Pre-Meal Effect of Whey Proteins on Metabolic Parameters in Subjects with and without Type 2 Diabetes: A Randomized, Crossover Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design
2.2. Subjects
2.3. Interventions
2.4. Blood Analysis
2.5. Statistical Analysis and Calculations
3. Results
3.1. Triglycerides, ApoB-48 and NEFA
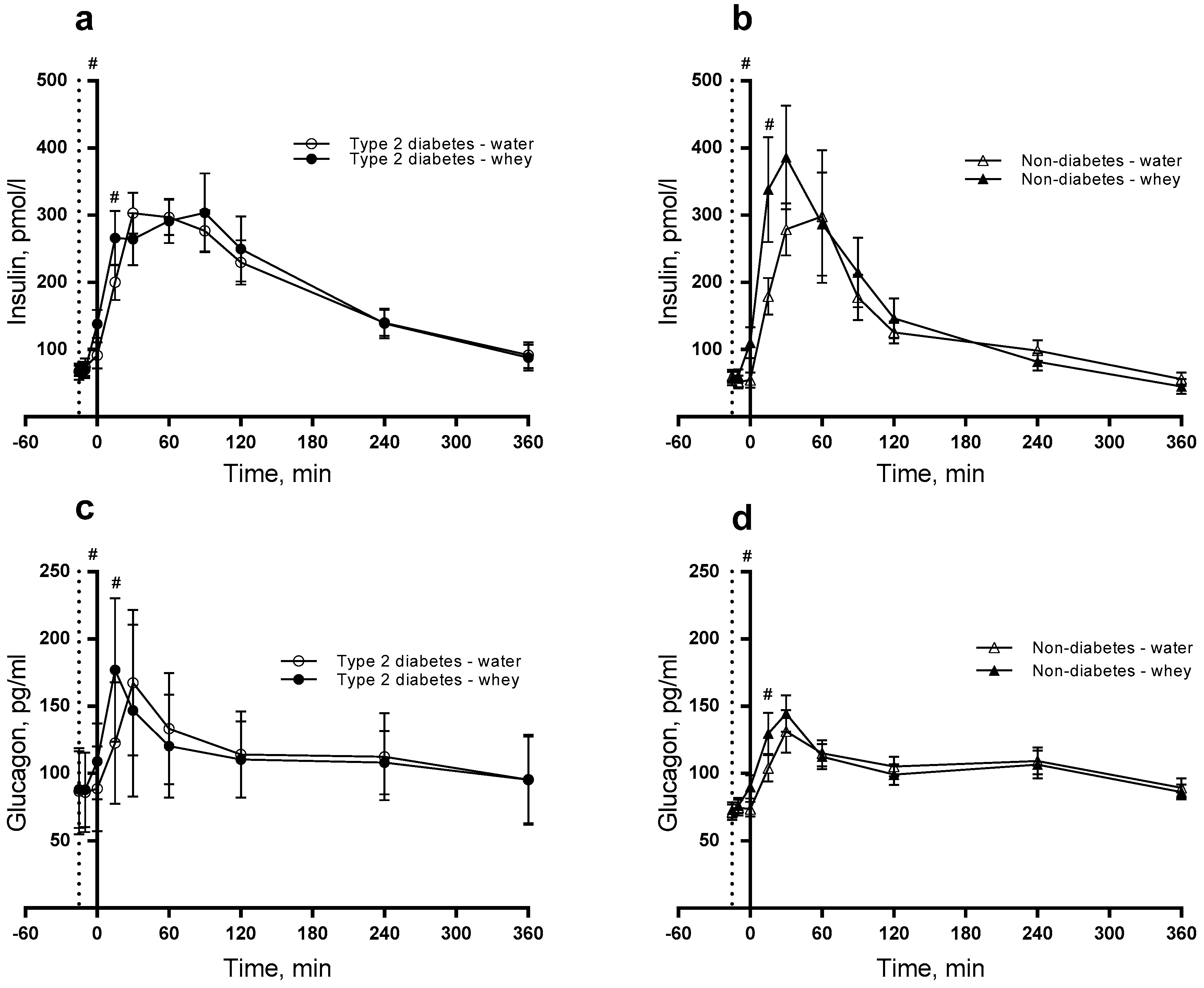
3.2. Insulin, Glucagon and Glucose
3.3. GIP and GLP-1
3.4. S-Paracetamol
3.5. Subjective Appetite
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ApoB-48 | Apolipoprotein B-48 |
| DPP-4 | Dipeptidyl peptidase 4 |
| EDTA | Ethylene-diamine-tetra acetic acid |
| ELISA | Enzyme-linked immunosorbent assay |
| E% | Energy percentage |
| GIP | Glucose-dependent insulinotropic peptide |
| GLP-1 | Glucagon-like peptide-1 |
| HbA1c | Haemoglobin A1c |
| iAUC | Incremental area under the curve |
| MUFA | Monounsaturated fatty acids |
| NEFA | Non-esterified fatty acids |
| PPL | Postprandial hypertriglyceridemia |
| PUFA | Polyunsaturated fatty acids |
| SEM | Standard error of the mean |
| SFA | Saturated fatty acids |
| TG | Triglyceride |
| T2D | Type 2 diabetes |
| VAS | Visual analogue scale |
| WP | Whey proteins |
References
- Kannel, W.B.; McGee, D.L. Diabetes and cardiovascular disease: The framingham study. JAMA 1979, 241, 2035–2038. [Google Scholar] [CrossRef] [PubMed]
- Taskinen, M.R. Diabetic dyslipidaemia: From basic research to clinical practice. Diabetologia 2003, 46, 733–749. [Google Scholar] [CrossRef] [PubMed]
- Hermansen, K.; Baekdal, T.A.; During, M.; Pietraszek, A.; Mortensen, L.S.; Jorgensen, H.; Flint, A. Liraglutide suppresses postprandial triglyceride and apolipoprotein B48 elevations after a fat-rich meal in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled, cross-over trial. Diabetes Obes. Metab. 2013, 15, 1040–1048. [Google Scholar] [CrossRef] [PubMed]
- Karpe, F. Postprandial lipoprotein metabolism and atherosclerosis. J. Intern. Med. 1999, 246, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Carstensen, M.; Thomsen, C.; Gotzsche, O.; Holst, J.J.; Schrezenmeir, J.; Hermansen, K. Differential postprandial lipoprotein responses in type 2 diabetic men with and without clinical evidence of a former myocardial infarction. Rev. Diabet. Stud. 2004, 1, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Nordestgaard, B.G.; Benn, M.; Schnohr, P.; Tybjaerg-Hansen, A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease and death in men and women. JAMA 2007, 298, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Zilversmit, D.B. Atherogenesis: A postprandial phenomenon. Circulation 1979, 60, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Lairon, D. Macronutrient intake and modulation on chylomicron production and clearance. Atheroscler. Suppl. 2008, 9, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Holmer-Jensen, J.; Mortensen, L.S.; Astrup, A.; de Vrese, M.; Holst, J.J.; Thomsen, C.; Hermansen, K. Acute differential effects of dietary protein quality on postprandial lipemia in obese non-diabetic subjects. Nutr. Res. 2013, 33, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, L.S.; Hartvigsen, M.L.; Brader, L.J.; Astrup, A.; Schrezenmeir, J.; Holst, J.J.; Thomsen, C.; Hermansen, K. Differential effects of protein quality on postprandial lipemia in response to a fat-rich meal in type 2 diabetes: Comparison of whey, casein, gluten and cod protein. Am. J. Clin. Nutr. 2009, 90, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Frid, A.H.; Nilsson, M.; Holst, J.J.; Bjorck, I.M. Effect of whey on blood glucose and insulin responses to composite breakfast and lunch meals in type 2 diabetic subjects. Am. J. Clin. Nutr. 2005, 82, 69–75. [Google Scholar] [PubMed]
- Ma, J.; Stevens, J.E.; Cukier, K.; Maddox, A.F.; Wishart, J.M.; Jones, K.L.; Clifton, P.M.; Horowitz, M.; Rayner, C.K. Effects of a protein preload on gastric emptying, glycemia and gut hormones after a carbohydrate meal in diet-controlled type 2 diabetes. Diabetes Care 2009, 32, 1600–1602. [Google Scholar] [CrossRef] [PubMed]
- Jakubowicz, D.; Froy, O.; Ahren, B.; Boaz, M.; Landau, Z.; Bar-Dayan, Y.; Ganz, T.; Barnea, M.; Wainstein, J. Incretin, insulinotropic and glucose-lowering effects of whey protein pre-load in type 2 diabetes: A randomised clinical trial. Diabetologia 2014, 57, 1807–1811. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, M.; Stenberg, M.; Frid, A.H.; Holst, J.J.; Bjorck, I.M. Glycemia and insulinemia in healthy subjects after lactose-equivalent meals of milk and other food proteins: The role of plasma amino acids and incretins. Am. J. Clin. Nutr. 2004, 80, 1246–1253. [Google Scholar] [PubMed]
- Akhavan, T.; Luhovyy, B.L.; Brown, P.H.; Cho, C.E.; Anderson, G.H. Effect of premeal consumption of whey protein and its hydrolysate on food intake and postmeal glycemia and insulin responses in young adults. Am. J. Clin. Nutr. 2010, 91, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Gunnerud, U.J.; Heinzle, C.; Holst, J.J.; Ostman, E.M.; Bjorck, I.M. Effects of pre-meal drinks with protein and amino acids on glycemic and metabolic responses at a subsequent composite meal. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, T.; Luhovyy, B.L.; Panahi, S.; Kubant, R.; Brown, P.H.; Anderson, G.H. Mechanism of action of pre-meal consumption of whey protein on glycemic control in young adults. J. Nutr. Biochem. 2014, 25, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, M.; Holst, J.J.; Bjorck, I.M. Metabolic effects of amino acid mixtures and whey protein in healthy subjects: Studies using glucose-equivalent drinks. Am. J. Clin. Nutr. 2007, 85, 996–1004. [Google Scholar] [PubMed]
- Bohl, M.; Bjornshave, A.; Rasmussen, K.V.; Schioldan, A.G.; Amer, B.; Larsen, M.K.; Dalsgaard, T.K.; Holst, J.J.; Herrmann, A.; O’Neill, S.; et al. Dairy proteins, dairy lipids and postprandial lipemia in persons with abdominal obesity (dairyhealth): A 12-Wk, randomized, parallel-controlled, double-blinded, diet intervention study. Am. J. Clin. Nutr. 2015, 101, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Kuhre, R.E.; Wewer Albrechtsen, N.J.; Hartmann, B.; Deacon, C.F.; Holst, J.J. Measurement of the incretin hormones: Glucagon-like peptide-1 and glucose-dependent insulinotropic peptide. J. Diabetes Complicat. 2015, 29, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Duez, H.; Lamarche, B.; Uffelman, K.D.; Valero, R.; Cohn, J.S.; Lewis, G.F. Hyperinsulinemia is associated with increased production rate of intestinal apolipoprotein B-48-containing lipoproteins in humans. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, J.P.; Maraninchi, M.; Beliard, S.; Padilla, N.; Duvillard, L.; Mancini, J.; Nicolay, A.; Xiao, C.; Vialettes, B.; Lewis, G.F.; et al. Absence of acute inhibitory effect of insulin on chylomicron production in type 2 diabetes. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Stanstrup, J.; Schou, S.S.; Holmer-Jensen, J.; Hermansen, K.; Dragsted, L.O. Whey Protein Delays Gastric Emptying and Suppresses Plasma Fatty Acids and their Metabolites Compared to Casein, Gluten and Fish Protein. J. Proteome Res. 2014, 13, 2396–2408. [Google Scholar] [CrossRef] [PubMed]
- Plummer, M.P.; Jones, K.L.; Cousins, C.E.; Trahair, L.G.; Meier, J.J.; Chapman, M.J.; Horowitz, M.; Deane, A.M. Hyperglycemia potentiates the slowing of gastric emptying induced by exogenous GLP-1. Diabetes Care 2015, 38, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Jesudason, D.R.; Stevens, J.E.; Keogh, J.B.; Jones, K.L.; Clifton, P.M.; Horowitz, M.; Rayner, C.K. Sustained effects of a protein ‘preload’ on glycaemia and gastric emptying over 4 weeks in patients with type 2 diabetes: A randomized clinical trial. Diabetes Res. Clin. Pract. 2015, 108, e31–e34. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, O.; Carr, R.D.; Holst, J.J.; Deacon, C.F.; Ahren, B. Dissociated incretin hormone response to protein versus fat ingestion in obese subjects. Diabetes Obes. Metab. 2011, 13, 863–865. [Google Scholar] [CrossRef] [PubMed]
- Meier, J.J.; Nauck, M.A. Clinical endocrinology and metabolism. Glucose-dependent insulinotropic polypeptide/gastric inhibitory polypeptide. Best Pract. Res. Clin. Endocrinol. Metab. 2004, 18, 587–606. [Google Scholar] [CrossRef] [PubMed]
- Holst, J.J.; McGill, M.A. Potential new approaches to modifying intestinal GLP-1 secretion in patients with type 2 diabetes mellitus: Focus on bile acid sequestrants. Clin. Drug Investig. 2012, 32, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Veldhorst, M.A.; Nieuwenhuizen, A.G.; Hochstenbach-Waelen, A.; van Vught, A.J.; Westerterp, K.R.; Engelen, M.P.; Brummer, R.J.; Deutz, N.E.; Westerterp-Plantenga, M.S. Dose-dependent satiating effect of whey relative to casein or soy. Physiol. Behav. 2009, 96, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Chungchunlam, S.M.; Moughan, P.J.; Henare, S.J.; Ganesh, S. Effect of time of consumption of preloads on measures of satiety in healthy normal weight women. Appetite 2012, 59, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.; Buring, J.E.; Rifai, N.; Mora, S.; Sacks, F.M.; Ridker, P.M. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA 2007, 298, 309–316. [Google Scholar] [CrossRef] [PubMed]



| Clinical Characteristic, Unit | Type 2 Diabetics (n = 12) | Non-Diabetics (n = 12) | P Value 2 |
|---|---|---|---|
| Gender, f/m | 3/9 | 3/9 | - |
| Age, years | 62.9 (57.0–68.8) | 62.8 (57.6–67.7) | 0.944 |
| Weight, kg | 89.1 (77.3–100.8) | 84.6 (78.1–91.1) | 0.473 |
| Fasting plasma glucose, mmol/L | 8.77 (7.6–10.0) | 5.7 (5.5–5.9) | <0.0001 |
| HbA1c, % | 49.6 (46.9–52.3) | 36.5 (33.8–39.2) | <0.0001 |
| Fasting triglycerides, mmol/L | 1.76 (0.88–2.28) | 1.33 (0.92–1.73) | 0.502 |
| HDL cholesterol, mmol/L | 1.3 (1.1–1.5) | 1.5 (1.2−1.9) | 0.226 |
| Type 2 Diabetes | Non-Diabetes | P-Value 3 | ||||||
|---|---|---|---|---|---|---|---|---|
| Parameter, Unit | Time, min | Whey Protein | Water | Whey Protein | Water | Diabetes × Intervention | Diabetes × Time | Intervention × Time |
| ApoB-48 2, µg/L | −15 (fasting) | 8596 (6847–10346) | 8750 (6968–10532) | 9141 (7304–10978) | 9348 (7470–11227) | 0.9282 | 0.0039 | 0.9416 |
| 240 | 23340 (18591–28090)x | 23758 (18920–28595)x | 18068 (14438–21699)y | 18478 (14766–22191)y | ||||
| 360 | 18029 (14360–21697)x | 18351 (14614–22088)x | 14202 (11349–17056)y | 14525 (11606–17443)y | ||||
| Triglycerides 2, mmol/L | −15 (fasting) | 1.47 (1.17–1.76) | 1.52 (1.22–1.82) | 1.25 (1.00–1.50) | 1.27 (1.02–1.52) | 0.9994 | 0.1011 | 0.9852 |
| 240 | 2.53 (2.02–2.03) | 2.62 (2.10–3.14) | 2.15 (1.72–2.58) | 2.19 (1.75–2.63) | ||||
| 360 | 2.04 (1.63–2.45) | 2.11 (1.69–2.53) | 1.74 (1.39–2.08) | 1.77 (1.41–2.11) | ||||
| NEFA, mmol/L | −15 (fasting) | 0.62 (0.58–0.66) | 0.60 (0.56–0.64) | 0.53 (0.49–0.57) | 0.52 (0.48–0.56) | 0.8925 | 0.7671 | 0.6513 |
| 120 | 0.26 (0.21–0.30) | 0.23 (0.19–0.28) | 0.17 (0.13–0.21) | 0.16 (0.11–0.20) | ||||
| 360 | 0.55 (0.51–0.59) | 0.53 (0.49–0.57) | 0.47 (0.42–0.51) | 0.44 (0.41–0.49) | ||||
| Insulin 2, pmol/L | −15 (fasting) | 60.9 (43.7–78.1) | 63.1 (45.5–80.6) | 50.3 (36.4–64.2) | 52.9 (28.3–67.5) | 0.2555 | <0.0001 | <0.0001 |
| 15 | 266.9 (192.7–341.2)x | 179.9 (129.9–230.0)y | 259.9 (188.3–331.5)x | 175.8 (127.4–224.4)y | ||||
| 120 | 218.5 (157.7–279.3) | 208.4 (150.4V–266.4) | 124.1 (89.9–158.2) | 118.8 (86.1–151.5) | ||||
| Glucagon 2, pg/mL | −15 (fasting) | 84.5 (76.9–92.1) | 83.3 (75.8–90.8) | 74.1 (67.5–80.7) | 71.9 (65.5–78.4) | 0.8994 | 0.1111 | <0.0001 |
| 15 | 153.2 (139.5–166.9)x | 118.5 (107.9–129.1)y | 134.3 (122.3–146.3)x | 102.3 (93.2–111.5)y | ||||
| 120 | 111.1 (101.1–121.0) | 117.9 (107.3–128.4) | 97.4 (88.7–106.1) | 101.8 (92.7–110.8) | ||||
| Glucose, mmol/L | −15 (fasting) | 8.47 (8.08–8.85) | 8.58 (8.19–8.96) | 5.61 (5.22–5.99) | 5.47 (5.08–5.9) | 0.0865 | <0.0001 | 0.5188 |
| 30 | 9.83 (9.44–10.21)x | 9.94 (9.55–10.32)x | 5.69 (5.58–6.35)y | 5.82 (5.44–6.21)y | ||||
| 120 | 9.85 (9.46–10.23)x | 9.96 (9.57–10.34)x | 5.46 (5.08–5.85)y | 5.33 (4.94–5.71)y | ||||
| GLP-1 2, pmol/L | −15 (fasting) | 21.6 (18.6–24.5) | 19.5 (16.8–22.3) | 14.2 (12.4–16.0) | 13.7 (11.9–15.5) | 0.4138 | 0.8896 | 0.4129 |
| 120 | 47.7 (41.2–54.3) | 43.3 (37.2–49.3) | 31.4 (27.3–35.5) | 30.4 (26.4–34.3) | ||||
| 360 | 26.0 (22.4–29.6) | 23.6 (20.2–26.9) | 17.1 (14.9–19.3) | 16.5 (14.4–18.7) | ||||
| GIP 2, pmol/L | −15 (fasting) | 9.7 (7.9–11.5) | 10.7 (8.8–12.7) | 8.5 (7.0–10.1) | 9.7 (7.9–11.5) | 0.9829 | 0.2080 | <0.0001 |
| 120 | 60.9 (49.7–72.1)x | 51.6 (42.1–61.1)y | 53.7 (43.9–63.6)x | 46.6 (38.0–55.2)y | ||||
| 360 | 28.8 (23.4–34.0) | 27.7 (22.6–32.8) | 25.3 (20.7–30.0) | 25.0 (20.4–29.6) | ||||
| S-paracetamol 2,4, µmol/L | 30 | 53.5 (33.8–73.2)x | 107.8 (67.0–148.6)y | 55.8 (35.3–76.3)x | 116.4 (72.3–160.4)y | 0.4115 | 0.0271 | 0.0390 |
| 120 | 56.9 (36.1–77.7) | 58.7 (37.4–80.2) | 60.0 (38.1–81.9) | 64.2 (40.75–9.6) | ||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bjørnshave, A.; Hermansen, K.; Holst, J.J. Pre-Meal Effect of Whey Proteins on Metabolic Parameters in Subjects with and without Type 2 Diabetes: A Randomized, Crossover Trial. Nutrients 2018, 10, 122. https://doi.org/10.3390/nu10020122
Bjørnshave A, Hermansen K, Holst JJ. Pre-Meal Effect of Whey Proteins on Metabolic Parameters in Subjects with and without Type 2 Diabetes: A Randomized, Crossover Trial. Nutrients. 2018; 10(2):122. https://doi.org/10.3390/nu10020122
Chicago/Turabian StyleBjørnshave, Ann, Kjeld Hermansen, and Jens Juul Holst. 2018. "Pre-Meal Effect of Whey Proteins on Metabolic Parameters in Subjects with and without Type 2 Diabetes: A Randomized, Crossover Trial" Nutrients 10, no. 2: 122. https://doi.org/10.3390/nu10020122






