, J.M.; Shearer, J. Coffee, glucose homeostasis, and insulin resistance: Physiological mechanisms and mediators. Appl. Physiol. Nutr. Metab. 2008, 33, 1290–1300. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-J.; Yoon, K.-Y.; Lee, B.-Y. Fucoidan regulate blood glucose homeostasis in C57BL/KSJ m+/+ db and C57BL/KSJ db/db mice. Fitoterapia 2012, 83, 1105–1109. [Google Scholar] [CrossRef] [PubMed]
- Hannan, J.; Ali, L.; Rokeya, B.; Khaleque, J.; Akhter, M.; Flatt, P.; Abdel-Wahab, Y. Soluble dietary fibre fraction of Trigonella foenum-graecum (fenugreek) seed improves glucose homeostasis in animal models of type 1 and type 2 diabetes by delaying carbohydrate digestion and absorption, and enhancing insulin action. Br. J. Nutr. 2007, 97, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-I.; Jin, Y.-J.; Ko, H.-C.; Choi, S.-Y.; Hwang, J.-H.; Whang, I.; Kim, M.-H.; Shin, H.-S.; Jeong, H.-B.; Kim, S.-J. Petalonia improves glucose homeostasis in streptozotocin-induced diabetic mice. Biochem. Biophys. Res. Commun. 2008, 373, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Pari, L.; Satheesh, M.A. Effect of pterostilbene on hepatic key enzymes of glucose metabolism in streptozotocin-and nicotinamide-induced diabetic rats. Life Sci. 2006, 79, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Soufi, F.; Sheervalilou, R.; Vardiani, M.; Khalili, M.; Alipour, M. Chronic resveratrol administration has beneficial effects in experimental model of type 2 diabetic rats. Endocr. Regul. 2012, 46, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, S.P.; Leibel, R.; Tortoriello, D.V. Dietary curcumin significantly improves obesity-associated inflammation and diabetes in mouse models of diabesity. Endocrinology 2008, 149, 3549–3558. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Kim, J.Y.; Choi, W.H.; Lee, S.S. Effects of seaweed supplementation on blood glucose concentration, lipid profile, and antioxidant enzyme activities in patients with type 2 diabetes mellitus. Nutr. Res. Pract. 2008, 2, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.-C.; Wijesinghe, W.; Lee, S.-H.; Kang, S.-M.; Ko, S.-C.; Yang, X.; Kang, N.; Jeon, B.-T.; Kim, J.; Lee, D.-H. Dieckol isolated from brown seaweed Ecklonia cava attenuates type ІІ diabetes in db/db mouse model. Food Chem. Toxicol. 2013, 53, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Miyashita, K. Dietary combination of fucoxanthin and fish oil attenuates the weight gain of white adipose tissue and decreases blood glucose in obese/diabetic KK-Ay mice. J. Agric. Food Chem. 2007, 55, 7701–7706. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.C.; Kang, N.; Kim, S.Y.; Lima, I.S.; Ko, S.C.; Kim, Y.T.; Kim, Y.B.; Jeung, H.D.; Choi, K.S.; Jeon, Y.J. Popular edible seaweed, Gelidium amansii prevents against diet-induced obesity. Food Chem. Toxicol. 2016, 90, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kim, K.J.; Koh, E.J.; Lee, B.Y. Gelidium elegans Regulates the AMPK-PRDM16-UCP-1 Pathway and Has a Synergistic Effect with Orlistat on Obesity-Associated Features in Mice Fed a High-Fat Diet. Nutrients 2017, 9, 342. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kim, K.-J.; Koh, E.-J.; Lee, B.-Y. Altered Gelidium elegans extract-stimulated beige-like phenotype attenuates adipogenesis in 3T3-L1 cells. J. Food Nutr. Res. 2016, 4, 448–453. [Google Scholar]
- Jeon, H.J.; Seo, M.J.; Choi, H.S.; Lee, O.H.; Lee, B.Y. Gelidium elegans, an Edible Red Seaweed, and Hesperidin Inhibit Lipid Accumulation and Production of Reactive Oxygen Species and Reactive Nitrogen Species in 3T3-L1 and RAW264.7 Cells. Phytother. Res. 2014, 28, 1701–1709. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kim, K.-J.; Koh, E.-J.; Seo, Y.-J.; Lee, B.-Y. Gelidium elegans Regulates Blood Glucose Homeostasis in ICR Mice. J. Food Nutr. Res. 2017, 5, 274–280. [Google Scholar]
- Kim, M.; Lim, J.; Youn, H.; Hong, Y.; Yang, K.; Park, H.; Chung, S.; Koh, S.; Shin, S.; Choi, B. Resveratrol prevents renal lipotoxicity and inhibits mesangial cell glucotoxicity in a manner dependent on the AMPK–SIRT1–PGC1α axis in db/db mice. Diabetologia 2013, 56, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Burke, S.J.; Batdorf, H.M.; Burk, D.H.; Noland, R.C.; Eder, A.E.; Boulos, M.S.; Karlstad, M.D.; Jason Collier, J. db/db Mice Exhibit Features of Human Type 2 Diabetes That Are Not Present in Weight-Matched C57BL/6J Mice Fed a Western Diet. J. Diabetes Res. 2017, 2017, 116–132. [Google Scholar] [CrossRef] [PubMed]
- Nishina, P.M.; Lowe, S.; Wang, J.; Paigen, B. Characterization of plasma lipids in genetically obese mice: The mutants obese, diabetes, fat, tubby, and lethal yellow. Metabolism 1994, 43, 549–553. [Google Scholar] [CrossRef]
- Harlow, E.; Lane, D. Antibodies: A Laboratory Manual; Cold Spring Harbor Press: Cold Spring Harbor, NY, USA, 1988; pp. 139–245. [Google Scholar]
- Worthington, D.R.; Brown, S.J. Diabetes Management System and Method for Controlling Blood Glucose. Patent USRE43316 E1, 17 April 2012. [Google Scholar]
- Ma, D.L.; Chen, F.Q.; Xu, W.J.; Yue, W.Z.; Yuan, G.; Yang, Y. Early intervention with glucagon-like peptide 1 analog liraglutide prevents tau hyperphosphorylation in diabetic db/db mice. J. Neurochem. 2015, 135, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Pruessner, J.C.; Kirschbaum, C.; Meinlschmid, G.; Hellhammer, D.H. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology 2003, 28, 916–931. [Google Scholar] [CrossRef]
- Nauck, M.A.; Heimesaat, M.M.; Orskov, C.; Holst, J.J.; Ebert, R.; Creutzfeldt, W. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J. Clin. Investig. 1993, 91, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Rohlfing, C.L.; Little, R.R.; Wiedmeyer, H.-M.; England, J.D.; Madsen, R.; Harris, M.I.; Flegal, K.M.; Eberhardt, M.S.; Goldstein, D.E. Use of GHb (HbA1c) in screening for undiagnosed diabetes in the US population. Diabetes Care 2000, 23, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Wallberg-Henriksson, H.; Zierath, J.R. GLUT4: A key player regulating glucose homeostasis? Insights from transgenic and knockout mice. Mol. Membr. Biol. 2001, 18, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-L.; Gibbs, E.M.; McCoid, S.C.; Milici, A.; Stukenbrok, H.A.; McPherson, R.K.; Treadway, J.L.; Pessin, J.E. Transgenic mice expressing the human GLUT4/muscle-fat facilitative glucose transporter protein exhibit efficient glycemic control. Proc. Natl. Acad. Sci. USA 1993, 90, 11346–11350. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, E.; Jansson, P.-A.; Nagaev, I.; Wenthzel, A.-M.; Smith, U. Insulin resistance with low cellular IRS-1 expression is also associated with low GLUT4 expression and impaired insulin-stimulated glucose transport. FASEB J. 2001, 15, 1101–1103. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, F.; Lavigne, C.; Jacques, H.; Marette, A. Defective insulin-induced GLUT4 translocation in skeletal muscle of high fat–fed rats is associated with alterations in both Akt/protein kinase B and atypical protein kinase C (ζ/λ) activities. Diabetes 2001, 50, 1901–1910. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.-J.; Yoon, K.-Y.; Koh, E.-J.; Choi, J.; Kim, K.-J.; Choi, H.-S.; Lee, B.-Y. Seapolynol and Dieckol Improve Insulin Sensitivity through the Regulation of the PI3K Pathway in C57BL/KsJ-db/db Mice. J. Food Nutr. Res. 2015, 3, 648–652. [Google Scholar] [CrossRef]
- Gogg, S.; Smith, U.; Jansson, P.-A. Increased MAPK activation and impaired insulin signaling in subcutaneous microvascular endothelial cells in type 2 diabetes: The role of endothelin-1. Diabetes 2009, 58, 2238–2245. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Xu, G.; Ma, S.; Li, F.; Yuan, M.; Xu, H.; Huang, K. Catalpol ameliorates high-fat diet-induced insulin resistance and adipose tissue inflammation by suppressing the JNK and NF-κB pathways. Biochem. Biophys. Res. Commun. 2015, 467, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Weyer, C.; Bogardus, C.; Mott, D.M.; Pratley, R.E. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J. Clin. Investig. 1999, 104, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Cline, G.W.; Petersen, K.F.; Krssak, M.; Shen, J.; Hundal, R.S.; Trajanoski, Z.; Inzucchi, S.; Dresner, A.; Rothman, D.L.; Shulman, G.I. Impaired glucose transport as a cause of decreased insulin-stimulated muscle glycogen synthesis in type 2 diabetes. New Engl. J. Med. 1999, 341, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Abel, E.D.; Peroni, O.; Kim, J.K.; Young-Bum, K. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature 2001, 409, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, E.; Kotani, K.; Peroni, O.D.; Kahn, B.B. Adipose-specific overexpression of GLUT4 reverses insulin resistance and diabetes in mice lacking GLUT4 selectively in muscle. Am. J. Physiol. Endocrinol. Metab. 2005, 289, E551–E561. [Google Scholar] [CrossRef] [PubMed]
- Kerouz, N.J.; Hörsch, D.; Pons, S.; Kahn, C.R. Differential regulation of insulin receptor substrates-1 and-2 (IRS-1 and IRS-2) and phosphatidylinositol 3-kinase isoforms in liver and muscle of the obese diabetic (ob/ob) mouse. J. Clin. Investig. 1997, 100, 3164–3172. [Google Scholar] [CrossRef] [PubMed]
- Khamzina, L.; Veilleux, A.; Bergeron, S.; Marette, A. Increased activation of the mammalian target of rapamycin pathway in liver and skeletal muscle of obese rats: Possible involvement in obesity-linked insulin resistance. Endocrinology 2005, 146, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, G.; Geng, F.; Zhang, Z.; Li, J.; Yang, M.; Dong, L.; Gao, F. Berberine reduces ischemia/reperfusion-induced myocardial apoptosis via activating AMPK and PI3K–Akt signaling in diabetic rats. Apoptosis 2014, 19, 946–957. [Google Scholar] [CrossRef] [PubMed]
- Schultze, S.M.; Hemmings, B.A.; Niessen, M.; Tschopp, O. PI3K/AKT, MAPK and AMPK signalling: Protein kinases in glucose homeostasis. Expert Rev. Mol. Med. 2012, 14, e1. [Google Scholar] [CrossRef] [PubMed]
- Ponugoti, B.; Dong, G.; Graves, D.T. Role of forkhead transcription factors in diabetes-induced oxidative stress. Exp. Diabetes Res. 2012, 2012, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Rondinone, C.M.; Wang, L.-M.; Lonnroth, P.; Wesslau, C.; Pierce, J.H.; Smith, U. Insulin receptor substrate (IRS) 1 is reduced and IRS-2 is the main docking protein for phosphatidylinositol 3-kinase in adipocytes from subjects with non-insulin-dependent diabetes mellitus. Proc. Natl. Acad. Sci. USA 1997, 94, 4171–4175. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, V.; Uchida, T.; Yenush, L.; Davis, R.; White, M.F. The c-Jun NH2-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser307. J. Biol. Chem. 2000, 275, 9047–9054. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; Thaete, F.L.; Simoneau, J.-A.; Kelley, D.E. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes 1997, 46, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Graziano, M.P.; Doebber, T.W.; Leibowitz, M.D.; White-Carrington, S.; Szalkowski, D.M.; Hey, P.J.; Wu, M.; Cullinan, C.A.; Bailey, P. Down-regulation of the expression of the obese gene by an antidiabetic thiazolidinedione in Zucker diabetic fatty rats and db/db mice. J. Biol. Chem. 1996, 271, 9455–9459. [Google Scholar] [CrossRef] [PubMed]
- Gross, J.L.; Kramer, C.K.; Leitão, C.B.; Hawkins, N.; Viana, L.V.; Schaan, B.D.; Pinto, L.C.; Rodrigues, T.C.; Azevedo, M.J. Effect of antihyperglycemic agents added to metformin and a sulfonylurea on glycemic control and weight gain in type 2 diabetes: A network meta-analysis. Ann. Intern. Med. 2011, 154, 672–679. [Google Scholar] [CrossRef] [PubMed]
| Nutrient | Ingredient | Content |
|---|---|---|
| Proximate composition (%) | Moisture | 5.1 |
| Crude ash | 24.1 | |
| Crude protein | 16.7 | |
| Carbohydrate | 47.6 |
| Group | Organ Mass (g) | |||||
|---|---|---|---|---|---|---|
| db/+ | db/db | |||||
| Parameters | GEE 0 * | GEE 0 * | GEE 0 * | GEE 50 * | GEE 200 * | |
| Metformin 0 * | Metformin 0 * | Metformin 140 * | Metformin 0 * | Metformin 0 * | ||
| Subcutaneous fat | 0.30 ± 0.04 d | 1.63 ± 0.13 b | 1.83 ± 0.21 a | 1.53 ± 0.16 bc | 1.45 ± 0.13 c | |
| Liver | 1.08 ± 0.07 d | 2.13 ± 0.18 a | 1.89 ± 0.15 bc | 2.05 ± 0.15 ab | 1.84 ± 0.19 c | |
| Heart | 0.16 ± 0.03 | 0.15 ± 0.03 | 0.16 ± 0.01 | 0.15 ± 0.02 | 0.15 ± 0.02 | |
| Lung | 0.18 ± 0.03 | 0.19 ± 0.04 | 0.19 ± 0.03 | 0.19 ± 0.03 | 0.19 ± 0.03 | |
| Group | Blood Biochemistry | |||||
|---|---|---|---|---|---|---|
| db/+ | db/db | |||||
| Parameters | GEE 0 * | GEE 0 * | GEE 0 * | GEE 50 * | GEE 200 * | |
| Metformin 0 * | Metformin 0 * | Metformin 140 * | Metformin 0 * | Metformin 0 * | ||
| Insulin | 0.17 ± 0.08 b | 1.06 ± 0.54 a | 0.52 ± 0.20 ab | 0.91 ± 0.20 a | 0.58 ± 0.30 ab | |
| C-peptide | 35.7 ± 25.8 b | 336.0 ± 168.0 a | 178.2 ± 97.3 ab | 159.3 ± 167.4 b | 145.2 ± 57.0 b | |
| HbA1c (ng/mL) | 14.6 ± 2.5 b | 28.0 ± 4.1 a | 19.7 ± 1.9 bc | 21.7 ± 2.0 b | 16.1 ± 4.2 cd | |
| LDL (mg/dL) | 14.7 ± 0.3 | 14.4 ± 1.0 | 14.5 ± 0.9 | 14.4 ± 1.4 | 14.1 ± 0.9 | |
| Triglyceride (mg/dL) | 75.3 ± 8.0 b | 341.5 ± 97.2 a | 282.2 ± 108.5 a | 237.5 ± 136.9 a | 315.8 ± 174.0 a | |
| T. cholesterol (mg/dL) | 95.1 ± 9.1 b | 177.8 ± 24.0 a | 156.7 ± 21.9 a | 157.8 ± 33.5 a | 152.7 ± 15.6 a | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.; Kim, K.-J.; Koh, E.-J.; Lee, B.-Y. Gelidium elegans Extract Ameliorates Type 2 Diabetes via Regulation of MAPK and PI3K/Akt Signaling. Nutrients 2018, 10, 51. https://doi.org/10.3390/nu10010051
Choi J, Kim K-J, Koh E-J, Lee B-Y. Gelidium elegans Extract Ameliorates Type 2 Diabetes via Regulation of MAPK and PI3K/Akt Signaling. Nutrients. 2018; 10(1):51. https://doi.org/10.3390/nu10010051
Chicago/Turabian StyleChoi, Jia, Kui-Jin Kim, Eun-Jeong Koh, and Boo-Yong Lee. 2018. "Gelidium elegans Extract Ameliorates Type 2 Diabetes via Regulation of MAPK and PI3K/Akt Signaling" Nutrients 10, no. 1: 51. https://doi.org/10.3390/nu10010051




